Introduction
Sevoflurane is a widely-used volatile anesthetic for
the induction and maintenance of general anesthesia, particularly
in pediatric anesthesia, due to its rapid induction and the rapid
recovery of patients. It has been reported that exposure of the
neonatal nervous system to sevoflurane may lead to widespread
neurodegeneration in a number of regions of the developing rat
brain and that it may cause learning abnormalities in mice
(1,2). The process of cell death triggered by
sevoflurane exhibits all the classical ultrastructural
characteristics of apoptosis (3–5) and
is known to be regulated by the Bax-dependent intrinsic pathway,
involving cytochrome c release from the mitochondria and
activation of the caspase cascade (6–8).
Currently there is no available prophylactic treatment to prevent
anesthesia-induced neuroapoptosis.
An Gong Niu Huang Pill is a well-known traditional
Chinese medicine used in the treatment of certain neurological
disorders. It contains Calculus bovis, Cornu bubali,
Cinnabar, Curcuma aromatica, Scutellaria, Coptidis
rhizoma and Fructus gardenia (30 g of each), Moschus and
Borneo camphor (7.5 g of each) and Concha margaritifera (15
g) (9). Xingnaojing (XNJ), an
extract of An Gong Niu Huang Pill, predominantly contains Moschus
(Moschus berezovskii F.; 7.5 g), Radix curcumae
(Curcuma wenyujin Y.H. Chen & C. Ling, Zingiberaceae; 30
g), Borneolum (Blumea balsamifera DC,
Compositae; 1 g) and Fructus gardenia (Gardenia
jasminoides J. Ellis, Rubiaceae; 30 g) (10). XNJ is a herbal preparation of
moschus, borneolum, Fructus gardenia and Radix
curcumae and is primarily used in the treatment of neurological
diseases, including brain ischemia, neurotoxic damage, cerebral
hemorrhage, bacterial and viral meningitis and vascular dementia
(10–12). It is unclear whether XNJ may
protect against sevoflurane-induced neuronal apoptosis in the
developing brain. Thus, the present study was designed to
investigate the possible neuroprotective effects of XNJ, and to
attempt to elucidate the underlying mechanisms of any such
effects.
Materials and methods
Preparation and quality control of
XNJ
XNJ was obtained from Wuxi Jiminkexin Shanhe
Pharmaceutical Co., Ltd. (Wuxi, China) with the Chinese Food and
Drug Administration number z32020563. It is registered by the
Ministry of Public Health of China under the Chinese traditional
patent formulation no. 17 (standard code, WS3-B-3353-98). The
experiments were repeated in five different batches (batch numbers:
120313, 121101, 121205, 120506 and 120103). The result were highly
similar with each batch. XNJ was prepared from four traditional
Chinese medicines, including Moschus, Borneolum, Radix
curcumae and Fructus gardenia (10). These herbs were identified by
Professor Yuning Yan of Beijing University of Chinese Medicine
(Beijing, China). Dried Radix curcumae and Fructus
gardenia (~30) were dissolved into 1.5 liters of water. The
solution was distilled, leaving 1 liter of liquid. Moschus (7.5 g)
and 250 ml distilled water were added for further distillation and
1 liter of liquid was collected. Borneolum (1 g) with 8 g
polysorbate 80 were added to the mixed solution. Finally, 8 g
sodium chloride was added. The solution was filtered, sterilized
and transferred into ampoules (13). In accordance with the corresponding
quality control standard, XNJ should contain no less than 0.7 g/l
Borneolum (molecular formula, C10H18O)
(13).
The chemical fingerprint of XNJ has been identified
in previous studies. The central active components of XNJ are
muscone (PubChem CID, 10947), L-borneol (PubChem CID, 6850744),
L-camphor (PubChem CID, 230921), isoborneol (PubChem CID, 6321405),
curdione (PubChem CID, 6441391), germacrone (PubChem CID, 6436348),
curcumin (PubChem CID, 969516) and geniposide (PubChem CID, 107848)
(10,14–17).
Animals
The study protocol was approved by the Animal Use
and Care Committee for Research and Education of Shanghai Jiao Tong
University (Shanghai, China). Sprague Dawley rats (Shanghai SLAC
Laboratory Animal Co., Ltd, Shanghai, China) used in this study
were maintained under a 12 h light/dark cycle (7am–7pm) with a room
temperature of 22 ± 1°C. Food and water were available ad
libitum for the lactating rats. Pups were divided into groups
with approximately equal numbers of males and females for all
experiments. The number of animals used and any suffering were
minimized.
Anesthesia treatment
On postnatal day seven (P7), rat pups
were placed into a chamber and exposed to 2.1% sevoflurane for 6 h.
The total gas flow was 1.5 l/min, using 70% O2 as a
carrier. The oxygen and anesthetic agent fractions were measured
using a gas analysis system (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA). During exposure to the anesthetic, the
chamber was maintained at 37 ± 1°C with an infrared heat lamp.
Animals were sacrificed following 6 h of anesthesia.
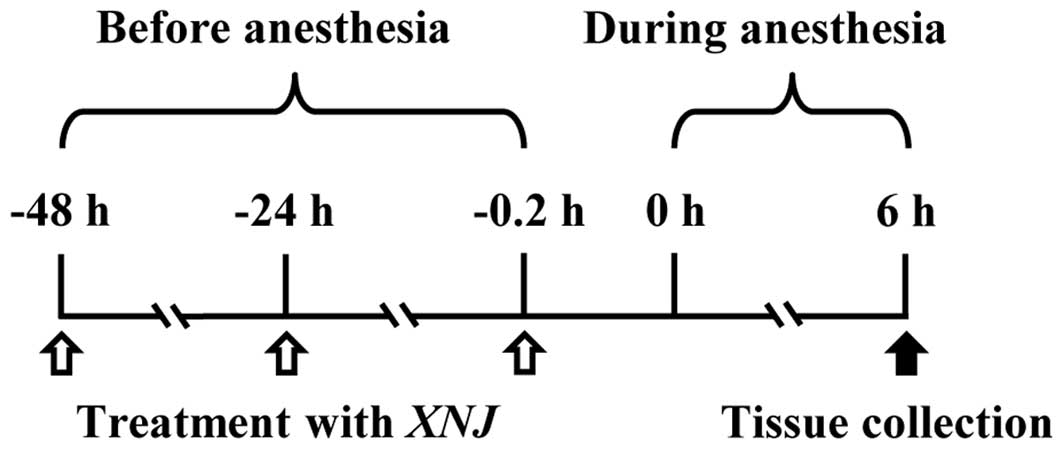
Pre-treatment strategies
Rat pups were injected intraperitoneally with 1 or
10 ml/kg XNJ (Henan New Century Pharmaceutical Co., Ltd., Henan,
China) at 0.2, 24 and 48 h prior to anesthesia. Rat pups in the
control group received an equal volume of saline (Fig. 1).
Western blot analysis
Striatal tissues were homogenized in
radioimmunoprecipitation assay lysis buffer, pH 7.4, containing 50
mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS), a protease
inhibitor cocktail (Thermo Fisher Scientific, Pittsburgh, PA, USA),
and phosphatase inhibitors (10 mM Na3VO4, 10
mM NaF). Following homogenization, samples were centrifuged at
15294 xg for 10 min. Equal quantities of protein lysates were
loaded onto a 10–15% SDS-polyacrylamide gel electrophoresis gel and
transferred onto a 0.2 μm polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked with 5%
non-fat milk to reduce nonspecific binding, and immunobloted
overnight using primary antibodies against Bax (1:2,000 dilution;
Epitomics, Burlingame, CA, USA), activated caspase 3 (1:500
dilution), phosphorylated protein kinase B (p-AKT; at site Thr308;
1:1,000 dilution), p-AKT (at site Ser473; 1:1,000 dilution), AKT
(1:5,000 dilution), phosphorylated extracellular-regulated protein
kinase (p-ERK; 1:1,000 dilution), ERK (1:1,000 dilution),
phosphorylated c-Jun N-terminal kinase (p-JNK; 1:5,000 dilution)
and JNK (1:1,000 dilution; all Cell Signaling Technology Inc.,
Danvers, MA, USA). Anti β-actin antibody (1:200 dilution; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was used as a control to
confirm equal loading. Immunocomplexes were visualized using an
enhanced chemiluminescence-detection reagent (EMD Millipore). Band
intensities were measured with Quantity One 4.4.0 software (Bio-Rad
Laboratories, Hercules, CA, USA).
Immunohistochemistry
The rats of sevo group and sevo plus pre-treatment
with XNJ group were exposed to 2.1% sevoflurane for 6 h. Soon after
they revived, the rats of all the groups, including the control
group, XNJ group, sevo group and sevo plus pre-treatment with XNJ
group, were anesthetized with sodium pentobarbital (60 mg/kg;
Harbin Pharmaceutical Group Co., Ltd., Harbin, China) to minimize
their suffering. The rats were sacrificed for immunohistochemistry
analysis. The brains were perfusion-fixed with 4% paraformaldehyde
and 0.1% picric acid in 0.1 M phosphate buffer (pH 7.4) followed by
immersion fixation in the same mixture overnight. Subsequently, one
hemisphere was kept in 20% sucrose in 0.1 M phosphate buffer for at
least two days at 4°C. Sagittal sections (20 μm) were cut using a
vibratome (Leica Microsystems GmBH, Wetzlar, Germany). Frozen
tissue sections were incubated for 10 min with 3%
H2O2 in 100% methanol to inactivate any
endogenous peroxidase. Tissues were permeabilized and non-specific
binding was blocked using 0.2% Triton X-100 and 5% heat-inactivated
donkey serum in phosphate-buffered saline (PBS). Primary rabbit
monoclonal antibodies against activated caspase 3 (Cell Signaling
Technology Inc., 1:200) were diluted in 5% donkey serum/PBS and
incubated overnight at 4°C. Specimens were incubated in
biotinylated goat anti-rabbit IgG (diluted 1:500 in PBS containing
1% normal goat serum; Beyotime Institute of Biotechnology,
Shanghai, China) for 3 h at room temperature. Sections were
developed using the VECTASTAIN ABC reagent (Vector Laboratories,
Inc., Burlingame, CA, USA) in PBS with 0.1% Tween-20 for 30 min. A
DAB substrate kit (Vector Laboratories Inc., Burlingame, CA, USA)
was used for immunohistochemical staining. Tissue sections were
examined under a light microscope (Leica DM400B; Leica Microsystems
GmBH). The number of activated caspase 3-positive neurons in the
striatum of the developing rats was counted with Image J 1.48u
software (National Institutes of Health, Bethesda, Maryland,
USA).
Statistical analysis
The experiments were performed with five different
batches of XNJ and repeated at least three times. Statistical
analyses were performed using SigmaPlot version 10.0 (Systat
Software Inc., San Jose, CA, USA). All data are expressed as the
mean ± standard error of the mean. Comparisons among multiple
groups involved one-way analysis of variance plus Newman-Keul’s
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Dose-dependent effect of XNJ on the
expression of activated caspase 3 in the striatum of the developing
rat brain following sevoflurane treatment
Caspases are cysteine proteases that are involved in
apoptosis. Increased expression and activation of caspase family
members is known to contribute to neuronal death (18). Western blot analysis was performed
to quantify the expression levels of activated caspase 3. Caspase 3
activity was significantly increased in the striatum of the
developing rats following 6 h of sevoflurane treatment compared
with the control group (Fig. 2).
The increase of caspase 3 activity was partially inhibited by
pre-treatment with 10 ml/kg XNJ (Fig.
2), but not by pre-treatment with the lower dose of 1 ml/kg
(Fig. 2). Furthermore, treatment
with XNJ alone did not increase the activity of caspase 3 in the
striatum of neonatal rats (Fig.
2).
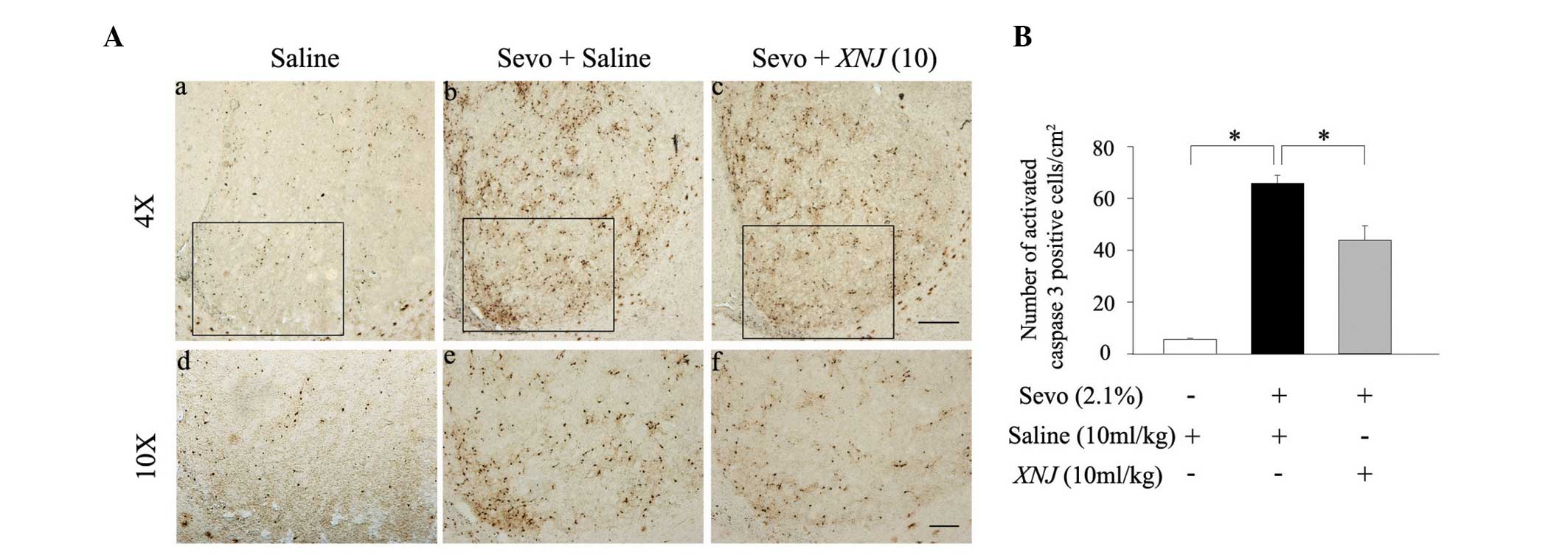
The expression of activated caspase 3 following 6 h
of sevoflurane treatment was also measured using
immunohistochemistry. There were few activated caspase 3-positive
neurons in the striatum of the developing rats that did not receive
sevoflurane anesthesia (Fig. 3Aa and
d). However, the number of activated caspase 3-positive neurons
was significantly increased following 6 h of administration with
sevoflurane (2.1%; Fig. 3Ab and
e). Pre-treatment with 10 ml/kg XNJ reversed the
sevoflurane-induced increase of activated caspase 3-positive
neurons in the striatum of neonatal rats (Fig. 3Ac and f). The statistical data from
this experiment concurred with the data from the western blot
analysis. (Fig. 3B). These results
show that sevoflurane-induces neuronal apoptosis in the striatum
and that this effect may be counteracted by administration of
XNJ.
Effect of XNJ on sevoflurane-induced
upregulation of apoptosis-related protein Bax levels in the
striatum of rat pups
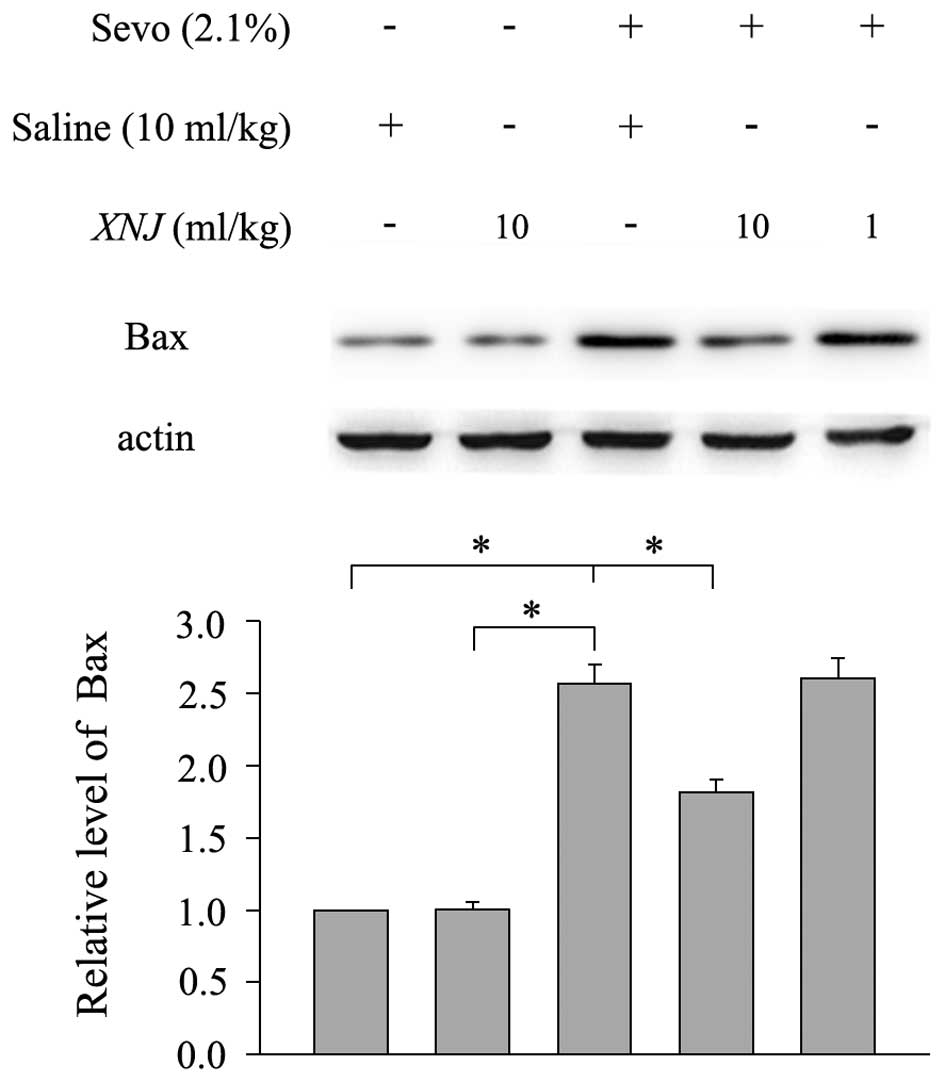
The proapoptotic protein, Bax, localizes to the
mitochondria in response to numerous apoptotic stimuli and leads to
the release of cytochrome c, which further activates the
caspase cascade (19). Therefore,
the expression level of Bax was measured in the striatum of
neonatal rats following 6 h of sevoflurane treatment in the absence
and presence of 10 ml/kg XNJ. The expression of Bax in the striatum
was upregulated following sevoflurane treatment compared with the
control group, whilst pre-treatment with 10 ml/kg XNJ significantly
reversed the sevoflurane-induced increase in Bax expression
(Fig. 4). However, pre-treatment
with 1 ml/kg XNJ had no significant protective effect on the
sevoflurane-induced upregulation of the Bax protein (Fig. 4).
These results show that treatment with sevoflurane
results in an increase in Bax expression, which may lead to
neuroapoptosis. However, pre-treatment with XNJ significantly
reduced this upregulation of Bax, which indicates a possible
neuroprotective effect.
Effect of XNJ and sevoflurane on Akt,
ERK1/2 and JNK expression
It is reported that the AKT, ERK and JNK pathways
are involved in the modulation of neuronal apoptosis (20). The present study aimed to
investigate the effects of pre-treatment with XNJ on these three
signaling cascades. Western blot analysis showed that sevoflurane
significantly reduced the levels of p-AKT (Ser473; Fig. 5A) and p-AKT (Thr308; Fig. 5B) in the striatum of rat pups.
Pre-treatment with 10 ml/kg XNJ reduced the suppressant effect of
sevoflurane on p-AKT levels (Fig. 5A
and B). XNJ at a dose of 1 ml/kg did not significantly affect
the expression of p-AKT (Fig. 5A and
B). Notably, sevoflurane anesthesia had no significant effect
on the levels of the unphosphorylated AKT protein (Fig. 5A and B).
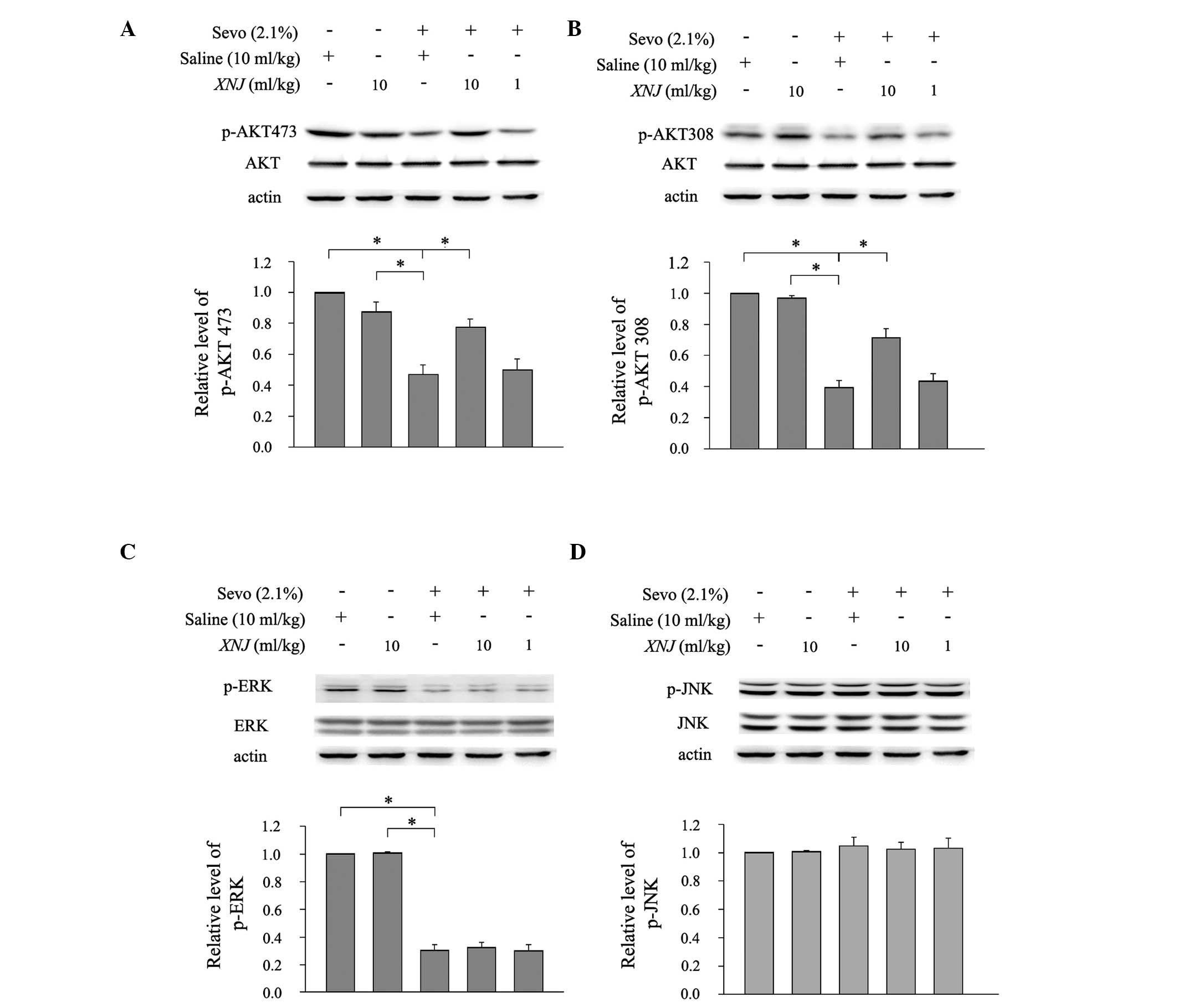 | Figure 5Effect of XNJ on the expression of
p-AKT, p-ERK and p-JNK in the striatum following sevoflurane
exposure. Upper panel, representative western blots; lower panel,
quantitative data showing expression of (A) p-AKT (Ser473), (B)
p-AKT (Thr308), (C) p-ERK and (D) p-JNK in the striatum of
seven-day-old rats in: Lane 1, the control group; lane 2, the XNJ
group; lane 3, the sevo group; lane 4, the sevo plus pre-treatment
with 10 ml/kg XNJ group; and lane 5, the sevo plus pre-treatment
with 1 ml/kg XNJ group. β-actin was used as a protein loading
control. Quantitative data are presented as the mean ± standard
deviation (n=5). *P<0.05. XNJ, xingnaojing; Sevo,
sevoflurane; p-AKT, phosphorylated protein kinase B; p-ERK,
phosphorylated extracellular-regulated kinase; p-JNK,
phosphorylated c-Jun N-terminal kinase. |
Sevoflurane anesthesia also reduced the expression
of p-ERK in the striatum of neonatal brain (Fig. 5C), which was in accordance with a
previous study (20). However,
pre-treatment with 1 or 10 ml/kg XNJ did not counteract this
decrease in p-ERK expression (Fig.
5C). In comparison to p-AKT and p-ERK, sevoflurane anesthesia
had no inhibitory effect on the expression of p-JNK (Fig. 5D).
These results indicate that sevoflurane-induced
neuronal apoptosis is modulated by the PI3K/AKT and ERK pathways.
Furthermore, administration of 10 mg/kg XNJ modulated the effect of
sevoflurane on the PI3K/AKT pathway, but not the ERK pathway.
Discussion
Exposure to sevoflurane (2.1%) for 6 h significantly
increased neuronal apoptosis in the striatum of P7 rat
brain. It has been shown that there is no evidence of hypoxia in
rat pups following treatment with sevoflurane at a concentration of
2.1% (21). The increased
apoptosis detected in the present study was therefore predominantly
a result of the anesthesia. The current study aimed to investigate
the effect of pre-treatment with XNJ on sevoflurane-induced
neuronal apoptosis in the neonatal rat brain. XNJ was administered
at 0.2, 24 and 48 h prior to anesthesia. The results showed that
pre-treatment with 10 ml/kg XNJ reversed in part the
sevoflurane-induced neuronal apoptosis in the striatum of the
neonatal rat brain.
It has been shown that neonatal exposure to
sevoflurane may cause deficits in a number of aspects of cognitive
function later in life, including maternal behavior, learning
disabilities, social behaviors, adaptability changes and behavior
in fear conditioning tests (2,22,23).
The striatum is composed of functional sub-units that are part of
cortico-striatal-thalamic circuits. Recent studies have focused on
the contribution of striatal sub-regions to planning and decision
making, declarative memory retrieval, learning of associations
between stimulation, actions and rewards, selection among
alternatives and modulation of motor behavior (24–26).
Thus, neuroprotection of the striatum against volatile anesthetics
is an important area of research, particularly in pediatric
anesthesia.
An Gong Niu Huang Pill is a well-known
traditional Chinese medicine. In traditional medicine, it is
believed to cure the illness, known as ‘shutting syndrome’, in
which the patient is thought to lose consciousness as a result of
too much sputum, fire or blot in the meridian. The herbs treat the
patient by keeping these factors away from the brain, something
that is termed the ‘Xingnaokaiqiao’ method (9). A previous study showed that An
Gong Niu Huang Pill promotes the recovery of neonatal
hypoxic-ischemic encephalopathy and answers the safety for the
newborn babies (27). XNJ, an
extract of An Gong Niu Huang Pill, has similar clinical
applications. A previous study investigated the pharmacokinetics of
muscone, borneol and geniposide, the main components of XNJ,
following injection into the tail veins of rats. The time taken to
reach their maximum concentrations in the brain was ~5 min for
muscone (28), 1 min for borneol
(29) and 1 min for geniposide
(30). The time taken to reach
their maximum concentrations in the brain when administered nasally
was ~3.4 min for borneol (29) and
3 min for geniposide (30). The
action of XNJ is rapid and relatively long-lasting in the brain
(31). The duration of XNJ
pre-treatment in the present study was based on these findings.
XNJ has a direct effect on the central nervous
system as it is able to cross the blood brain barrier (32). It has been shown to be effective in
a number of neurological diseases, including cerebrovascular
disorders, central nervous system infections, cerebral injury,
alcoholism and seizure disorders (17,33–35).
XNJ enhances learning and memory, and facilitates cognitive
function in older animals following ketamine treatment (36,37).
Furthermore, XNJ may exert an antiapoptotic effect (11). These studies led to the hypothesis
that XNJ may inhibit apoptosis triggered by sevoflurane in the
developing rat brain.
The mechanism of neuronal apoptosis has been well
investigated. Bax and activated caspase 3 are likely to be involved
in neuronal apoptosis during synaptogenesis (38,39).
Caspase 3 is a cysteine protease that is involved in mediating cell
apoptosis. It results in cell shrinkage, DNA degradation and the
formation of apoptotic bodies (40). The proapoptotic protein, Bax,
localizes to the mitochondria in response to numerous apoptotic
stimuli and leads to the release of cytochrome c, which
further activates the caspase cascade. Therefore Bax is important
in a number of apoptotic signaling pathways (41). It has been shown that anesthetic
exposure may expedite physiological apoptosis by elevating the
expression of Bax and activated caspase 3 (38). The results from the present study
supported this finding and showed that pre-conditioning with XNJ
reduced these changes in rat striatum.
To further investigate the effects of XNJ, the
activity of the MAPK and PI3K/AKT pathways, which have potential
neuroprotective actions, was investigated. AKT, ERK and JNK kinases
are known to be involved in regulating diversified physiological
and pathological processes in the brain, including neuronal
survival, apoptosis, proliferation, differentiation, development
and synaptic plasticity (42).
High levels of growth factors, particularly those that activate
PI3K, may favor the survival of certain cell types with the aid of
AKT (43). The PI3K/AKT signaling
pathway, which triggers the phosphorylation of the serine/threonine
kinase AKT, protects cells from apoptosis by increasing the
activity of proteins that promote cell survival and suppressing
caspases involved in promoting apoptosis (44,45).
AKT is primarily phosphorylated at two sites: Thr308 and Ser473
(46). In the present study,
sevoflurane treatment inhibited the expression of p-AKT (Thr308)
and p-AKT (Ser473) in the striatum of rat neonatal brain, although
it had no significant effect on the expression of the AKT protein.
These results were consistent with those from previous studies
(20,38). It was found that pre-treatment with
10 mg/kg XNJ prior to anesthesia reduced the inhibitory effect of
sevoflurane on the expression of p-AKT (at Ser473 and Thr308), but
did not alter the expression of the AKT protein in the striatum of
rat neonatal brain. Although sevoflurane inhibited the
phosphorylation of ERK, pre-treatment with 10 mg/kg XNJ had no
significant effect on ERK phosphorylation in the striatum. It was
also demonstrated that sevoflurane anesthesia did not alter the
activity of JNK in the striatum. Notably, treatment with 10 mg/kg
XNJ alone had no significant effect on the activity of AKT, ERK or
JNK in the striatum of neonatal rat brain, which implies it may be
safe to use in neonatal rodents.
It has been reported that a combination of two or
more kinase systems may be required in order to trigger apoptosis.
AKT suppression leads to apoptosis when the ERK pathway is
suppressed simultaneously (20,47).
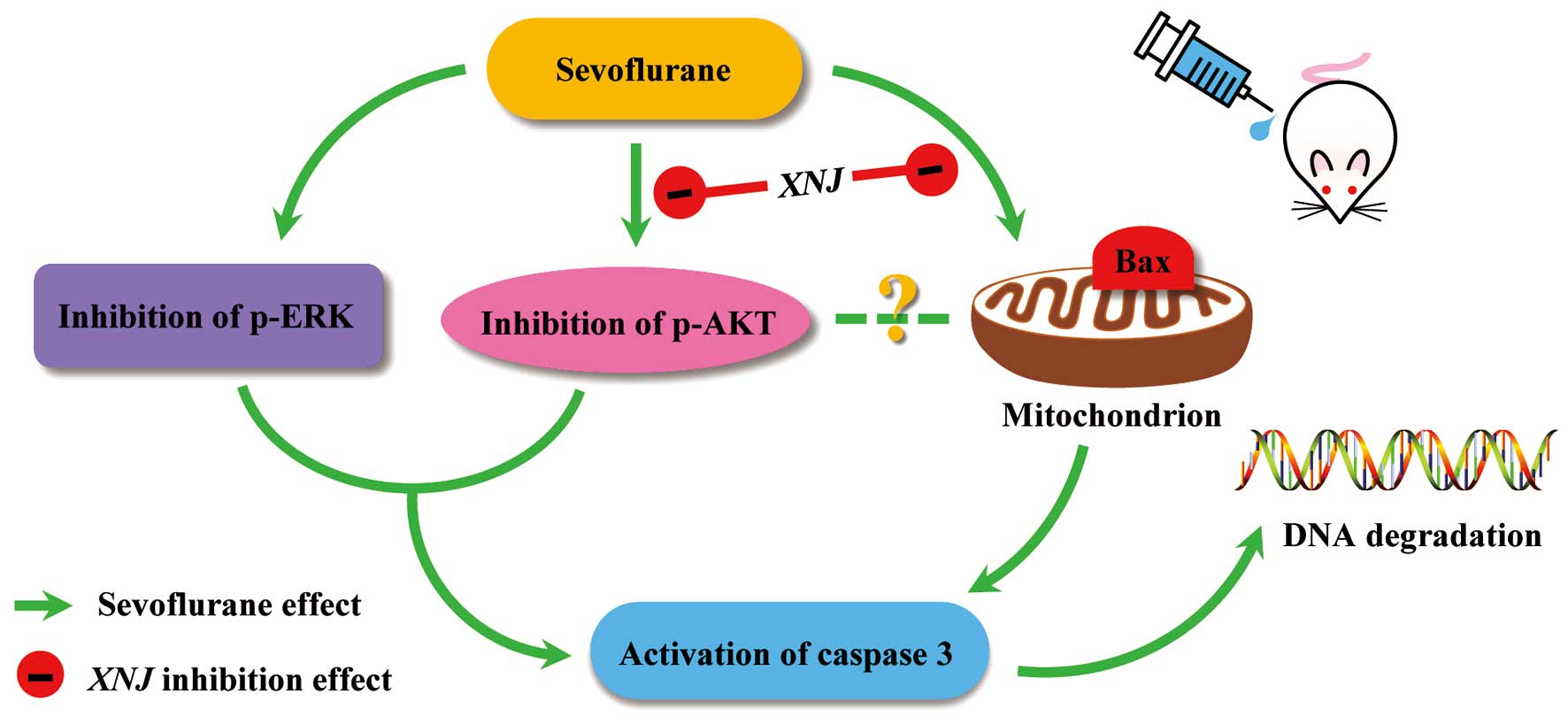
It is therefore likely that sevoflurane-induced neuroapoptosis
results from suppression of the p-AKT and p-ERK pathways. In
addition, the current study suggested that there may be a point at
which XNJ interferes with the apoptotic signaling pathway.
Pre-treatment with XNJ reversed the sevoflurane-induced reduction
in p-AKT, but not p-ERK expression. Thus phosphorylation of AKT may
be the step at which XNJ exerts its antiapoptotic and
neuroprotective effects. To the best of our knowledge, there are no
previous studies showing that the effect of XNJ on p-AKT expression
protects against drug-induced neuroapoptosis. Elucidation of the
exact mechanism requires further investigation. Furthermore,
activation of AKT results in phosphorylation of the proapoptotic
protein, Bax. This interferes with the stability of Bax, abrogates
its proapoptotic function and aids in promoting cell survival
(48). The present study found
that pre-conditioning with XNJ reduced neuronal apoptosis via
regulation of Bax, but did not confirm the association between
p-AKT and Bax, which requires further investigation (Fig. 6).
To date, a number of approaches to combat
anesthesia-induced neonatal brain injury have been addressed in
numerous animal models, including pre-conditioning with pyruvate,
lithium, nicotinamide, N-stearoyl-l-tyrosine and nucleosome
assembly protein (20,38,49,50).
Although these drugs have yielded favorable responses, the adverse
effects of these compounds on the brains of infants remain unclear
(20). In traditional Chinese
medicine, XNJ is believed to act on the patients by the principle
of ‘jun-chen-zuo-shi’, on a systematic level, in which the active
components interact with each other, enhancing the efficacy and
reducing the side effects of this compound. It has traditionally
been used in China and no significant adverse effects are known.
The current study showed the neuroprotective effect of
sevoflurane-induced neuronal apoptosis in neonatal rat brain,
indicating that XNJ may be effective against anesthesia-induced
neurotoxicity in the infant brain. However, the underlying
mechanisms of this effect remain unclear and further investigation
is required in order to demonstrate its safety and efficacy in
human infants.
In conclusion, pre-treatment with 10 ml/kg XNJ may
have a neuroprotective effect against sevoflurane-induced neuronal
apoptosis in the striatum of neonatal rat brain. The effect of XNJ
on the phosphorylation of AKT may contribute to this
neuroprotective effect.
Acknowledgements
This study was supported by the fund of Shanghai
Science and Technology Commission (grant no. 11DZ1974000), the
National Natural Science Foundation of China (Beijing) Grant (grant
no. 81171169) and the Shanghai New 100-Talent Program Grant (grant
no. XBR2011023).
References
|
1
|
Lerman J, Sikich N, Kleinman S and Yentis
S: The pharmacology of sevoflurane in infants and children.
Anesthesiology. 80:814–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Satomoto M, Satoh Y, Terui K, et al:
Neonatal exposure to sevoflurane induces abnormal social behaviors
and deficits in fear conditioning in mice. Anesthesiology.
110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dikranian K, Ishimaru MJ, Tenkova T, et
al: Apoptosis in the in vivo mammalian forebrain. Neurobiol Dis.
8:359–379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dikranian K, Qin YQ, Labruyere J, Nemmers
B and Olney JW: Ethanol-induced neuroapoptosis in the developing
rodent cerebellum and related brain stem structures. Brain Res Dev
Brain Res. 155:1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olney JW, Tenkova T, Dikranian K, Qin YQ,
Labruyere J and Ikonomidou C: Ethanol-induced apoptotic
neurodegeneration in the developing C57BL/6 mouse brain. Brain Res
Dev Brain Res. 133:115–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olney JW, Tenkova T, Dikranian K, et al:
Ethanol-induced caspase-3 activation in the in vivo developing
mouse brain. Neurobiol Dis. 9:205–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young C, Klocke BJ, Tenkova T, et al:
Ethanol-induced neuronal apoptosis in vivo requires BAX in the
developing mouse brain. Cell Death Differ. 10:1148–1155. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Young C, Roth KA, Klocke BJ, et al: Role
of caspase-3 in ethanol-induced developmental neurodegeneration.
Neurobiol Dis. 20:608–614. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv L, Liu Y, Shi HF and Dong Q:
Qingkailing injection attenuates apoptosis and neurologic deficits
in a rat model of intracerebral hemorrhage. J Ethnopharmacol.
125:269–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu P, Du SY, Lu Y, et al: The effect of
stroke and other components in Xing-Nao-Jing on the
pharmacokinetics of geniposide. J Ethnopharmacol. 152:302–307.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei G, Chen DF, Lai XP, et al: Muscone
exerts neuroprotection in an experimental model of stroke via
inhibition of the fas pathway. Nat Prod Commun. 7:1069–1074.
2012.PubMed/NCBI
|
|
12
|
Yang XL, Xu WY, G YX, LYP and Sun Y:
Studies on pharmacological action of Xingnaojing injection. China
Pharmacy. 22–23. 1993.(In Chinese).
|
|
13
|
Ministry of Public Health. Drug Standard
of Ministry of Public Health of the Peoples Republic of China.
People’s Medical Publishing House; Beijing: 1998
|
|
14
|
Zhang QD, Liu DH, Lin WW, Wei G, Huang YC
and Feng XQ: Analysis of Xingnaojing injection by chiral capillary
gas chromatography. Traditional Chinese Drug Research &
Clinical Pharmacology. 23:338–341. 2012.(In Chinese).
|
|
15
|
Wang LQ, Wang SL and SU DS: Quality
control study of Xingnaojing injection. Chinese Traditional Patent
Medicine. 26:289–291. 2004.(In Chinese).
|
|
16
|
Yang LX, Liu WW, Gan GF, Zhou FK, He XR
and Li C: Simultaneous determination of curdione and germacrone in
Xingnaojing injection using high performance liquid chromatography.
Chinese Journal of Experimental Traditional Medical Formulae.
17:136–139. 2013.(In Chinese).
|
|
17
|
Xu M, Su W, Xu QP and Huang WD: Effect of
Xingnaojing injection on cerebral edema and blood-brain barrier in
rats following traumatic brain injury. Chinese J Traumatol.
13:158–162. 2010.
|
|
18
|
Rupinder SK, Gurpreet AK and Manjeet S:
Cell suicide and caspases. Vascular Pharmacol. 46:383–393. 2007.
View Article : Google Scholar
|
|
19
|
Reed JC: Bcl-2 family proteins. Oncogene.
17:3225–3236. 1998. View Article : Google Scholar
|
|
20
|
Straiko MM, Young C, Cattano D, et al:
Lithium protects against anesthesia-induced developmental
neuroapoptosis. Anesthesiology. 110:862–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edwards DA, Shah HP, Cao W, Gravenstein N,
Seubert CN and Martynyuk AE: Bumetanide alleviates epileptogenic
and neurotoxic effects of sevoflurane in neonatal rat brain.
Anesthesiology. 112:567–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takaenoki Y, Satoh Y, Araki Y, et al:
Neonatal exposure to sevoflurane in mice causes deficits in
maternal behavior later in adulthood. Anesthesiology. 120:403–415.
2014. View Article : Google Scholar
|
|
23
|
Zheng SQ, An LX, Cheng X and Wang YJ:
Sevoflurane causes neuronal apoptosis and adaptability changes of
neonatal rats. Acta Anaesthesiol Scand. 57:1167–1174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furuyashiki T and Deguchi Y: Roles of
altered striatal function in major depression. Brain Nerve.
64:919–926. 2012.(In Japanese). PubMed/NCBI
|
|
25
|
Liljeholm M and O’Doherty JP:
Contributions of the striatum to learning, motivation, and
performance: an associative account. Trends Cogn Sci. 16:467–475.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scimeca JM and Badre D: Striatal
contributions to declarative memory retrieval. Neuron. 75:380–392.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su WD, Huang YD, Qu EL, Zhang Y, Ye W and
Bao M: Effect of angong niuhuang pill as an adjuvant treatment on
moderate or severe neonatal hypoxic-ischemic Encephalopathy.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:652–654. 2005.(In Chinese).
PubMed/NCBI
|
|
28
|
Chen WK, Huang YF and Wang HD: An
experimental study on distribution of musk into the brain through
blood brain barrier. Zhong Xi Yi Jie He Xue Bao. 2:288–291. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao JY, Lu Y, Du SY, Song X, Bai J and
Wang Y: Comparative pharmacokinetic studies of borneol in mouse
plasma and brain by different administrations. J Zhejiang Univ Sci
B. 13:990–996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu Y, Du S, Bai J, Li P, Wen R and Zhao X:
Bioavailability and brain-targeting of geniposide in
gardenia-borneol co-compound by different administration routes in
mice. Int J Mol Sci. 13:14127–14135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang MR, Liu QD, Huang TL, Zhang YQ and
Ou WP: The pharmacokinetic characteristics of borneol in serum and
brain tissue of rats. Traditional Chinese Drug Research &
Clinical Pharmacology. 38–40. 621993.(In Chinese).
|
|
32
|
Tao W, Xu X, Wang X, et al: Network
pharmacology-based prediction of the active ingredients and
potential targets of Chinese herbal Radix Curcumae formula for
application to cardiovascular disease. J Ethnopharmacol. 145:1–10.
2013. View Article : Google Scholar
|
|
33
|
Bai J, Zeng Q and Chai Z: Clinical and
experimental study on treatment of acute alcohol intoxication with
xiangnaojing injection. Zhongguo Zhong Xi Yi Jie He Za Zhi.
18:607–609. 1998.(In Chinese).
|
|
34
|
Guo F, Lu XW and Xu QP: Protective effect
of Xingnaojing and Xuesaitong injections on cerebral ischemic
reperfusion injury in rats. Zhonghua Yi Xue Za Zhi. 90:1645–1647.
2010.PubMed/NCBI
|
|
35
|
Wang LC and Liu HY: Clinical observation
on acupuncture combined with Xingnaojing injection for treatment of
cerebral hemorrhage at acute stage. Zhongguo Zhen Jiu. 26:253–255.
2006.(In Chinese). PubMed/NCBI
|
|
36
|
Wen HM, Lin SY, Gao J, et al: Protective
effect and mechanism of Xingnaojing injection on ketamine-induced
impairment of learning and memory. Guangdong Medical Journal.
33:1546–1549. 2012.(In Chinese).
|
|
37
|
Li GC and Mai SD: Effects of Fufang
Shexiang injection on dysfunction of learning and memory
post-anesthesia with ketamine in aged rats. China Healthcare
Innovation. 3:12–13. 2008.(In Chinese).
|
|
38
|
Wang WY, Yang R, Hu SF, Wang H, Ma ZW and
Lu Y: N-stearoyl-L-tyrosine ameliorates sevoflurane induced
neuroapoptosis via MEK/ERK1/2 MAPK signaling pathway in the
developing brain. Neurosci Lett. 541:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuribayashi K, Mayes PA and El-Deiry WS:
What are caspases 3 and 7 doing upstream of the mitochondria?
Cancer Biol Ther. 5:763–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dohare P, Garg P, Sharma U, Jagannathan NR
and Ray M: Neuroprotective efficacy and therapeutic window of
curcuma oil: in rat embolic stroke model. BMC Complement Altern
Med. 8:552008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dummler B and Hemmings BA: Physiological
roles of PKB/Akt isoforms in development and disease. Biochem Soc
Trans. 35:231–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cardone MH, Roy N, Stennicke HR, et al:
Regulation of cell death protease caspase-9 by phosphorylation.
Science. 282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dudek H, Datta SR, Franke TF, et al:
Regulation of neuronal survival by the serine-threonine protein
kinase Akt. Science. 275:661–665. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marushige K and Marushige Y: Changes in
the mitogen-activated protein kinase and phosphatidylinositol
3-kinase/Akt signaling associated with the induction of apoptosis.
Anticancer Res. 19:3865–3871. 1999.
|
|
48
|
Xin M and Deng X: Nicotine inactivation of
the proapoptotic function of Bax through phosphorylation. J Biol
Chem. 280:10781–10789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ishrat T, Sayeed I, Atif F, Hua F and
Stein DG: Progesterone is neuroprotective against ischemic brain
injury through its effects on the phosphoinositide 3-kinase/protein
kinase B signaling pathway. Neuroscience. 210:442–450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ullah N, Ullah I, Lee HY, et al:
Protective function of nicotinamide against ketamine-induced
apoptotic neurodegeneration in the infant rat brain. J Mol
Neurosci. 47:67–75. 2012. View Article : Google Scholar
|