Introduction
The liver plays a central role in nutritional
metabolism and, therefore, metabolic disorders, including amino
acid imbalances and trace element deficiencies, occur frequently in
patients with chronic liver disease (1). Branched-chain amino acids (BCAAs) are
essential amino acids that play a crucial role in albumin synthesis
and ammonia detoxification in patients with cirrhosis (2). In the clinical setting, reductions in
serum BCAA levels can be assessed by measurement of the serum
BCAA-to-tyrosine ratio (BTR) (1).
Low serum BTR, along with low serum albumin levels, have recently
been reported to be a predictive factor in the development of
hepatocellular carcinoma (HCC), and confer a poor prognosis in
patients with cirrhosis (3,4).
Zinc is a trace element that activates >300
metalloenzymes, including DNA polymerase (5). In addition, zinc stabilizes zinc
finger proteins, which bind to DNA and modulate the transcription
of target genes, including tumor suppressor genes (6). In patients with chronic liver
disease, serum zinc levels decrease as the severity of liver
disease increases (7), and low
serum zinc levels are known to be associated with the development
of HCC and poor prognosis in hepatitis C virus (HCV)-infected
patients (8,9). Thus, serum zinc levels are a
significant metabolic prognosticator in HCV-infected patients.
BCAAs and zinc are pharmacological nutrients that
exert diverse biological effects (1). A decreased risk of
hepatocarcinogenesis and mortality has been reported in patients
with chronic liver disease treated with BCAA (4,10,11)
or zinc (8,9) supplementation. Therefore, a
supplement containing both BCAAs and zinc may have the potential to
improve prognostic factors in patients with chronic liver disease.
Aminofeel® is a commercially available BCAA and
zinc-enriched supplement. We previously examined the usefulness of
the supplement and demonstrated that it improves insulin
resistance, taste sensitivity and adherence to interferon therapy
in HCV-infected patients (12–16).
However, it remains unclear whether BCAA and zinc-enriched
supplementation improves prognostic factors, including the platelet
count, serum albumin levels, homeostasis model assessment for
insulin resistance (HOMA-IR) value, serum BTR and zinc levels in
HCV-infected patients. In addition, to the best of our knowledge,
the impact of the supplement on serum α-fetoprotein (AFP) levels
has never been investigated. Therefore, we performed a prospective
multicenter randomized controlled trial to investigate the effects
of a BCAA and zinc-enriched supplement on prognostic factors in
HCV-infected patients.
Subjects and methods
Ethical considerations
This study was designed in 2009 by the steering
committee of Research on Hepatitis, The Ministry of Health, Labour
and Welfare of Japan (principal investigator, Michio Sata, MD), to
evaluate the usefulness of BCAA and zinc supplementation in
patients with chronic HCV infection. The study protocol was
approved by the Ethical Committee of Human Experimentation in
Kurume University School of Medicine (approval no. 09152,
UMIN000012815), and is in accordance with the Helsinki Declaration
of 1975, as revised in 1983. Written informed consent for
participation in the study was obtained from each subject.
Subjects
HCV-infected patients aged 65 years or older who had
serum albumin levels ≥3.5 and <4.0 g/dl were recruited for the
study. The exclusion criteria were as follows: i) currently
undergoing interferon therapy; ii) positivity for hepatitis B
surface antigen or serum hepatitis B virus DNA; iii) presence of
autoimmune hepatitis, alcoholic liver disease (ethanol consumption
>50 g/day), primary biliary cirrhosis, primary sclerosing
cholangitis, hemochromatosis or Wilson’s disease; iv) presence of
cardiac or renal disease or severe psychiatric disease; v) presence
of HCC or within a year of treatment for HCC; vi) presence of
esophageal or gastric varices at risk of rupture; vii) presence of
diabetes mellitus with anti-diabetic medication; viii) history of
consumption of the BCAA and zinc-enriched supplement; ix) having
taken a BCAA-related medication or a BCAA-containing supplement
within the preceding 90 days; and x) currently taking a trace
element-related medication or a trace element-containing
supplementation.
Study design and participants
We performed a prospective multicenter randomized
controlled trial in an outpatient setting in six medical
institutions in Japan. From 2010 to 2012, 54 HCV-infected patients
who fulfilled the inclusion criteria were enrolled in the study.
One patient withdrew consent for participation before
randomization. The stratified randomization method was used to
achieve balance among groups in terms of subjects’ baseline
characteristics. Randomization was performed centrally, and both
patients and investigators were blinded to the patients’ group
assignment. The patients were allocated at a 1:1 ratio to the
placebo (n=27) or supplement group (n=26). Two patients in the
placebo group were withdrawn from the study due to withdrawal of
consent (n=1) and anemia (n=1). Two patients in the supplement
group were withdrawn due to general fatigue (n=1) and the
appearance of a rash (n=1). Thus, four patients were excluded from
the statistical analysis on day 60 owing to lack of data. Finally,
92.6% (25/27) of the patients in the placebo group (age, 74±5
years; female/male, 17/8) and 92.3% (24/26) of patients in the
supplement group (age, 74±5 years, female/male, 15/9) completed the
60-day treatment period, and the efficacy and safety of the
treatment were assessed (Fig.
1).
Intervention and assessment
protocols
In the supplement group, the patients were given two
sachets of the BCAA and zinc-enriched supplement containing 6,400
mg/day BCAAs and 10 mg/day zinc (Aminofeel, Seikatsu Bunkasya Co.,
Inc., Chiba, Japan), once after breakfast and again at bedtime
(Fig. 2). In the placebo group,
the patients were administered a sachet of placebo after breakfast
and another at bedtime. Although the BCAAs, trace elements and
vitamins were replaced with corn starch in the placebo (Table I), the appearance and taste of the
placebo were similar to those of the supplement.
 | Table IContents of one sachet of placebo and
one sachet of the branch-chain amino acid and zinc-enriched
supplement. |
Table I
Contents of one sachet of placebo and
one sachet of the branch-chain amino acid and zinc-enriched
supplement.
| Placebo | Supplement |
|---|
| Valine (mg) | 0.0 | 800.0 |
| Leucine (mg) | 0.0 | 1,600.0 |
| Isoleucine
(mg) | 0.0 | 800.0 |
| Zinc (mg) | 0.0 | 5.0 |
| Calcium (mg) | 0.0 | 21.1 |
| Magnesium (mg) | 0.0 | 12.6 |
| Copper (mg) | 0.0 | 0.2 |
| Selenium (μg) | 0.0 | 49.6 |
| Chromium (μg) | 0.0 | 14.4 |
| Vitamin A (μg) | 0.0 | 315.0 |
| Vitamin D (μg) | 0.0 | 3.0 |
| Vitamin E (mg) | 0.0 | 6.4 |
| Vitamin K (μg) | 0.0 | 29.6 |
| Vitamin C (mg) | 0.0 | 40.0 |
| Vitamin B1
(mg) | 0.0 | 2.4 |
| Vitamin B2
(mg) | 0.0 | 2.6 |
| Niacin (mg) | 0.0 | 12.0 |
| Vitamin B6
(mg) | 0.0 | 2.4 |
| Vitamin B12
(μg) | 0.0 | 10.0 |
| Folic acid
(μg) | 0.0 | 0.2 |
| Pantothenic acid
(mg) | 0.0 | 6.8 |
| Corn starch
(mg) | 3,487.0 | 0.0 |
On days 0 and 60, we evaluated body mass index
(BMI), subjective symptoms (fatigue, sleeplessness, muscle cramps,
loss of appetite and taste disorders) using a visual analog scale
(Fig. 2). A visual analog scale is
a horizontal line, 100 mm in length, anchored by word descriptors
for subjective symptoms at each end. The patients marked on the
line the point that they felt represented their current subjective
symptom. The visual analog scale score was determined by measuring
the distance (in mm) from the left-hand end of the line to the
point that the patient had marked, on which 0 mm indicates an
absence of symptoms and 100 mm indicates the worst symptom.
The following blood biochemical parameters were
measured in all the patients on days 0 and 60: White blood cell
count, hemoglobin levels, platelet count, total protein levels,
albumin levels, BTR, prothrombin time (international normalized
ratio), ammonia levels, zinc levels, fasting blood glucose levels,
hemoglobin A1c levels, fasting immune reactive insulin levels,
HOMA-IR, total cholesterol levels, aspartate aminotransferase
levels, alanine aminotransferase levels, γ-glutamyl transpeptidase
levels, alkaline phosphatase levels, blood urea nitrogen levels,
creatinine levels, AFP levels and HCV RNA levels. All the
biochemical parameters were measured by standard clinical methods
using venous blood samples taken the morning after a 12-h overnight
fast, as previously described (17).
Outcomes
The primary outcomes were the following known
prognostic factors for patients with chronic liver disease:
Platelet count, serum albumin levels, serum AFP levels, HOMA-IR
value, serum BTR and serum zinc levels. The secondary outcomes were
BMI, subjective symptoms and biochemical examinations, including
liver and renal functions, glucose metabolism and HCV RNA
levels.
Stratification analysis according to
serum AFP levels
As the serum AFP levels had a wide distribution, we
performed a stratification analysis to assess the effects of the
BCAA and zinc-enriched supplement on changes in serum AFP levels.
The patients in both the placebo and supplement groups were further
classified into two groups based on their serum AFP levels at
baseline as follows: One group with AFP levels within the reference
range (≤8.7 ng/ml) and another group with elevated serum AFP levels
(>8.7 ng/ml). Changes in serum AFP levels were expressed as ΔAFP
(day 60 AFP level vs. day 0 AFP level).
Safety monitoring
Safety was assessed in terms of vital signs,
physical examination results, laboratory test results and clinical
adverse events. When 19 patients (n=9 in the placebo group; n=10 in
the supplement group) had completed the study, the data monitoring
committee evaluated the safety and disadvantages of the groups and
confirmed that there were no severe adverse events or disadvantages
observed in either the placebo or supplement group. This evaluation
was conducted to produce an interim report at the meeting of
Research on Hepatitis (Ministry of Health, Labour and Welfare of
Japan; principal investigator, Michio Sata).
Considerations relating to the sample
size and treatment period
Based on the BTR data in our previous pilot study
(12), the sample size was
calculated using Power and Sample Size Calculation v 3.0, as
previously described (18).
Briefly, we planned to study a continuous response variable in
independent controls and experimental subjects with one control per
experimental subject. In our previous pilot study, the response
within each subject group was normally distributed with a standard
deviation of 0.9 (12). If the
true difference between the experimental and control means is 1, we
would need to study a total of 36 subjects (18 experimental
subjects and 18 control subjects) to be able to reject the null
hypothesis that the population means of the experimental and
control groups are equal with a probability (power) of 0.9. The
type I error probability associated with testing this null
hypothesis is 0.05.
The treatment period was also based on BTR data from
our previous pilot study (12). In
the previous study, we examined the effects of the BCAA and
zinc-enriched supplement on serum BTR 30, 60 and 90 days after
treatment. Considering that the most significant efficacy was
observed 60 days after treatment, a 60-day treatment period was
used in this study (12).
Statistical analysis
All the data are expressed as the number or mean ±
standard deviation. Differences between the placebo and supplement
groups were analyzed using the χ2 test or Mann-Whitney U
test. Statistical comparisons between multiple groups were
performed using the Kruskal-Wallis test. P<0.05 was considered
to indicate a statistically significant difference. All analyses
were performed using JMP 10.0.2 (SAS Institute Inc., Cary, NC,
USA).
Results
Patient characteristics
At baseline, there were no significant differences
in age, gender and BMI between the placebo and supplement groups
(Table II). No significant
differences were observed in any subjective symptoms, including
fatigue, sleeplessness, muscle cramps, loss of appetite and taste
disorders between the two groups (Table II).
 | Table IIPatient characteristics at
baseline. |
Table II
Patient characteristics at
baseline.
| Placebo group | Supplement
group | P-value |
|---|
| No. of
patients | 25 | 24 | - |
| Age (years) | 74±5 | 74±5 | 0.7480 |
| Gender
(female/male) | 17/8 | 15/9 | 0.7688 |
| Body mass index
(kg/m2) | 22.9±3.7 | 22.6±3.3 | 0.7820 |
| Subjective symptoms
evaluated by visual analog scale (mm) |
| Fatigue | 20±21 | 21±22 | 0.9679 |
| Sleeplessness | 19±24 | 19±25 | 0.9272 |
| Muscle cramp | 14±25 | 20±29 | 0.7054 |
| Loss of
appetite | 11±18 | 20±24 | 0.2278 |
| Taste
disorder | 5±14 | 8±16 | 0.1828 |
| Biochemical
prognosticators |
| Platelet count
(104/μl) | 13.6±5.8 | 14.2±4.4 | 0.4776 |
| Albumin
(g/dl) | 3.79±0.12 | 3.79±0.20 | 0.6448 |
| α-fetoprotein
(ng/ml) | 15.6±32.1 | 8.0±8.6 | 0.3372 |
| HOMA-IR | 2.99±3.81 | 2.89±2.38 | 0.7491 |
| BCAA-to-tyrosine
ratio | 4.43±1.06 | 4.93±1.33 | 0.3731 |
| Zinc (μg/dl) | 68±9 | 68±7 | 0.8281 |
| Other biochemical
parameters |
| White blood cell
count (/μl) | 4182±1092 | 4504±1132 | 0.3787 |
| Total lymphocyte
count (/μl) | 1449±658 | 1515±826 | 0.7501 |
| Hemoglobin
(g/dl) | 12.8±1.4 | 13.1±1.4 | 0.4007 |
| Aspartate
aminotransferase (U/l) | 51±18 | 47±16 | 0.5283 |
| Alanine
aminotransferase (U/l) | 47±23 | 37±20 | 0.0799 |
| γ-glutamyl
transpeptidase (U/l) | 33±15 | 36±34 | 0.3650 |
| Alkaline
phosphatase (U/l) | 340±132 | 318±113 | 0.6170 |
| Total protein
(g/dl) | 7.55±0.63 | 7.44±0.62 | 0.5552 |
| Prothrombin time
(international normalized ratio) | 1.05±0.12 | 1.02±0.06 | 0.9366 |
| Fasting blood
glucose (mg/dl) | 102±12 | 109±23 | 0.3123 |
| Hemoglobin Alc
(%) | 5.2±0.4 | 5.5±0.6 | 0.0932 |
| Fasting immune
reactive insulin (μU/ml) | 11.0±10.8 | 10.1±6.4 | 0.8204 |
| Total cholesterol
(mg/dl) | 160±23 | 158±28 | 0.6671 |
| Ammonia
(μg/dl) | 32±13 | 30±16 | 0.5598 |
| Blood urea
nitrogen (mg/dl) | 16.2±4.8 | 16.9±4.0 | 0.3916 |
| Creatinine
(mg/dl) | 0.65±0.29 | 0.73±0.25 | 0.0872 |
| HCV RNA (log
copy/ml) | 6.1±1.0 | 6.2±0.8 | 0.9544 |
There were no significant differences in any of the
prognostic factors, including platelet count, serum albumin and AFP
levels, HOMA-IR value, serum BTR and serum zinc levels between the
two groups (Table II). Moreover,
no significant difference was observed in any of the biochemical
examinations, including tests for liver and renal function, glucose
metabolism and HCV RNA levels, between the groups at baseline
(Table II).
Effects of BCAA and zinc-enriched
supplementation on primary outcomes
On day 60, we evaluated the effects of the BCAA and
zinc-enriched supplementation on the prognostic factors in the
HCV-infected patients. There were no significant differences in
platelet count, serum albumin and AFP levels, and HOMA-IR value
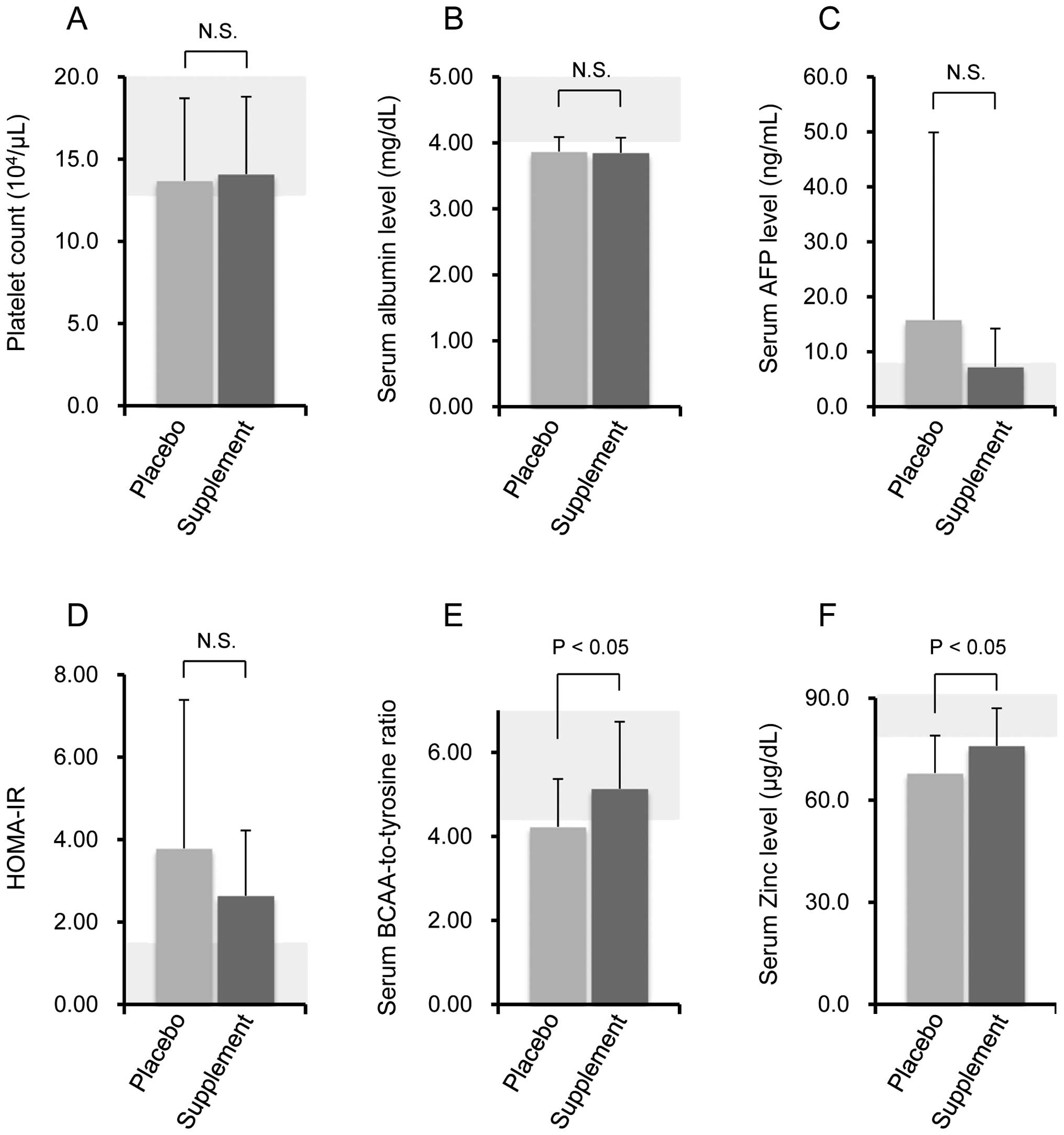
between the placebo and supplement groups (Fig. 3A–D). Conversely, a significant
increase in serum BTR was observed in the supplement group compared
with the placebo group (Fig. 3E).
In addition, a significant increase was observed in serum zinc
levels in the supplement group compared with the placebo group
(Fig. 3F).
Effects of the BCAA and zinc-enriched
supplementation on secondary outcomes
On day 60, we also evaluated the effects of the BCAA
and zinc-enriched supplement on BMI, subjective symptoms and
biochemical parameters in the HCV-infected patients. There was no
significant difference in BMI between the placebo and supplement
groups (Table III). There was
also no significant difference in fatigue, sleeplessness, muscle
cramps, loss of appetite or taste disorders between the groups
(Table III).
 | Table IIIEffects of the branch-chain amino
acid and zinc-enriched supplement on body mass index and subjective
symptoms. |
Table III
Effects of the branch-chain amino
acid and zinc-enriched supplement on body mass index and subjective
symptoms.
| Placebo group | Supplement
group | P-value |
|---|
| No. of
patients | 25 | 24 | |
| Body mass index
(kg/m2) | 23.2±3.6 | 22.8±3.2 | 0.7505 |
| Subjective symptoms
evaluated by a visual analog scale, mm |
| Fatigue | 18±17 | 18±25 | 0.5486 |
| Sleeplessness | 25±26 | 18±24 | 0.3095 |
| Muscle cramp | 16±23 | 23±33 | 0.5357 |
| Loss of
appetite | 15±19 | 16±19 | 0.6210 |
| Taste
disorder | 10±21 | 4±5 | 0.4153 |
There was no significant difference in liver
function test results, renal function test results, glucose
metabolism or blood ammonia levels between the placebo and
supplement groups (Table IV). In
addition, no significant difference in HCV RNA levels was observed
between the two groups (Table
IV).
 | Table IVEffects of the branch-chain amino
acid and zinc-enriched supplement on biochemical examinations. |
Table IV
Effects of the branch-chain amino
acid and zinc-enriched supplement on biochemical examinations.
| Placebo group | Supplement
group | P-value |
|---|
| No. of
patients | 25 | 24 | |
| White blood cell
count (/μl) | 4142±1010 | 4475±965 | 0.2711 |
| Total lymphocyte
count (/μl) | 1494±650 | 1478±708 | 0.9915 |
| Hemoglobin
(g/dl) | 13.1±1.4 | 13.0±2.0 | 0.8100 |
| Aspartate
aminotransferase (U/l) | 52±17 | 54±21 | 0.9521 |
| Alanine
aminotransferase (U/l) | 48±21 | 45±28 | 0.4064 |
| γ-glutamyl
transpeptidase (U/l) | 34±16 | 37±40 | 0.2417 |
| Alkaline
phosphatase (U/l) | 355±132 | 308±109 | 0.2846 |
| Total protein
(g/dl) | 7.77±0.71 | 7.57±0.57 | 0.4412 |
| Prothrombin time
(international normalized ratio) | 1.02±0.09 | 1.00±0.05 | 0.8915 |
| Fasting blood
glucose (mg/dl) | 105±14 | 109±25 | 0.8886 |
| Hemoglobin A1c
(%) | 5.2±0.4 | 5.6±0.6 | 0.0402 |
| Fasting immune
reactive insulin (μU/ml) | 14.0±10.9 | 9.5±4.6 | 0.2155 |
| Total cholesterol
(mg/dl) | 165±26 | 163±29 | 0.8258 |
| Ammonia
(μg/dl) | 35±13 | 29±15 | 0.1906 |
| Blood urea nitrogen
(mg/dl) | 14.9±4.5 | 16.9±4.5 | 0.0927 |
| Creatinine
(mg/dl) | 0.64±0.27 | 0.72±0.25 | 0.0871 |
| HCV RNA (log
copy/ml) | 5.9±1.5 | 6.1±0.9 | 0.7837 |
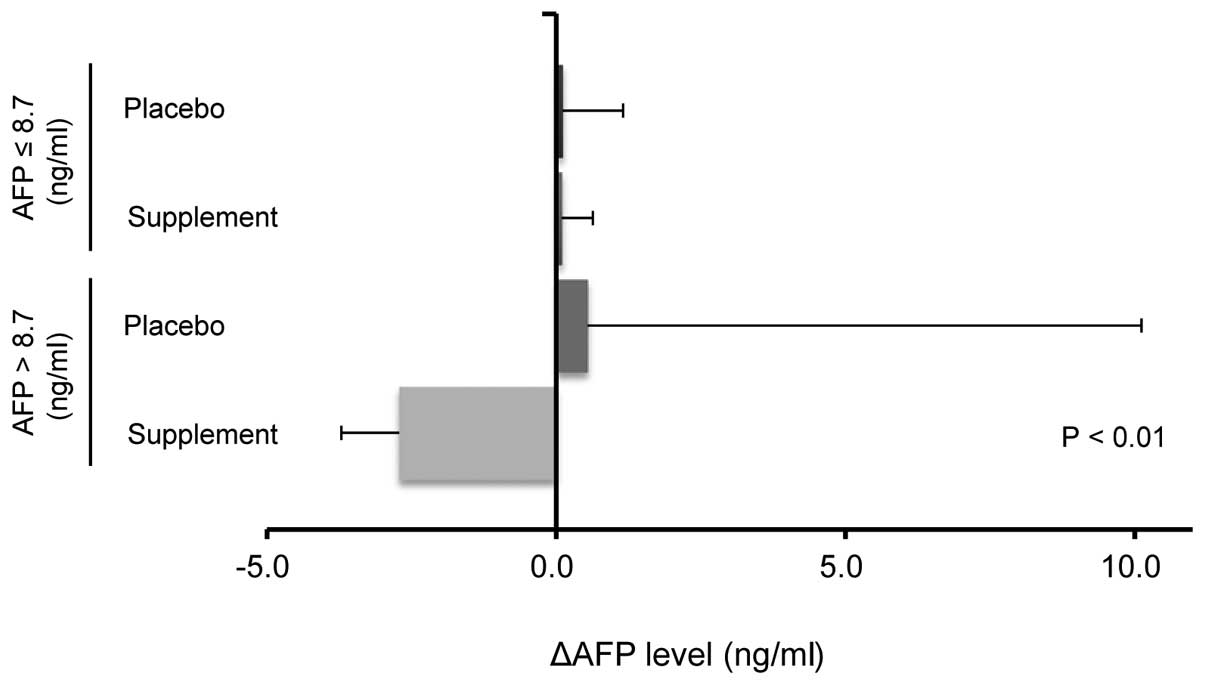
Stratification analysis according to
serum AFP levels at baseline
As the serum AFP levels were widely distributed, a
stratification analysis was performed according to the serum AFP
levels at baseline to assess the effects of BCAA and zinc-enriched
supplementation on changes in the serum AFP levels. A significant
reduction in the ΔAFP levels was observed in the supplement group
with an elevation in the AFP levels compared with the other groups
(Fig. 4).
Safety
The incidence rates of adverse events during the
study were 3.7% (1/27) and 7.7% (2/26) in the placebo and
supplement groups, respectively. Two subjects discontinued the
placebo due to withdrawal of consent (n=1) and anemia (Grade 2;
n=1). Two subjects in the supplement group discontinued the
treatment due to general fatigue (Grade 2; n=1) and rash (Grade 3;
n=1). There was no significant deterioration in any biochemical
parameters including liver and renal function. No Grade 4 or higher
adverse events occurred during the study period.
Discussion
This was a multicenter randomized controlled trial
designed to examine the effects of a BCAA and zinc-enriched
supplement on prognostic factors in HCV-infected patients. No
changes were observed in the platelet counts, serum albumin levels
or HOMA-IR values; however, a significant increase was noted in the
serum BTR and zinc levels in the patients administered the
supplement for 60 days. Furthermore, the stratification analysis
revealed a significant reduction in the ΔAFP levels in the
supplement group with an elevation in the AFP levels compared with
the other groups.
No significant increase was observed in the serum
albumin levels in this study. However, to the best of our
knowledge, we demonstrated for the first time that 6,400 mg/day
BCAAs administered for 60 days was sufficient to increase the serum
BTR in patients in the early stages of HCV-related chronic liver
disease. The dose of BCAAs used in this study may have been
insufficient to increase serum albumin levels over the period of 60
days. However, a lower serum BTR can predict decreases in serum
albumin levels (19), suggesting
that long-term administration of the supplement may maintain
constant serum albumin levels. In addition, low serum BTRs have
been recently reported to be a predictive factor in the development
of HCC, intrahepatic distant recurrence of HCC and poor prognosis
in patients with cirrhosis (2,3).
Moreover, BCAA supplementation is known to increase serum BTR,
leading to the suppression of hepatocarcinogenesis and improvement
in survival in patients with cirrhosis (4,10,11).
Taken together, it would be worthwhile to test the effect of the
long-term administration of the supplement on hepatocarcinogenesis
and prognosis in HCV-infected patients.
We also demonstrated that the administration of 10
mg/day zinc was sufficient to increase the serum zinc levels in
patients in the early stages of HCV-related chronic liver disease.
In patients with cirrhosis, zinc upregulates hepatic ornithine
transcarbamylase activity, a key enzyme in the urea cycle, and
enhances hepatic ammonia detoxification (20). A recent meta-analysis by
Chavez-Tapia et al (21)
demonstrated that oral zinc supplementation improved performance in
the number connection test, a test for hepatic encephalopathy.
Thus, the supplement tested here may be beneficial for patients
with hyperammonemia. In addition, a decrease in serum zinc levels
is known to predict the development of HCC and poor prognosis in
HCV-infected patients (8,9). Moreover, decreased risks of
hepatocarcinogenesis and mortality have been observed in
HCV-infected patients with increased serum zinc levels owing to
zinc supplementation (8,9). The data reported here indicate that
the zinc-enriched supplement has the potential to suppress the
onset of HCC and improve prognosis in HCV-infected patients.
When all of the data were analyzed, the serum AFP
levels were not significantly decreased in the patients
administered the BCAA and zinc-enriched supplement. However, the
stratification analysis revealed a significant reduction in the
ΔAFP levels in the supplement group with elevated AFP levels at
baseline compared with the other groups. Although the reasons for
the supplement-induced reductions in serum AFP levels are unclear,
our findings are supported by previously published studies. First,
Hagiwara et al (22)
reported that BCAAs induce apoptosis in HCC cell lines by promoting
a negative feedback loop from the mammalian target of rapamycin
complex 1/S6K1 to the PI3K/Akt pathway and by suppressing the
mammalian target of rapamycin complex 2 kinase activity towards
Akt. Second, zinc stabilizes zinc finger proteins, which bind to
DNA, and Nakao et al, as well as Xie et al, reported
that zinc fingers and homeoboxes 2 and zinc finger and BTB
domain-containing protein 20 repress the postnatal expression of
AFP by interacting with the AFP gene promoter regions (23,24).
Thus, BCAAs and zinc may independently contribute to a reduction in
serum AFP levels by causing apoptosis of hepatoma cells and
repressing AFP expression.
In this study, the BCAA and zinc-enriched supplement
did not affect the platelet count, HOMA-IR value or HCV RNA levels.
Conversely, previous basic studies demonstrated that valine, a
BCAA, increased blood platelet counts in carbon
tetrachloride-treated cirrhotic rats (25). Leucine and isoleucine have been
shown to improve insulin resistance in mice fed a high-fat diet
(26,27). Valine has been shown to suppress
HCV genome replication in a dose-dependent manner (28). Although the reason for the
discrepancy between these previous studies and our study remains
unknown, BCAAs may exert beneficial effects on the platelet count,
HOMA-IR value and serum HCV RNA levels only under specific
conditions. We also demonstrated that no subjective symptoms were
significantly improved by the BCAA and zinc-enriched
supplementation. BCAAs and zinc have been previously reported to
improve muscle cramps and taste disorders (16,29,30),
respectively. However, these symptoms were mild in the study
subjects at baseline. This may explain why significant changes in
muscle cramps and taste disorders were not evident in this
study.
In conclusion, we examined the effects of a BCAA and
zinc-enriched supplement on prognostic factors in HCV-infected
patients. There were no significant changes in platelet count,
serum albumin levels or HOMA-IR values. However, serum BTR and zinc
levels were significantly improved by the supplementation. In
addition, a stratification analysis revealed a significant
reduction in ΔAFP levels in the supplement group, with an increase
in AFP levels compared with the other groups. In light of these
results, we conclude that the BCAA and zinc-enriched supplement may
improve prognosis in HCV-infected patients by improving amino acid
imbalance, reducing zinc deficiencies and partly downregulating AFP
expression.
Acknowledgements
The authors thank Dr Tatsuya Ide (Kurume University
School of Medicine), Dr Tatsuo Kanda (Chiba University) and Dr
Makoto Arai (Chiba University) for the collection of data.
Abbreviations:
|
BCAAs
|
branched-chain amino acids
|
|
BTR
|
BCAA-to-tyrosine ratio
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
HOMA-IR
|
homeostasis model assessment for
insulin resistance
|
|
AFP
|
α-fetoprotein
|
|
BMI
|
body mass index
|
References
|
1
|
Kawaguchi T, Izumi N, Charlton MR and Sata
M: Branched-chain amino acids as pharmacological nutrients in
chronic liver disease. Hepatology. 54:1063–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawaguchi T, Taniguchi E and Sata M:
Effects of oral branched-chain amino acids on hepatic
encephalopathy and outcome in patients with liver cirrhosis. Nutr
Clin Pract. 28:580–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishikawa T, Kubota T, Horigome R, Kimura
N, Honda H, Iwanaga A, et al: Branched-chain amino acids to
tyrosine ratio (BTR) predicts intrahepatic distant recurrence and
survival for early hepatocellular carcinoma.
Hepatogastroenterology. 60:2013.PubMed/NCBI
|
|
4
|
Kawaguchi T, Shiraishi K, Ito T, Suzuki K,
Koreeda C, Ohtake T, et al: Branched-chain amino acids prevent
hepatocarcinogenesis and prolong survival of patients with
cirrhosis. Clin Gastroenterol Hepatol. in press. 2014. View Article : Google Scholar
|
|
5
|
Auld DS, Kawaguchi H, Livingston DM and
Vallee BL: RNA-dependent DNA polymerase (reverse transcriptase)
from avian myeloblastosis virus: a zinc metalloenzyme. Proc Natl
Acad Sci USA. 71:2091–2095. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar R, Manning J, Spendlove HE,
Kremmidiotis G, McKirdy R, Lee J, et al: ZNF652, a novel zinc
finger protein, interacts with the putative breast tumor suppressor
CBFA2T3 to repress transcription. Mol Cancer Res. 4:655–665. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moriyama M, Matsumura H, Fukushima A,
Ohkido K, Arakawa Y, Nirei K, et al: Clinical significance of
evaluation of serum zinc concentrations in C-viral chronic liver
disease. Dig Dis Sci. 51:1967–1977. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katayama K, Sakakibara M, Imanaka K,
Ohkawa K, Matsunaga T, Naito M, et al: Effect of zinc
supplementation in patients with type C liver cirrhosis. O J Gas.
1:22–28. 2011.
|
|
9
|
Matsumura H, Nirei K, Nakamura H, Arakawa
Y, Higuchi T, Hayashi J, et al: Zinc supplementation therapy
improves the outcome of patients with chronic hepatitis C. J Clin
Biochem Nutr. 51:178–184. 2012.PubMed/NCBI
|
|
10
|
Muto Y, Sato S, Watanabe A, Moriwaki H,
Suzuki K, Kato A, et al: Effects of oral branched-chain amino acid
granules on event-free survival in patients with liver cirrhosis.
Clin Gastroenterol Hepatol. 3:705–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muto Y, Sato S, Watanabe A, Moriwaki H,
Suzuki K, Kato A, et al: Overweight and obesity increase the risk
for liver cancer in patients with liver cirrhosis and long-term
oral supplementation with branched-chain amino acid granules
inhibits liver carcinogenesis in heavier patients with liver
cirrhosis. Hepatol Res. 35:204–214. 2006.PubMed/NCBI
|
|
12
|
Kawaguchi T, Nagao Y, Matsuoka H, Ide T
and Sata M: Branched-chain amino acid-enriched supplementation
improves insulin resistance in patients with chronic liver disease.
Int J Mol Med. 22:105–112. 2008.PubMed/NCBI
|
|
13
|
Kawaguchi T, Taniguchi E, Itou M, Sumie S,
Oriishi T, Matsuoka H, et al: Branched-chain amino acids improve
insulin resistance in patients with hepatitis C virus-related liver
disease: report of two cases. Liver Int. 27:1287–1292.
2007.PubMed/NCBI
|
|
14
|
Nagao Y, Kawaguchi T, Ide T and Sata M:
Effect of branched-chain amino acid-enriched nutritional
supplementation on interferon therapy in Japanese patients with
chronic hepatitis C virus infection: a retrospective study. Virol
J. 9:2822012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagao Y, Kawaguchi T, Kakuma T, Ide T and
Sata M: Post-marketing surveillance study for efficacy and safety
of Aminofeel®, a branched chain amino acids-enriched
supplement including zinc. J New Rem & Clin. 60:198–215.
2011.(In Japanese).
|
|
16
|
Nagao Y, Matsuoka H, Kawaguchi T and Sata
M: Aminofeel® improves the sensitivity to taste in
patients with HCV-infected liver disease. Med Sci Monit.
16:PI7–P12. 2010.PubMed/NCBI
|
|
17
|
Kawaguchi T, Yoshida T, Harada M, Hisamoto
T, Nagao Y, Ide T, et al: Hepatitis C virus down-regulates insulin
receptor substrates 1 and 2 through up-regulation of suppressor of
cytokine signaling 3. Am J Pathol. 165:1499–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dupont WD and Plummer WD Jr: Power and
sample size calculations. A review and computer program. Control
Clin Trials. 11:116–128. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki K, Koizumi K, Ichimura H, Oka S,
Takada H and Kuwayama H: Measurement of serum branched-chain amino
acids to tyrosine ratio level is useful in a prediction of a change
of serum albumin level in chronic liver disease. Hepatol Res.
38:267–272. 2008. View Article : Google Scholar
|
|
20
|
Riggio O, Merli M, Capocaccia L, Caschera
M, Zullo A, Pinto G, et al: Zinc supplementation reduces blood
ammonia and increases liver ornithine transcarbamylase activity in
experimental cirrhosis. Hepatology. 16:785–789. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chavez-Tapia NC, Cesar-Arce A,
Barrientos-Gutierrez T, Villegas-Lopez FA, Mendez-Sanchez N and
Uribe M: A systematic review and meta-analysis of the use of oral
zinc in the treatment of hepatic encephalopathy. Nutr J. 12:742013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagiwara A, Nishiyama M and Ishizaki S:
Branched-chain amino acids prevent insulin-induced hepatic tumor
cell proliferation by inducing apoptosis through mTORC1 and
mTORC2-dependent mechanisms. J Cell Physiol. 227:2097–2105. 2012.
View Article : Google Scholar
|
|
23
|
Nakao K and Ichikawa T: Recent topics on
alpha-fetoprotein. Hepatol Res. 43:820–825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Z, Zhang H, Tsai W, Zhang Y, Du Y,
Zhong J, et al: Zinc finger protein ZBTB20 is a key repressor of
alpha-fetoprotein gene transcription in liver. Proc Natl Acad Sci
USA. 105:10859–10864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakanishi C, Doi H, Katsura K and Satomi
S: Treatment with L-valine ameliorates liver fibrosis and restores
thrombopoiesis in rats exposed to carbon tetrachloride. Tohoku J
Exp Med. 221:151–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Guo K, LeBlanc RE, Loh D,
Schwartz GJ and Yu YH: Increasing dietary leucine intake reduces
diet-induced obesity and improves glucose and cholesterol
metabolism in mice via multimechanisms. Diabetes. 56:1647–1654.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikehara O, Kawasaki N, Maezono K, Komatsu
M and Konishi A: Acute and chronic treatment of L-isoleucine
ameliorates glucose metabolism in glucose-intolerant and diabetic
mice. Biol Pharm Bull. 31:469–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishida H, Kato T, Takehana K, Tatsumi T,
Hosui A, Nawa T, et al: Valine, the branched-chain amino acid,
suppresses hepatitis C virus RNA replication but promotes
infectious particle formation. Biochem Biophys Res Commun.
437:127–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kugelmas M: Preliminary observation: oral
zinc sulfate replacement is effective in treating muscle cramps in
cirrhotic patients. J Am Coll Nutr. 19:13–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sako K, Imamura Y, Nishimata H, Tahara K,
Kubozono O and Tsubouchi H: Branched-chain amino acids supplements
in the late evening decrease the frequency of muscle cramps with
advanced hepatic cirrhosis. Hepatol Res. 26:327–329. 2003.
View Article : Google Scholar : PubMed/NCBI
|