Introduction
At present, ultrasound contrast agents (UCAs) are
frequently used in order to expand the scope of ultrasound
diagnostics, in particular for tumors (1–3). The
materials used in the fabrication of UCAs are lipids, proteins,
carbohydrates and polymers. The polymers, including
poly(lactic-co-glycolic acid) (PLGA), have been extensively studied
in recent years (4,5). PLGA was selected for the present
study due to its good biodegradability and biocompatibility as
reported in previous studies, which is also the reason why the Food
and Drug Administration approved its usage in sutures and drug
delivery devices (4).
PLGA microcapsules are the most common type of UCA
available at present. The polymeric shell improves the stability of
the capsules, compared to that of those stabilized by a
monomolecular layer of surfactant (5,6).
Furthermore, PLGA microcapsules contain gas, which increases their
scattering power and leads to a high echogenicity due to the high
compressibility and low density of the gases (5,7).
However, the large scale of PLGA microcapsules limits their
applications. Sun et al (8)
reported that the superparamagnetic PLGA-iron oxide microcapsules
for dual-modality ultrasound/magnetic resonance imaging had an
average diameter of 885.6 nm. The endothelial gap of a tumor was
found to be ~400–600 nm, which makes it difficult for microcapsules
to penetrate the vasculature and detect tumors (7). To overcome this limitation, studies
have focused on developing PLGA capsules on a nanoscale (9,10).
Néstor et al (9) prepared
air-filled PLGA nanocapsules with a mean diameter of 370±96 nm and
evaluated their echogenic power and stability in vitro. Kohl
et al (10) prepared and
evaluated multifunctional PLGA nanoparticles for photoacoustic
imaging. PLGA nanobubbles, of mean diameter 268 nm, were developed
by Xu et al (11) for
cancer targeting and imaging. In these previous studies, the
imaging effects of PLGA nanoparticles were examined in vitro
and the results suggested that the PLGA shell may improve
nanocapsule stability. However, there were almost no systematic
studies investigating the in vitro imaging capacity of PLGA
nanoparticles under varying conditions. Furthermore, to the best of
our knowledge, few studies have been conducted to investigate the
properties of PLGA nanoparticles in in vivo ultrasound
imaging (7,9–11).
The present study was therefore designed to:
i) Prepare perfluoropropane
(C3F8)-filled nanoparticles with a
biodegradable polymeric shell composed of PLGA and ii)
investigate the feasibility of using PLGA nanoparticles to enhance
ultrasound imaging in vitro and in vivo.
Materials and methods
Materials
Poly(D,L-lactic-co-glycolic acid) (PLGA; 50:50;
molecular weight, 40,000) was purchased from Jinan Daigang
Biomaterial Co., Ltd (Jinan, China). Polyvinyl alcohol (PVA; 88%
mole hydrolyzed) and (D+)-camphor were purchased from Aladdin
Chemistry Co., Ltd (Shanghai, China). Methylene chloride,
isopropanol, mannitol and phosphotungstic acid were purchased from
Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Deionized
water used in all experiments was purified using a Milli-Q Plus 185
water purification system (Millipore, Bedford, MA, USA) with
resistivity >18 MΩ cm. All chemical reagents were of at least
analytical grade and were used directly without further
purification.
Animals
A total of ten male Wistar rats (three months old;
mean weight, 150 g) were purchased from the Shanghai Laboratory
Animal Center (Shanghai Laboratory Animal Co., Ltd, Shanghai,
China) and housed in pathogen-free facilities with a 12-hour
light/dark cycle (6am–6pm) and provided with rodent diet and tap
water ad libitum. Animal studies were conducted according to
the guidelines of the Renji Hospital, School of Medicine, Shanghai
Jiao Tong University. Animal procedures were approved by the
Institutional Animal Care and Use Committee, which is certified by
the Shanghai Association for Accreditation of Laboratory Animal
Care (Shanghai, China).
Preparation of
C3F8-filled PLGA nanoparticles
PLGA hollow nanoparticles were prepared based on a
modified double-emulsion solvent evaporation method (12). For the first emulsion, 125 mg PLGA
and 12.5 mg camphor were dissolved in 5 ml methylene chloride. The
polymer was fully dissolved prior to the addition of 1 ml PVA (3%,
w/v). The mixture was subsequently emulsified in an ice bath using
a Ultrasonic processing 04717 (Cole-Parmer, Vernon Hills, IL, USA)
at 130 W for 180 sec, in pulse mode with sonication turned off for
2 sec and on for 4 sec to prevent heat stacking. For the second
emulsion, the emulsified solution was added to 20 ml PVA (3%, w/v)
and sonicated for 180 sec at 4 sec on, 2 sec off. Following the
double emulsion, the resulting water-in-oil-in-water emulsion was
added to 100 ml isopropyl alcohol (5%, v/v) and continually stirred
for 1 h to evaporate any organic solvent. Following evaporation,
samples were collected using centrifugation (17,800 × g for 10 min)
and washed three times with 5 ml deionized water. The precipitation
was subsequently flash frozen and lyophilized for 48 h. As the
mixture underwent the freeze-drying process, camphor sublimed out
of the particles, leaving a void in their place. This hollow core
was filled with C3F8 gas (Renjieling Optical
Instrument Co, Ltd., Shanghai, China) when later exposed to
C3F8 pressure.
Characterization of
C3F8-filled PLGA nanoparticles
The surface morphology of the nanoparticles was
investigated using an S-4800 field emission scanning electron
microscope (FE-SEM; Hitachi, Tokyo, Japan). The PLGA nanoparticle
samples were dispersed in deionized water, spread over a piece of
aluminum foil and dried at room temperature. The samples were
subsequently sputter coated with a layer of gold using a fine coat
ion sputter (JFC-1100; JEOL, Ltd, Tokyo, Japan) prior to FE-SEM
imaging.
Transmission electron microscopy was performed using
a JEOL JEM-2100 low- to high-resolution transmission electron
microscope (HR-TEM; Hitachi). The PLGA nanoparticle suspension was
dropped onto a formvar film-coated copper grid for 2 min, excess
solution was removed using filter paper and phosphotungstic acid
solution (1%, w/v) was used to stain the sample for 2 min (13). Finally, the grids were air-dried
prior to observation.
The samples were dispersed in deionized water to
obtain a uniform suspension. Dynamic laser scattering (DLS;
Zetasizer Nano ZS model ZEN3690; Malvern Instruments, Ltd, Malvern,
UK) was used to evaluate the mean size and size-distribution of the
PLGA nanoparticles dispersed in deionized water. The laser light
wavelength was 633 nm and measurements were obtained at a 90°
fixed-angle for 180 sec at 25°C. Measurements were made in
triplicate for all batches prepared.
In vitro ultrasound imaging of
C3F8-filled PLGA nanoparticles
A preliminary evaluation of the ultrasound contrast
behavior of PLGA nanoparticles was performed. Various
concentrations of PLGA nanoparticles in deionized water were imaged
in a tank filled with degassed and deionized water at ~37°C. Images
of degassed and deionized water were acquired to serve as
background controls. The mechanical index (MI) was 0.06. The
samples were scanned using an ultrasonic diagnostic instrument
(MyLab Twice; Esaote SpA, Genova, Italy) in conventional B mode.
The center frequencies of the transducers were 13 and 22 MHz,
respectively.
In vivo ultrasound imaging of
C3F8-filled PLGA nanoparticles
In vivo investigation was performed on
healthy male rats, randomly divided into two groups (n=5). The rats
were anesthetized with an intraperitoneal injection of 10% chloral
hydrate, and then fixed on a board. The PLGA nanoparticles were
suspended in deionized water at a concentration of 2 mg/ml and 200
μl PLGA nanoparticle solution was injected through a 1-ml syringe
into the right testicle. The testicles of the rats were scanned by
the MyLab Twice scanner (Esaote SpA) prior to and following the
administration of PLGA nanoparticles. A total of 200 ml deionized
water was also injected into the control group.
Statistical analysis
Values are presented as the mean ± standard
deviation. The nanoparticles were analyzed using DLS (14,15).
Results
Preparation and characterization of
C3F8-filled PLGA nanoparticles
The capsular structure of
C3F8-filled nanocapsules was evaluated by
electron microscopy. The examination of
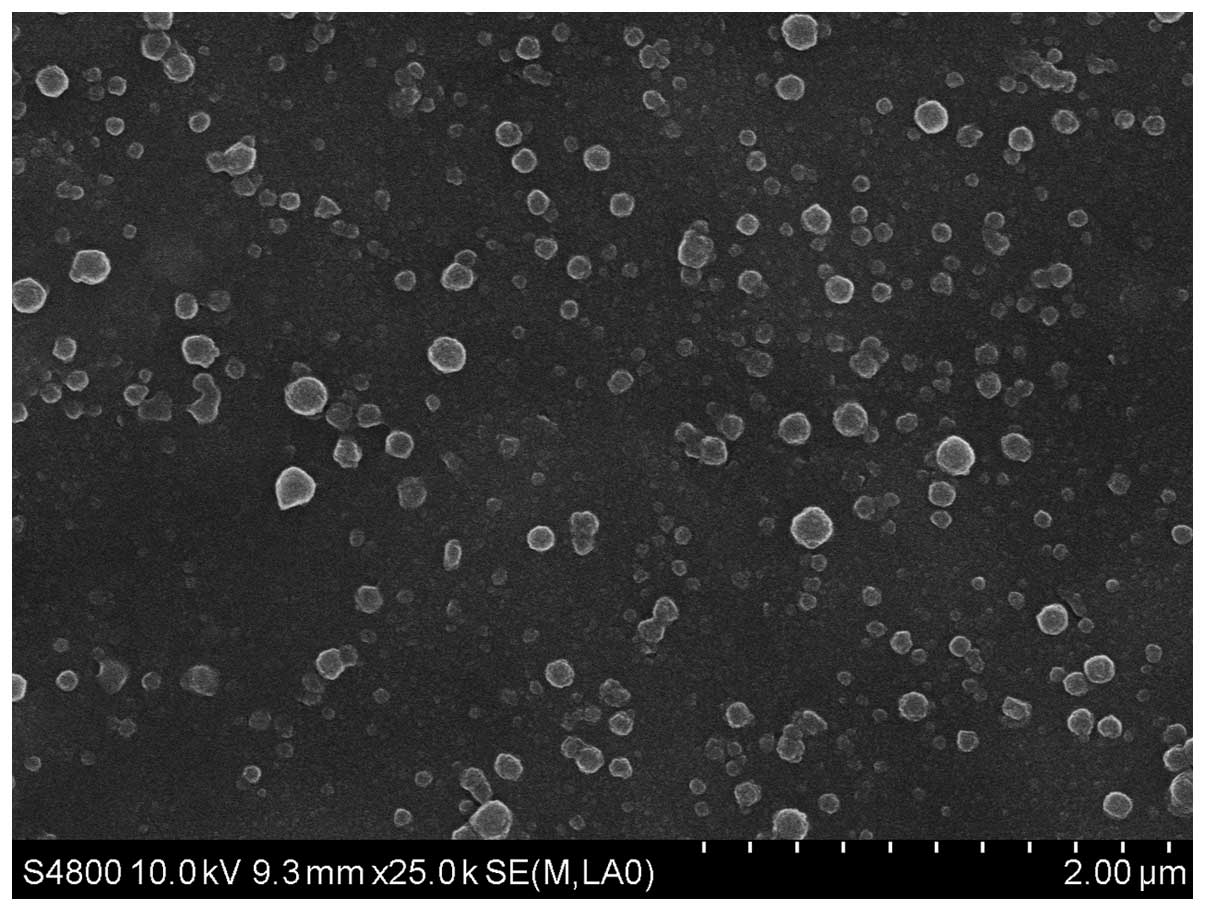
C3F8-filled nanocapsules by SEM revealed
spherical particles with smooth surfaces (Fig. 1). TEM revealed the typical
core-shell structure of C3F8-filled
nanocapsules with a thin shell, where the shell appeared darker and
the gas core appeared gray due to the differences in electron
density (Fig. 2). As indicated in
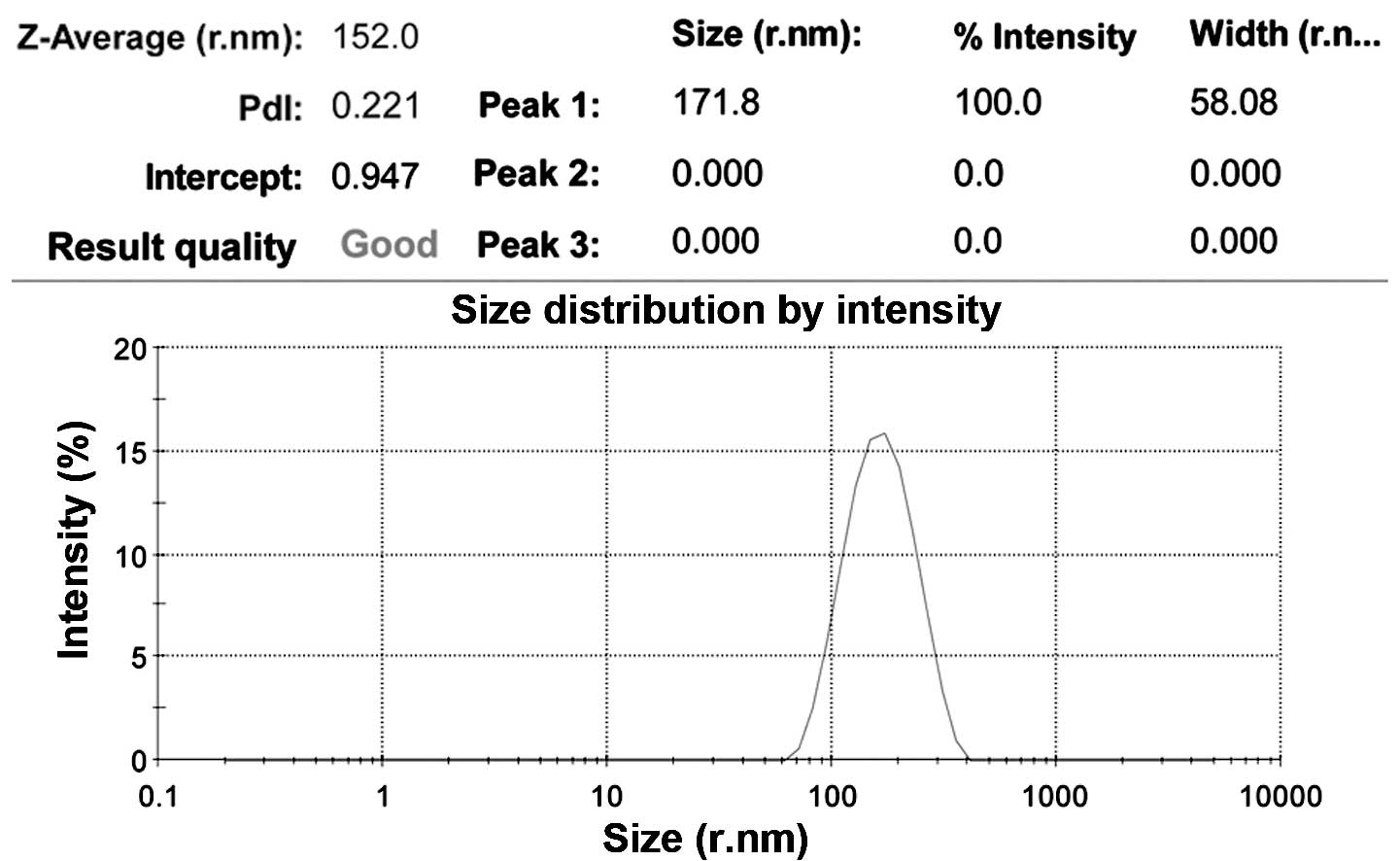
Fig. 3, the average size of
nanoparticles prepared in the present study was 152.0±58.08 nm and
the polydispersity index was 0.221, revealing uniform size and good
dispersion of the PLGA nanoparticles.
In vitro imaging
The capability of C3F8-filled
PLGA nanoparticles as a contrast agent for ultrasound imaging was
assessed in vitro using the MyLab Twice scanner system at
various concentrations and frequencies and at MI 0.06. Three
concentrations of C3F8-filled PLGA
nanoparticles (3, 2 and 1 mg/ml) were evaluated in the first
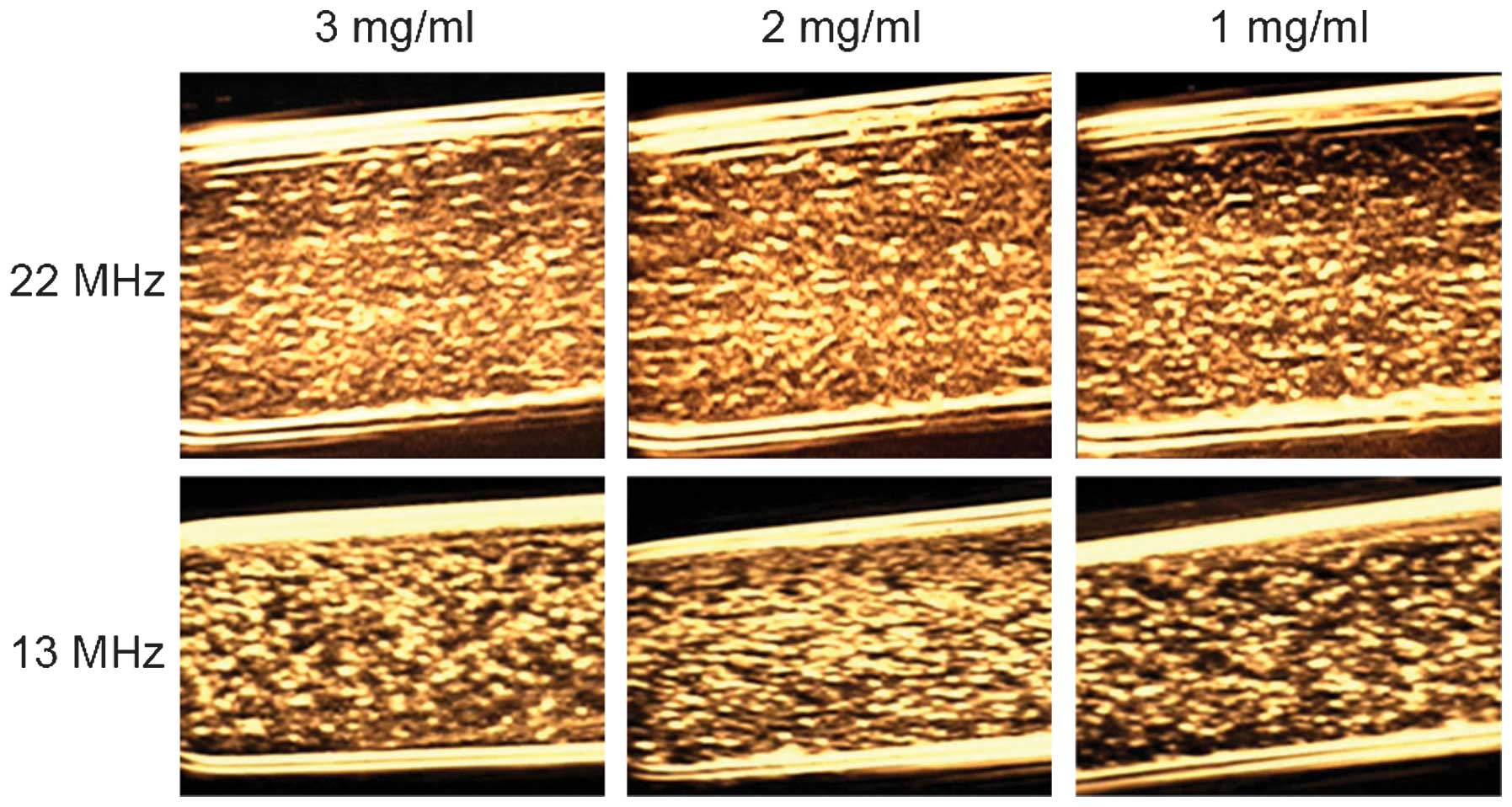
experiment. As shown in Fig. 4,
there was almost no visible difference between 3 and 2 mg/ml at 22
or 13 MHz. However, the echo signal of 1 mg/ml C3F8-filled PLGA
nanoparticles was a little weaker than those of 2 and 3 mg/ml
nanoparticles. For the prepared C3F8-filled
PLGA nanoparticles, the optimal ultrasound imaging concentration
was 2 mg/ml. Accordingly, 2 mg/ml was selected as the maximum
concentration in the subsequent study of the imaging effects of
PLGA nanoparticles.
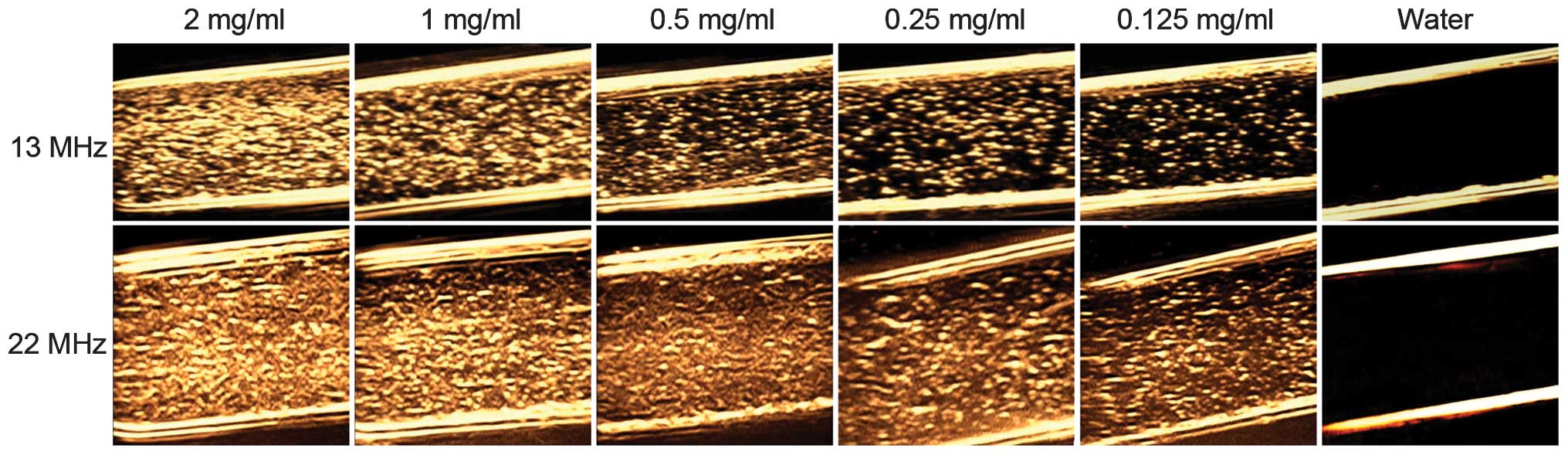
As indicated in Fig.
5, the ultrasound signals gradually decreased with decreasing
concentrations of PLGA nanoparticles at 13 and 22 MHz. The signal
intensity was strongest at 2 mg/ml and remained relatively strong
at the low concentration of 0.125 mg/ml. These in vitro
results demonstrated that the PLGA nanoparticles were able to be
used as the contrast agents for efficient ultrasound imaging.
Identical concentrations of PLGA nanoparticles imaged more
effectively at 22 MHz compared with imaging at 13 MHz. Therefore,
the optimal ultrasound imaging conditions were 2 mg/ml
nanoparticles at a frequency of 22 MHz.
In addition, the length of time during which the
signal enhancement was produced by different concentrations of PLGA
nanoparticles at 22 MHz was sustained was investigated. As
indicated in Fig. 6, the signal
intensity of 2 mg/ml PLGA nanoparticles remained strong until 60
sec, there was slight decrease at 120 sec and signal enhancement
could still be detected at 180 sec. At 0.125 mg/ml the signal
intensity gradually decreased with the extension of time. The
results indicated that the PLGA nanoparticles were able to image at
low concentrations for at least two minutes and that the higher the
concentration of PLGA nanoparticles was, the longer the potential
imaging time persisted.
In vivo imaging
In order to evaluate the acoustic behavior of
C3F8-filled PLGA nanoparticles in
vivo, testicular ultrasound imaging was performed using the
B-Scan imaging mode at a frequency of 22 MHz and an MI of 0.06. Two
groups of five male rats were anesthetized intraperitoneally with
10% chloral hydrate prior to agent injection. The transducer was
placed on the testicle for real-time monitoring and 200 μl PLGA
nanoparticles dispersed in deionized water were intra-testicularly
injected. Ultrasound imaging was recorded during the injection.
Fig. 7A and B indicated that the
local intensity of the acoustic signal continued to increase during
PLGA nanoparticle-injection into the testicle and the ability of
hollow PLGA nanoparticles to enhance ultrasound imaging in
vivo was demonstrated. The results revealed that the
enhancement image of testicular tissue following injection of
hollow PLGA nanoparticles was able to be sustained for ≥5 min.
Fig. 7C and D demonstrated the
imaging behavior of deionized water injected into the testicle of
rats in the control group. There was almost no echo difference
detected prior to and following injection of degassed and deionized
water.
Discussion
Various nano-scale UCAs, including solid
nanoparticles, liquid-core nanoagents and gaseous-core nanoagents
have been reported (16–19). Although the feasibility of the use
of solid nanoparticles to enhance high-frequency ultrasound B-mode
images was reported by Liu et al (16), the biodegradation and
biocompatibility of solid nanoparticles have remained major issues.
Liquid-core nanoagents have also been evaluated as UCAs (18); however, drawbacks include poor
echogenicity due to low compressibility and the use of high
frequencies of insonification. Gas-filled nanobubbles were
developed to further enhance the ultrasound signal (9). A biodegradable polymer shell was more
rigid than surfactants and lipids and was easily modified with
various ligands to achieve active targeting to specific tissues and
cells (18,19). Therefore, the present study aimed
to synthesize a novel C3F8-filled polymer UCA
using PLGA as the shell.
The micro-scale method of using a porogen to create
voids for accommodating gas in the particles was adapted for use in
the present study to produce hollow nanocapsules. Camphor and other
substances that are able to sublime or decompose rapidly without
the generation of toxic reactions have the ability to form hollows
within particles. Kwon and Wheatley (20) produced gas-loaded PLA nanoparticles
for use as UCAs by using camphor. Néstor et al (9) prepared air-filled PLGA nanocapsules
using ammonium bicarbonate as sublimable porogens. The PLGA
nanoparticles used in the present study were prepared using camphor
as the sublimable porogen, which produced a large hollow in the
particles. The hollows were subsequently filled with
C3F8 gas, producing higher echo reflection
(5).
In the present study, methylene chloride was used as
a solvent, with high-power emulsification and high-speed
centrifugation, to produce PLGA nanoparticles markedly smaller than
the microbubbles prepared using the traditional double-emulsion
method (21,22). The average diameter of the
nanoparticles fabricated was 152.0±58.08 nm, markedly smaller than
the endothelial gap. In theory, particles <400–600 nm are able
to penetrate the endothelial gap of tumor feeding vessels (23). It was therefore possible for the
PLGA nanoparticles prepared in the present study to image the
target tissue outside the vascular structures, overcoming the
drawbacks of conventional ultrasound contrast agents that are only
able to image within the blood pool.
The preliminary in vitro studies performed
confirmed the imaging effects of various concentrations of PLGA
nanoparticles at 22 and 13 MHz. The results indicated that the
identical concentration of PLGA nanoparticles imaged better at a
frequency of 22 MHz than at 13 MHz, which indicated that the PLGA
nanoparticles were more suitable for use in high frequencies of
insonification. Furthermore, low concentrations (0.125 mg/ml) of
PLGA nanoparticles were able to image for at the 120th sec,
suggesting higher stability compared with that of UCAs made of
lipid or protein. Therefore, there was also sufficient time for the
PLGA nanoparticles to penetrate the endothelial cell gap into the
tumor tissue and detect tumors efficiently when circulating in
blood (24).
The PLGA nanoparticles were also imaged effectively
in the rat testis compared with imaging using degassed water. This
result indicated that the prepared
C3F8-filled PLGA nanoparticles had potential
to be used as a novel UCA that may be modified for use in in
vivo targeted molecular imaging (22,25,26).
In conclusion, C3F8-filled
PLGA nanoparticles for efficient ultrasound imaging were generated
via a modified double-emulsion solvent evaporation method. Results
of in vitro and preliminary in vivo studies
demonstrated that C3F8-filled PLGA
nanoparticles exhibited effective ultrasound imaging capabilities.
Therefore, these prepared C3F8-filled PLGA
nanoparticles have potential applications as a novel UCA. Further
study is required in order to investigate the imaging capacity of
functionalized PLGA nanoparticles in tumor models. Further study is
required in order to investigate the imaging capacity of
functionalized PLGA nanoparticles in tumor models, and much more
qutitative analysis is needed.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81102014). The authors
would like to thank Ms. Jin Sun, Ms. Yan Yang, Mr. Jun Wang and Mr.
Li Wang for their help with the in vitro and in vivo
experiments.
References
|
1
|
Lencioni R, Piscaglia F and Bolondi L:
Contrast-enhanced ultrasound in the diagnosis of hepatocellular
carcinoma. J Hepatol. 48:848–857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nemec U, Nemec SF, Novotny C, Weber M,
Czerny C and Krestan CR: Quantitative evaluation of
contrast-enhanced ultrasound after intravenous administration of a
microbubble contrast agent for differentiation of benign and
malignant thyroid nodules: assessment of diagnostic accuracy. Eur
Radiol. 22:1357–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kornmann LM, Reesink KD, Reneman RS and
Hoeks AP: Critical appraisal of targeted ultrasound contrast agents
for molecular imaging in large arteries. Ultrasound Med Biol.
36:181–191. 2010. View Article : Google Scholar
|
|
4
|
Shive MS and Anderson JM: Biodegradation
and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv
Rev. 28:5–24. 1997. View Article : Google Scholar
|
|
5
|
Pisani E, Tsapis N, Paris J, et al:
Polymeric nano/microcapsules of liquid perfluorocarbons for
ultrasonic imaging: physical characterization. Langmuir.
22:4397–4402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basude R, Duckworth JW and Wheatley MA:
Influence of environmental conditions on a new surfactant-based
contrast agent: ST68. Ultrasound Med Biol. 26:621–628. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oeffinger BE and Wheatley MA: Development
and characterization of a nano-scale contrast agent. Ultrasonics.
42:343–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Zheng Y, Ran H, et al:
Superparamagnetic PLGA-iron oxide microcapsules for dual-modality
US/MR imaging and high intensity focused US breast cancer ablation.
Biomaterials. 33:5854–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Néstor MM, Kei NP, Guadalupe NA, Elisa ME,
Adriana GQ and David QG: Preparation and in vitro evaluation of
poly(D,L-lactide-co-glycolide) air-filled nanocapsules as a
contrast agent for ultrasound imaging. Ultrasonics. 51:839–845.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohl Y, Kaiser C, Bost W, et al:
Preparation and biological evaluation of multifunctional
PLGA-nanoparticles designed for photoacoustic imaging.
Nanomedicine. 7:228–237. 2011. View Article : Google Scholar
|
|
11
|
Xu JS, Huang J, Qin R, et al: Synthesizing
and binding dual-mode poly (lactic-co-glycolic acid) (PLGA)
nanobubbles for cancer targeting and imaging. Biomaterials.
31:1716–1722. 2010. View Article : Google Scholar
|
|
12
|
Xu RX, Huang J, Xu JS, et al: Fabrication
of indocyanine green encapsulated biodegradable microbubbles for
structural and functional imaging of cancer. J Biomed Opt.
14:0340202009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunoqrot S, Bae JW, Jin SE, Pearson MR,
Liu Y and Hong S: Kinetically controlled cellular interactions of
polymer-polymer and polymer-liposome nanohybrid systems. Bioconjug
Chem. 22:466–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu B: Laser Light Scattering: Basic
Principles and Practice. 2nd edition. Academic Press; 1991
|
|
15
|
Xu R: Particle Characterization: Light
Scattering Methods. Kluwer Academic Publications; 2000
|
|
16
|
Liu J, Levine AL, Mattoon JS, et al:
Nanoparticles as image enhancing agents for ultrasonography. Phys
Med Biol. 51:2179–2189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Li J, Rosol TJ, Pan X and Voorhees
JL: Biodegradable nanoparticles for targeted ultrasound imaging of
breast cancer cells in vitro. Phys Med Biol. 52:4739–4747. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu H, Zhou H, Du J, et al: Biocompatible
hollow silica microsphere as a novel ultrasound contrast agent for
in vivo imaging. J Mater Chem. 21:6576–6583. 2011. View Article : Google Scholar
|
|
19
|
Doiron AL, Homan KA, Emelianov S and
Brannon-Peppas L: Poly(lactic-co-glycolic) acid as a carrier for
imaging contrast agents. Pharm Res. 26:674–682. 2009. View Article : Google Scholar
|
|
20
|
Kwon S and Wheatley MA: Gas-loaded PLA
nanoparticles as ultrasound contrast agents. IFMBE Proceedings.
14:275–278. 2007. View Article : Google Scholar
|
|
21
|
Wheatley MA, Forsberg F, Oum K, et al:
Comparison of in vitro and in vivo acoustic response of a novel
50:50 PLGA contrast agent. Ultrasonics. 44:360–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenbrey JR, Burstein OM, Kambhampati R,
Forsberg F, Liu JB and Wheatley MA: Development and optimization of
a doxorubicin loaded poly(lactic acid) contrast agent for
ultrasound directed drug delivery. J Control Release. 143:38–44.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan F, Dellian M, Fukumura D, et al:
Vascular permeability in a human tumor xenograft: molecular size
dependence and cutoff size. Cancer Res. 55:3752–3756.
1995.PubMed/NCBI
|
|
24
|
Moore A, Marecos E, Bogdanov A Jr and
Weissleder R: Tumoral distribution of long-circulating
dextran-coated iron oxide nanoparticles in a rodent model.
Radiology. 214:568–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou S, Liao X, Li X, Deng X and Li H:
Poly-D,L-lactide-co-poly (ethylene glycol) microspheres as
potential vaccine delivery systems. J Control Release. 86:195–205.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng J, Teply BA, Sherifi I, et al:
Formulation of functionalized PLGA-PEG nanoparticles for in vivo
targeted drug delivery. Biomaterials. 28:869–876. 2007. View Article : Google Scholar
|