Introduction
Biliary tract cancer includes types of cancer which
originate from epithelial cells of the intrahepatic or extrahepatic
bile ducts and anatomically comprise intrahepatic
cholangiocarcinoma, perihilar cholangiocarcinoma, gallbladder
cancer and distal cholangiocarcinoma (1). Though biliary tract cancer is an
uncommon type of tumor, the incidence and mortality of biliary
tract cancer has been increasing worldwide (2–4).
Patients with biliary tract cancer are usually asymptomatic or may
present with nonspecific clinical features, including fever,
fatigue, anorexia, mild abdominal pain, weight loss and occasional
jaundice late in the course of the disease (1). Patients with biliary tract cancer
have a high mortality rate and poor prognosis, with a five-year
survival rate of <5% due to its late clinical presentation
(5).
The majority of tumors are at an advanced stage at
the time of diagnosis, which is one of the reasons for the high
mortality rate and poor prognosis of biliary tract cancer (6). Confirmation of the diagnosis of
biliary tract cancer is often difficult and the diagnosis is based
on a combination of serum tumor markers, radiological imaging and
histological verification; however, each of these approaches has
its own drawbacks (7–11). Tissue diagnosis has been accepted
as the ‘gold standard’. Percutaneous fine-needle aspiration biopsy,
brush and scrape biopsy and cytological examination of bile have
all been used to establish a tissue diagnosis; however, the
sensitivity in detecting a malignancy is low and the possibility
that a benign result is unreliable requires consideration (12,13).
Tissue diagnosis cannot generally be performed due to the location
and size of the tumor (7).
Percutaneous fine-needle aspiration biopsy cannot be used in a
number of cases when the tumors are located in the hepatic hilum
and accompanied by important arteries and veins (8). Although serum tumor markers,
including carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic
antigen (CEA) have been widely used to assist in the diagnosis of
biliary tract cancer, they have poor sensitivity (50–60%) and these
serum tumor markers can also be elevated in other types of
malignancy and certain benign conditions (9–11).
A novel approach, termed ‘metabolomics’ or
‘metabonomics’, deals with the diverse properties of low molecular
metabolites in the body. This novel approach has been demonstrated
to be an effective tool for biomarker screening, disease diagnosis
and prognosis as well as in the characterization of the metabolic
network (14–16). Metabolomics can be applied to any
bio-fluid, including serum, urine, saliva or bile and has been
identified as a promising and reliable novel diagnostic approach
for several types of tumor, including breast cancer, prostate
cancer, hepatocellular carcinoma and pancreatic carcinoma (17–20).
However, few studies have investigated the application of
metabolomics in biliary tract cancer or chlangiocarcinoma.
In the present study, bile was obtained from 115
individuals and the metabolite patterns of these bile samples were
examined using a liquid chromatography/mass spectrometry
(LC/MS)-based metabolomic method. Potential biomarkers were
identified for distinguishing between patients with biliary tract
cancer, patients with benign biliary tract disease and healthy
individuals at an early stage to improve the diagnosis and
prognosis of biliary tract cancer.
Materials and methods
Patient recruitment and sample
collection
Informed consent was obtained from each individual
and the present study was approved by the Ethics Committee of The
First Affiliated Hospital of Zhejiang University (Hangzhou, China).
A total of 115 individuals were enrolled in the present study,
which were divided into three groups: 32 patients with biliary
tract cancer (male/female, 5:3), 61 patients with benign biliary
tract diseases (male/female, 1:1) and 22 normal controls
(male/female, 10:1). The 22 normal control individuals were all
liver donors for transplant at the First Affiliated Hospital of
Zhejiang University. Bile samples were prospectively obtained from
patients with biliary tract cancer or benign biliary tract diseases
either during surgery or endoscopic retrograde
cholangiopancreatography (ERCP) and from normal control individuals
during the donor liver transplantation surgery. The collected bile
samples were then immediately frozen at −80°C.
Patient characteristics
The 115 enrolled individuals consisted of 32
patients with biliary tract cancer, 61 patients with benign biliary
tract diseases and 22 normal controls. The 32 patients in the
biliary tract cancer group included 13 cases of intrahepatic
cholangiocarcinoma, 4 cases of Klatskin tumor, 2 cases of
gallbladder cancer, 11 cases of common bile duct cancer and 2 cases
of a combination of gallbladder cancer and common bile duct cancer.
The 61 patients in the benign biliary tract disease group included
40 cases of biliary calculi, 12 cases of benign biliary tract
stricture and 9 cases of choledochal cyst. The patient
characteristics of the three groups differed due to the
epidemiology of the different diseases. The average age was 59
years in the cancer group, 58 years in the benign biliary tract
disease group and 26 years in the normal control group. The ratio
of males to females was 5:3 in the cancer group, ~1:1 in the benign
biliary tract diseases group and almost 10:1 in the normal control
group.
Bile specimen pretreatment
Prior to LC/MS analysis, 2,200 μl acetonitrile
Sigma-Aldrich (St.Louis, MO, USA) was added to bile samples (200
μl) and vortex was performed for 2 min at room temperature. The
sample mixture was left to stand for 5 min and centrifuged at
18,000 × g for 10 min at 4°C. Subsequently, 1,500 μl supernatant
was used in the LC/MS analysis.
LC separation
Chromatographic separations were performed on an
ACQUITY Ultra Performance LC system using an ACQUITY UPLC BEH C18
analytical column (internal dimension, 2.1×100 mm; particle size,
1.7 μm; pore size, 130 Å; Waters MS Technologies, Manchester, UK).
A mixture of water and formic acid (99.9:0.1, v/v; Sigma-Aldrich)
was used as mobile phase A and acetonitrile with formic acid
(99.9:0.1, v/v) as mobile phase B at a flow rate of 300 μl/min. The
linear gradient LC system was optimized as follows: The composition
of mobile phase B was increased from 3 to 30% over 2 min, to 60%
over 8 min, 90% over 1 min and 100% over 6 min, then leveled for 8
min. The column was maintained at 50°C and the sample manager was
set at 4°C. A 2 μl injection of each sample was made into the
column.
MS assay
MS was performed using a Q-Tof Premier Mass
Spectrometer (Waters MS Technologies) operating in positive ion
mode. The nebulization gas was set to 600 l/h at a temperature of
350°C, the cone gas was set to 0 l/h and the source temperature was
set to 120°C. The capillary voltage was set at 2.8 kV and the
sampling cone voltage was set at 40 V (Waters MS Technologies). The
Q-Tof Premier acquisition rate was set to 0.5 sec, with a 0.1 sec
inter-scan delay. The instrument was used with the first resolving
quadrupole in a wide pass mode with the collision cell operating
with two alternating collision energies, 5 eV and 50 eV. The data
were collected into two separate data channels, with the instrument
spending 0.5 sec on data acquisition for each channel with a 0.05
sec inter-channel delay. The instrument was previously calibrated
with sodium formate, the lock mass spray for precise mass
determination was set using leucine enkephalin at a mass to charge
ratio (m/z) of 554.2615 (0.5 ng/l) in the positive ion mode, which
increased the maximum relative error up to 10 ppm. Data were
collected in centroid mode with a lock spray frequency of 5 sec and
the average from 10 scans was obtained. All analyses were acquired
using lock spray to ensure accuracy and reproducibility.
Data processing and statistical
analysis
The raw data files obtained from the LC/MS runs were
analyzed using MassLynx v4.1 and MarkerLynx v4.1 software (Waters
MS Technologies). MarkerLynx extracted components from the exact
mass chromatograms and listed the detected peaks as their mass,
retention time and associated intensities. Chromatographic peaks in
the raw data files were detected by extracting nominal mass
chromatograms and tracking the apex of the peaks in the
chromatograms. The spectra from each of the detected peaks were
listed as the retention time, exact mass pairs and associated
intensities, which were saved as either normalized or absolute
intensities. Following extraction of all the data, it was aligned
within user-defined mass (0.05 Da) and retention time windows (0.2
min). The resulting multivariate dataset, consisting of the peak
number based on the retention time, m/z, sample name and the
normalized peak intensity, was exported and analyzed by partial
least squares projection to latent structures with discriminant
analysis (PLS-DA) and orthogonal projection to latent structures
with discriminant analysis (OPLS-DA) using SIMCA-P 12.0 software
(Umetrics, Umeå, Sweden).
Results
Metabonomic profiling of bile samples and
multivariate analysis
The bile samples were characterized by LC/MS in the
positive ion mode and the mass spectra of the bile samples were
obtained from all three groups. The mass spectral data were then
processed by multivariate analysis. The PLS-DA model was
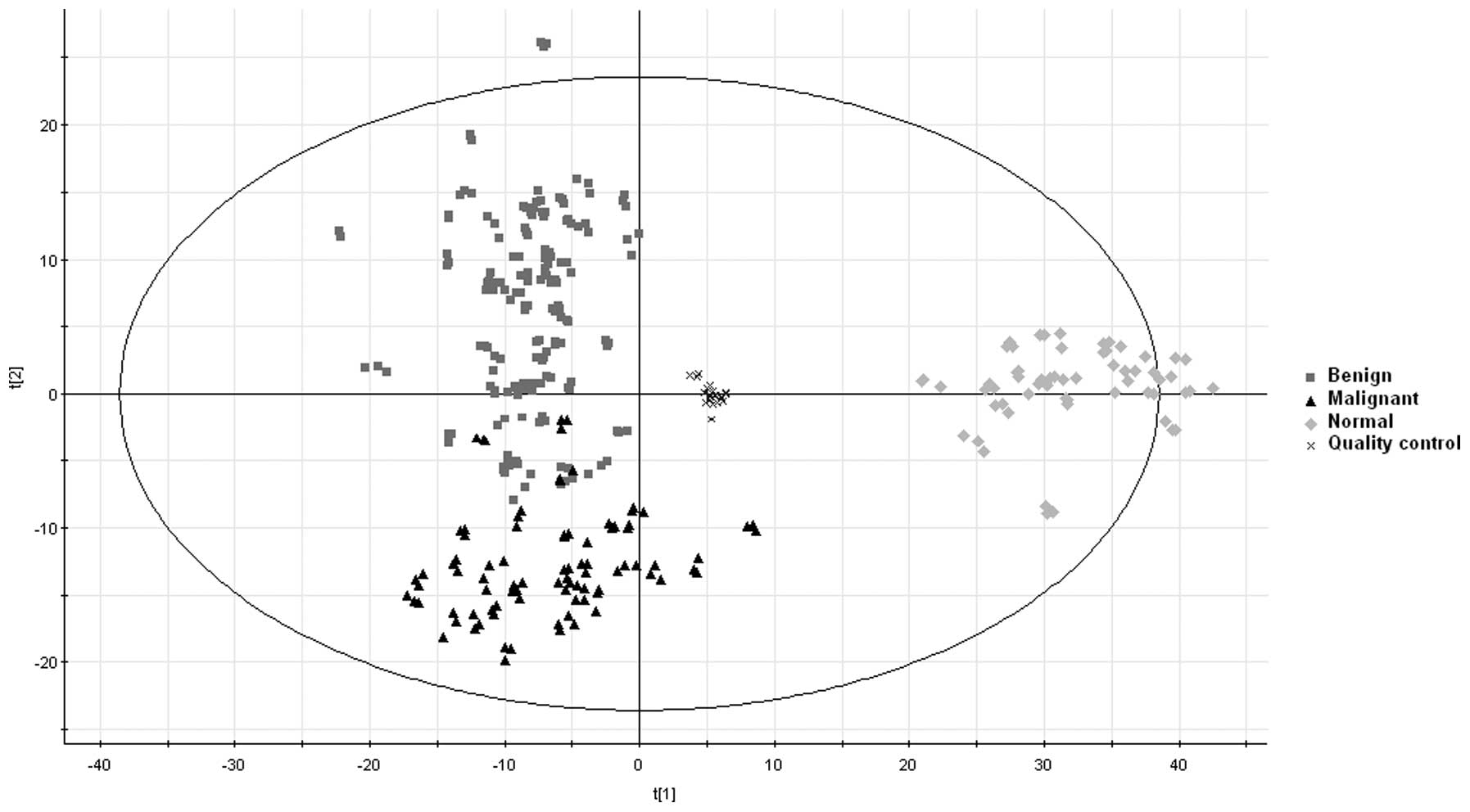
subsequently used for data analyses. The results revealed
high-grade quality control and clear separation between the disease
groups (biliary tract cancer and benign biliary tract diseases) and
the normal control group and the results also demonstrated clear
separation between the biliary tract cancer group and the benign
biliary tract disease group in the metabolomic 2D scores plot. The
majority of the cancer and benign samples appeared clustered in
their respective regions with only a few overlaps between them
(Fig. 1).
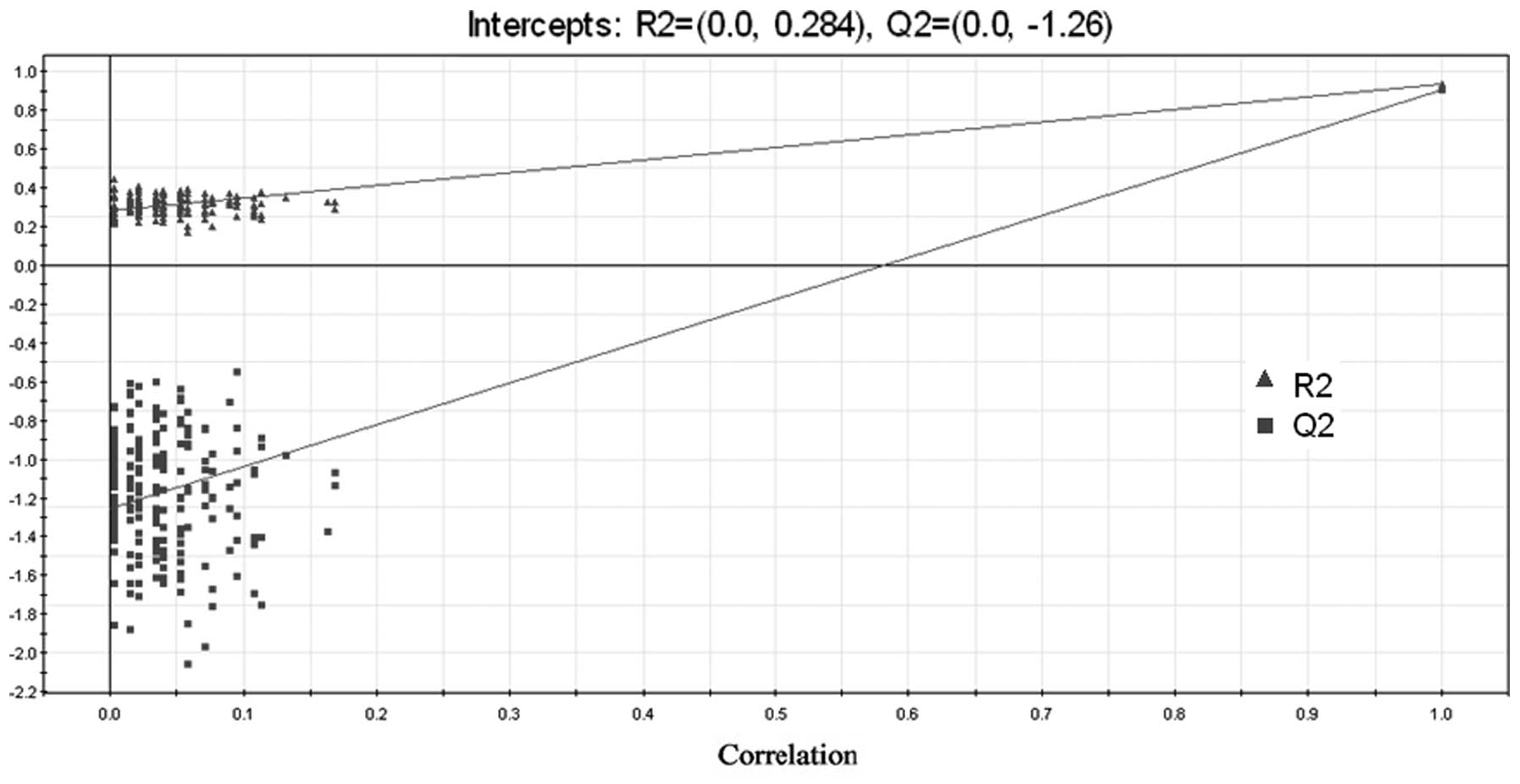
The 3D scores plot demonstrated similar results. A
validated PLS-DA model was used to evaluate the goodness of fit of
the model. Generally, a model is considered to have a good fit if
R2 intercepts are <0.4 and Q2 is <0.05 in the permutation
assessment, with 200 iterations (21,22).
In the present study, the R2 intercepts were <0.4 and the Q2 was
<0 using PLS-DA, demonstrating that the model was well-fit and
statistically valid (Fig. 2).
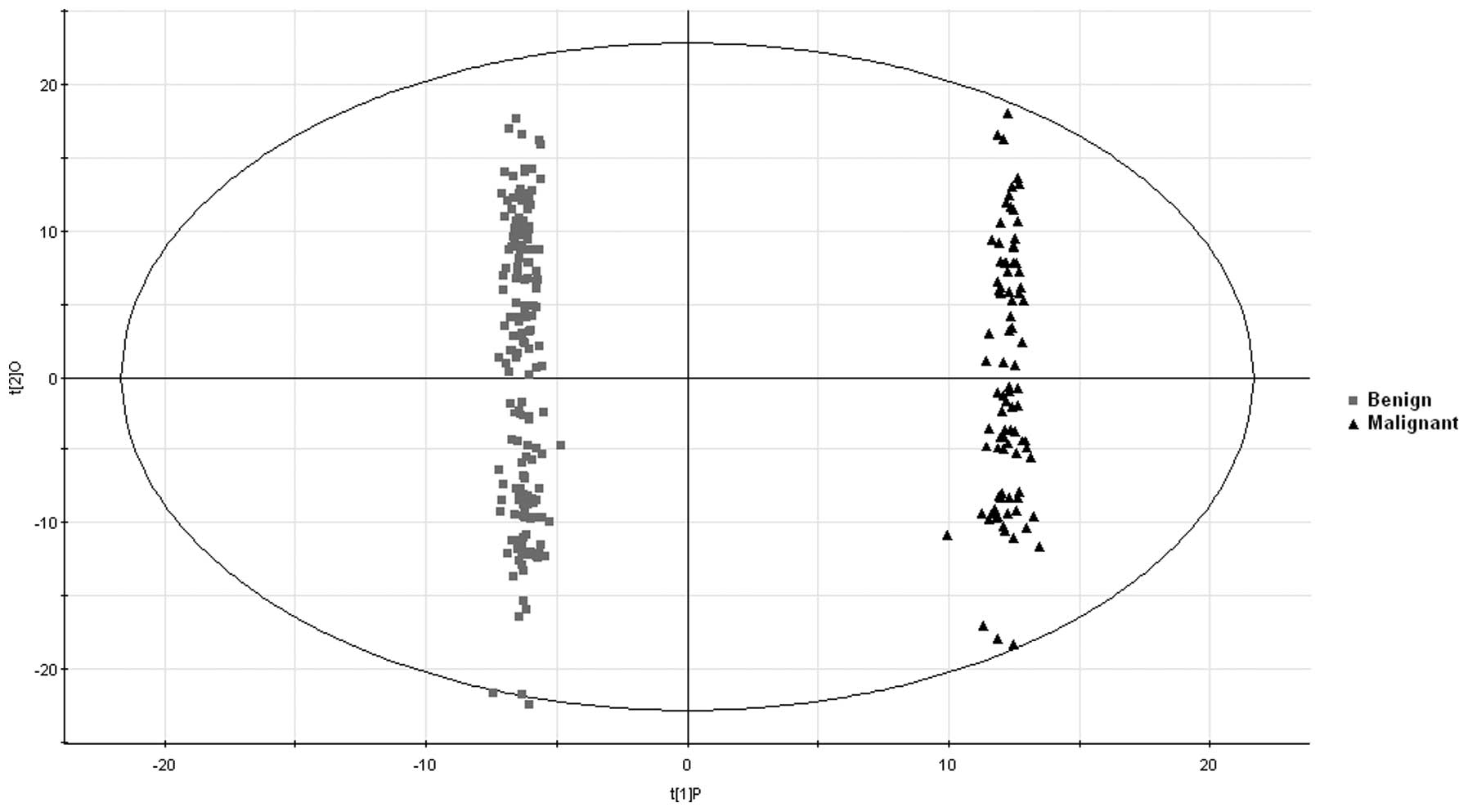
To further address the substantial clinical
difficulties in differentiating between biliary tract cancer and
benign biliary tract diseases, an OPLS-DA model was used for
discrimination of the two disease groups and to select potential
biomarkers. The analytical results revealed that the cancer and
benign groups were well differentiated (Fig. 3).
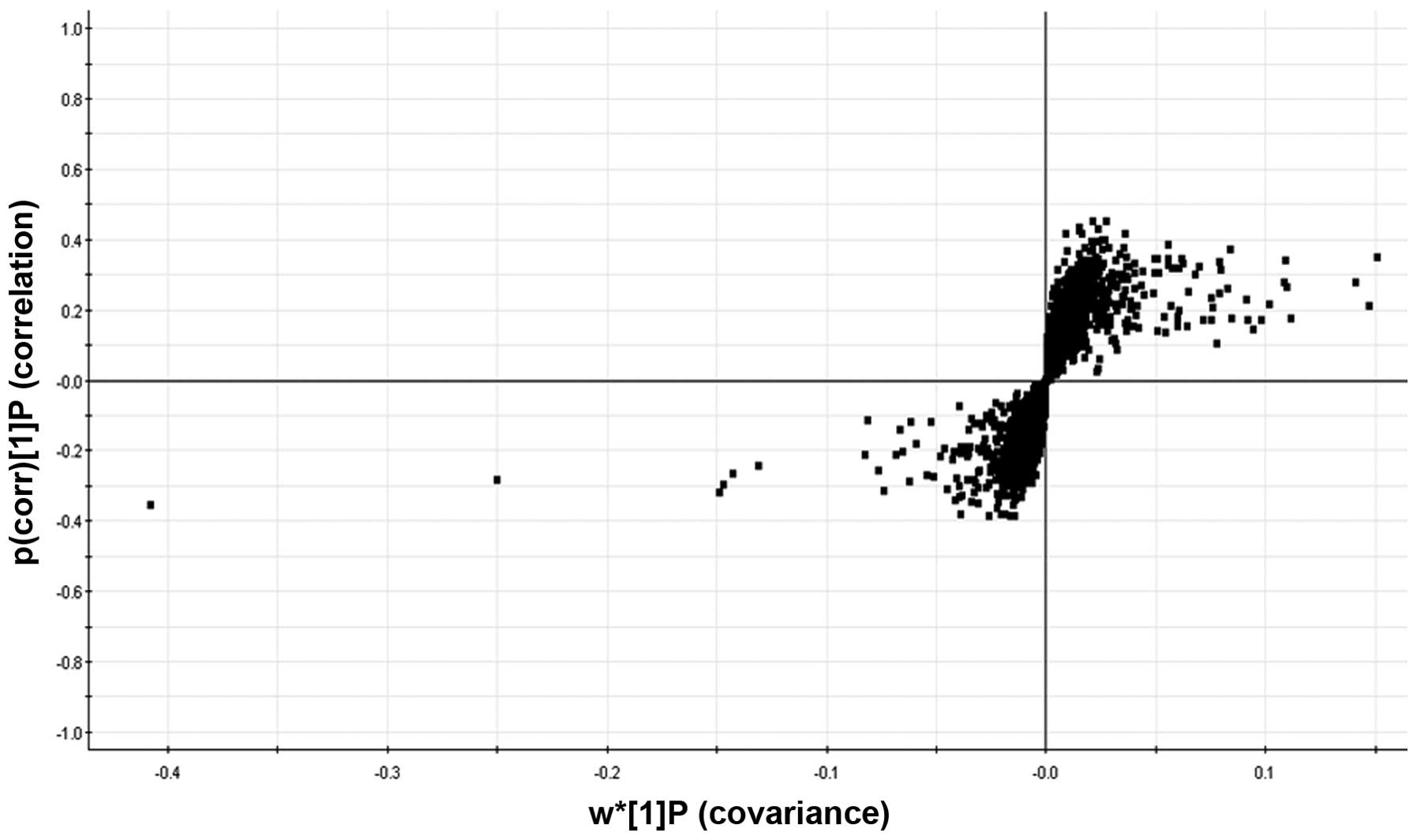
The S-plot, which visualizes the covariance and
correlation among metabolites, is usually used to identify
important metabolites (23). As
shown in Fig. 4, the S-plot
revealed the metabolites which most reliably assisted in the
differentiation of the two groups.
The variable importance in the projection (VIP) in
the OPLS-DA model is a predominant parameter for the detection of
potential biomarkers (24) and, in
the present study, the different metabolite VIP values reflect the
correlation between the metabolites and the discrimination of the
biliary tract cancer and the benign biliary tract disease groups.
The potential biomarkers, which discrimination was mainly
attributed to, were selected from the S-plot according to the VIP
values and, using the Metlin Metabolite Database (http://metlin.scripps.edu), Human Metabolome Database
(http://www.hmdb.ca/), relevant literature
(25,26) and comparison with a standard
substance, the potential biomarkers were identified (Table I).
 | Table ISummary of the metabolites indicating
significant changes in bile between the benign and cancer
groups. |
Table I
Summary of the metabolites indicating
significant changes in bile between the benign and cancer
groups.
| RT (min)_m/z | VIP | Identification
results | Cancer (vs.
benign) |
|---|
| 11.58_496.3385 | 21.4074 | Lyso-PC 16:0,
[M+H]+ | ↓ |
| 11.53_991.6724 | 13.1144 | Lyso-PC 16:0,
[M+H]+ | ↓ |
| 11.32_496.3387 | 7.8017 | Lyso-PC 16:0
isomer, [M+H]+ | ↓ |
| 7.57_899.6366 | 7.71738 | GCDCA,
[2M+H]+ | ↑ |
| 12.29_524.3703 | 7.69633 | Lyso-PC 18:0,
[M+H]+ | ↓ |
| 4.51_462.2664 | 7.39132 | TUCA,
[M-3H2O+H]+ | ↑ |
| 2.02_120.0801 | 6.87389 | Phenylalanine,
[M+H]+ | ↓ |
| 5.71_464.2823 | 5.85991 | TUDCA,
[M-2H2O]+ | ↑ |
| 7.56_450.3208 | 5.34161 | GCDCA,
[M+H]+ | ↑ |
| 5.54_412.2835 | 4.93109 | GCA,
[M-3H2O+H] | ↑ |
| 5.53_430.2947 | 4.82189 | GCA,
[M-2H2O+H]+ | ↑ |
| 3.97_480.2770 | 4.38365 | TUCA isomer,
[M-2H2O]+ | ↑ |
| 4.51_480.2769 | 4.15222 | TUCA,
[M-2H2O]+ | ↑ |
| 7.57_414.2993 | 4.08688 | GCDCA,
[M-2H2O+H] | ↑ |
| 3.49_286.2004 | 4.0158 |
2-Octenoylcarnitine,
[M+H]+ | ↓ |
| 6.06_464.2821 | 3.74324 | TCDCA,
[M-2H2O+H]+ | ↑ |
| 11.09_520.3395 | 3.59733 | Lyso-PC 18:2,
[M+H]+ | ↓ |
| 2.28_188.0698 | 3.42225 | Tryptophan,
[M+H]+ | ↓ |
| 4.52_498.2880 | 3.3963 | TUCA,
[M-H2O]+ | ↑ |
| 5.54_948.6544 | 3.36625 | GCA,
[2M+NH4]+ | ↑ |
The representative variable averages of the
potential biomarkers in the biliary tract cancer group and in the
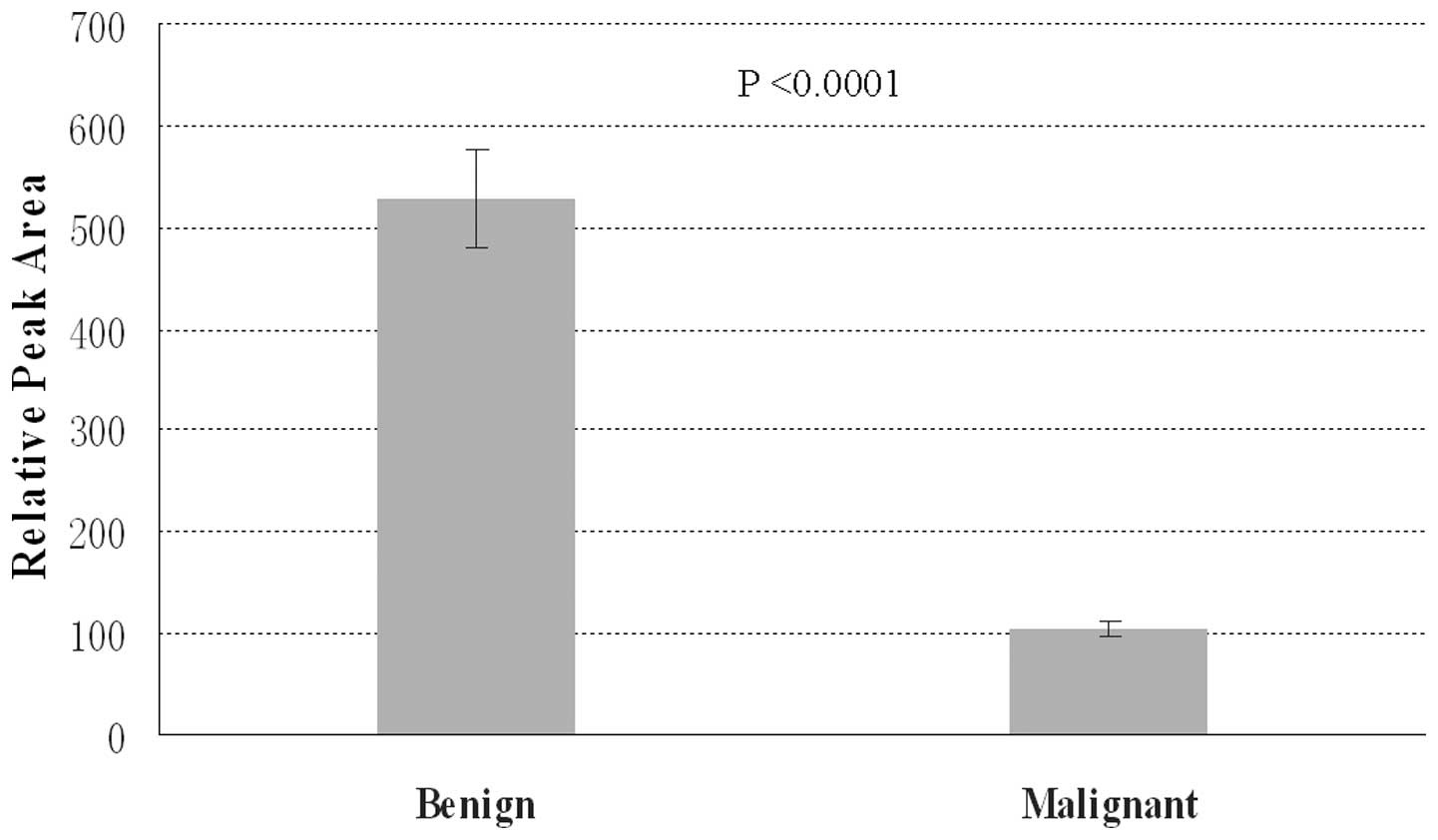
benign biliary tract disease group are shown in Figs. 5 and 6. As shown in Fig. 5, the variable averages of
lysophosphatidylcholine (lyso-PC) 16:0 were markedly reduced in the
biliary tract cancer group compared with those in the benign
biliary tract disease group. Conversely, the variable averages of
glycochenodeoxycholic acid (GCDCA) were significantly augmented in
the biliary tract cancer group compared with those in the benign
biliary tract disease group (Fig.
6).
Discussion
Biliary tract cancer comprises a group of malignant
biliary diseases, which have a high mortality rate (2–4).
Surgical excision of the tumor is the only option to improve the
survival rate of patients with these diseases, due to the
insensitivity or lack of response to chemotherapy or radiotherapy.
However, the prognosis of patients who suffer from biliary tract
cancer remains poor as diagnosis of the disease is generally late.
This late diagnosis is partly the result of the disease
characteristics, which include asymptomatic traits or nonspecific
clinical features; however, it is mainly attributed to the lack of
powerful and sensitive diagnostic tools (6). Previously, the diagnosis was
primarily dependent on the serum tumor markers CA19-9 and CEA as
well as radiological imaging, which have low levels of sensitivity
and specificity. Tissue diagnosis was also rare due to the location
and size of the tumor and the risk of possible hemorrhage or bile
leakage (7,8). In recent years, brush and scrape
biopsy and cytological examinations of bile have been used to
establish a tissue diagnosis with widespread application of ERCP;
however, their use for differentiating between cancer and benign
diseases is limited due to insufficient sensitivity and benign
results are often considered to be unreliable (27–30).
Therefore, novel diagnostic methods are required and of high
importance.
Metabolomics is a novel and promising tool, which
has emerged in recent years. It has been identified as an effective
tool for the screening of biomarkers and disease diagnosis
(14,16). Techniques used in metabolomic
studies generally include nuclear magnetic resonance spectroscopy,
Fourier-transform infrared spectroscopy and gas chromatography/mass
spectrometry or LC/MS (31). LC/MS
is regarded as an ideal tool for organic metabolites and the
screening of biomarkers among them due to its prominent advantages,
including reproducible quantitative analysis and the ability to
analyze biofluids with high levels of molecular complexity
(32). Previous studies have
demonstrated the potential for metabolomics as a novel reliable
diagnostic approach for hepatocellular carcinoma as well as
pancreatic, breast and prostate cancer (18–20).
The few previous investigations into the application of the
metabolomic approach on biliary tract cancer consisted of small
sample sizes, discrepant results and different methods (33–35).
In the present study, an LC/MS-based metabolomic method was used to
examine the metabolite patterns of bile samples from a large study
sample of 115 individuals. In addition, normal bile samples were
included as a control, which, to the best of our knowledge, had not
been included in previous similar metabolomic investigations of
bile. The metabolomic analytic results demonstrated a clear
separation between the disease groups (biliary tract cancer and
benign biliary tract diseases) and the normal control group in the
PLS-DA model. The results also revealed clear separation between
the biliary tract cancer group and benign biliary tract disease
group in the metabolomic 2D and 3D score plots. These results
reflected the evident efficacy of the present study and the applied
model, which was further confirmed using a validated model in the
permutation assessment with 200 iterations, R2 intercepts <0.4
and the Q2<0, demonstrating that the model was good-fit and
statistically valid.
In diagnosing biliary tract diseases, the
differential diagnosis between biliary tract cancer and benign
biliary tract diseases is particularly challenging. It is often
difficult to provide an exact diagnosis for a patient with biliary
tract stricture as biliary tract cancer or long term biliary
inflammation can lead to biliary tract stricture (36). The present study aimed to assist in
overcoming this clinical difficulty. The OPLS-DA model was further
applied to discriminate between the disease groups in the present
study and the results revealed that the cancer and benign groups
were well differentiated. Furthermore, potential biomarkers with
the greatest contribution to this discrimination were then selected
from the S-plot according to the VIP values and were identified.
The results revealed significantly lower levels of lyso-PC 16:0,
lyso-PC 16:0 isomer, lyso-PC 18:0, phenylalanine,
2-octenoylcarnitine, lyso-PC 18:2 and tryptophan and significantly
higher levels of GCDCA, tauroursocholic acid (TUCA),
tauroursodeoxycholic acid (TUDCA), glycocholic acid (GCA), TUCA
isomer and taurochenodeoxycholic acid (TCDCA) in the bile from
biliary tract cancer patients compared with that of patients with
benign biliary tract diseases.
Lyso-PC is one of the major lysophospholipids and is
mainly generated by hydrolysis of phosphatidylcholine.
Phosphatidylcholine reduction has been observed in the bile from
patients with biliary tract cancer or cholangiocarcinoma (34,35,37).
Phosphatidylcholine is considered to be the major and dominant
biliary phospholipid and is essential for membrane structure,
signal transduction and lipoprotein metabolism (38). It is synthesized in the hepatocytes
and is subsequently transported into the biliary canaliculus by
flippase multidrug-resistant protein 3 (39). Phosphatidylcholine is
cytoprotective towards the biliary epithelium and reduces the
cellular toxicity of bile acids (40,41).
The reduction of phosphatidylcholine in the bile exposes the
biliary epithelium to ‘toxic’ bile and predisposes it to biliary
malignancies (42). The
correlation between lyso-PC and biliary malignancy remains to be
elucidated; however, a significant reduction in lyso-PC was
observed in the bile of patients with biliary tract cancer patients
compared with that of patients with benign biliary tract diseases
in the present study. The mechanism underlying this finding
requires further elucidation, which following investigations aim to
focus on.
Phenylalanine and tryptophan are two of the
essential amino acids involved in protein synthesis in the human
body, which are aromatic amino acids. No previous studies have
investigated the role of phenylalanine in biliary tract cancer,
whereas one revealed that the expression of tryptophan hydroxylase
1 increases and monoamine oxidase A decreases in
cholangiocarcinoma, resulting in increased secretion of serotonin
from the cholangiocarcinoma and increased serotonin in the bile
from cholangiocarcinoma patients (43). No other observations have been made
regarding tryptophan and biliary tract cancer and the previous
tryptophan study did explain the significantly lower level of
tryptophan in the bile from patients with biliary tract cancer,
which was observed in the present study. Thus, to the best of our
knowledge, the present study was the first to demonstrate a
correlation between phenylalanine and tryptophan and biliary tract
cancer.
Of note, only a few previous studies have
investigated 2-octenoylcarnitine and no studies have examined its
role in biliary tract cancer (44,45).
In the present study, 2-octenoylcarnitine was markedly decreased in
the bile from patients with biliary tract cancer and was examined
as a potential biomarker for biliary tract cancer for the first
time, although similar studies are required to repeat and verify
the findings and to examine the detailed mechanism.
GCDCA, TUCA, TUDCA, GCA, TUCA isomer and TCDCA are
all taurine- or glycine-conjugated bile acids. Compared with the
reduced metabolites, a significant elevation of these bile acids in
the bile from patients with biliary tract cancer compared with that
from patients with benign biliary tract diseases has been observed
in previous studies. Sharif et al (34) reported that primary bile acids and
their glycine-conjugates are significantly increased in patients
with cholangiocarcinoma compared with those in benign disease
groups. AbdAlla et al (35)
observed a similar result to that of the present study, with
taurine- and glycine-conjugated bile acids significantly elevated
in bile from patients with cholangiocarcinoma. It is understood
that an imbalance of lipids and bile acids in bile may have a
pathogenic role in cholangiocarcinogenesis. Markedly increasing
bile acids disrupt the balance and cellular toxicity of bile acids,
which may lead to carcinogenesis through oxidative DNA damage and
DNA mutation (46–48).
In conclusion, the present study aimed to assist in
overcoming the substantial difficulties faced by clinicians in the
differential diagnosis between biliary tract cancer and benign
biliary tract diseases. The results of the present study
demonstrated that the LC/MS-based metabolomic method is a potent
and promising approach for discriminating between biliary tract
cancer and benign biliary tract diseases and in identifying
specific metabolites, which may have potential as a novel
biomarkers for the early detection of biliary tract cancer.
Acknowledgements
The authors would like to thank Dr. Kwabena Kobby
(Division of Hepatobiliary and Pancreatic Surgery, Department of
Surgery, The First Affiliated Hospital, School of Medicine,
Zhejiang University, Hangzhou, Zhejiang, China) for his important
comments and suggestions. This study was sponsored by grants from
the National High Technology Research and Development Program 863
(grant no. 2012AA021002), the Special Fund for Health Research in
the Public Welfare (no. 201302009) and the National Science and
Technology Major Project (no. 2012ZX10002017).
References
|
1
|
Ravi SC and Shimul AS: Malignant biliary
disease. Sabiston Textbook of Surgery. Townsend CM, Beauchamp RD,
Evers BM, et al: Saunders Elsevier; Philadelphia, PA: 2007
|
|
2
|
Taylor-Robinson SD, Toledano MB, Arora S,
et al: Increase in mortality rates from intrahepatic
cholangiocarcinoma in England and Wales 1968–1998. Gut. 48:816–820.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel T: Worldwide trends in mortality
from biliary tract malignancies. BMC Cancer. 2:102002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SA, Taylor-Robinson SD, Toledano MB,
et al: Changing international trends in mortality rates for liver,
biliary and pancreatic tumours. J Hepatol. 37:806–813. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan SA, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno N, Sano T, Kanamaru T, et al:
Adenosquamous cell carcinoma arising from the papilla major. Oncol
Rep. 9:317–320. 2002.PubMed/NCBI
|
|
8
|
Matsumoto A, Imamura M, Akagi Y, et al: A
case report of disseminated recurrence of inferior bile duct
carcinoma in PTCD fistula. Kurume Med J. 49:71–75. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel AH, Harnois DM, Klee GG, LaRusso NF
and Gores GJ: The utility of CA19-9 in the diagnoses of
cholangiocarcinoma in patients without primary sclerosing
cholangitis. Am J Gastroenterol. 95:204–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perkins GL, Slater ED, Sanders GK and
Prichard JG: Serum tumor markers. Am Fam Physician. 68:1075–1082.
2003.PubMed/NCBI
|
|
11
|
Carpelan-Holmström M, Louhimo J, Stenman
UH, Alfthan H and Haglund C: CEA, CA 19-9 and CA 72-4 improve the
diagnostic accuracy in gastrointestinal cancers. Anticancer Res.
22:2311–2316. 2002.PubMed/NCBI
|
|
12
|
Harewood GC, Baron TH, Stadheim LM, et al:
Prospective, blinded assessment of factors influencing the accuracy
of biliary cytology interpretation. Am J Gastroenterol.
99:1464–1469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malhi H and Gores GJ: Review article: the
modern diagnosis and therapy of cholangiocarcinoma. Aliment
Pharmacol Ther. 23:1287–1296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wua H, Xue R, Lub C, et al: Metabolomic
study for diagnostic model of oesophageal cancer using gas
chromatography/mass spectrometry. J Chromatogr B Analyt Technol
Biomed Life Sci. 877:3111–3117. 2009. View Article : Google Scholar
|
|
15
|
Wu H, Xue R, Dong L, et al: Metabolomic
profiling of human urine in hepatocellular carcinoma patients using
gas chromatography/mass spectrometry. Anal Chim Acta. 648:98–104.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bogdanov M, Matson WR, Wang L, et al:
Metabolomic profiling to develop blood biomarkers for Parkinson’s
disease. Brain. 131:389–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goodacre R, Vaidyanathan S, Dunn WB,
Harrigan GG and Kell DB: Metabolomics by numbers: acquiring and
understanding global metabolite data. Trends Biotechnol.
22:245–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Claudino WM, Quattrone A, Biganzoli L, et
al: Metabolomics: available results, current research projects in
breast cancer, and future applications. J Clin Oncol. 25:2840–2846.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen F, Xue J, Zhou L, Wu S and Chen Z:
Identification of serum biomarkers of hepatocarcinoma through
liquid chromatography/mass spectrometry-based metabonomic method.
Anal Bioanal Chem. 401:1899–1904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
OuYang D, Xu J, Huang H and Chen Z:
Metabolomic profiling of serum from human pancreatic cancer
patients using 1 H NMR spectroscopy and principal component
analysis. Appl Biochem Biotechnol. 165:148–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu N, Wei D, Chen F and Yang ST: Lipidomic
profiling and discovery of lipid biomarkers in snow alga
Chlamydomonas nivalis under salt stress. Eur J Lipid Sci Technol.
114:253–265. 2012. View Article : Google Scholar
|
|
22
|
Yan XJ, Xu JL, Chen JJ, et al: Lipidomics
focusing on serum polar lipids reveals species dependent stress
resistance of fish under tropical storm. Metabolomics. 8:299–309.
2012. View Article : Google Scholar
|
|
23
|
Wiklund S, Johansson E, Sjöström L, et al:
Visualization of GC/TOF-MS based metabolomics data for
identification of biochemically interesting compounds using OPLS
class models. Anall Chem. 80:115–122. 2008. View Article : Google Scholar
|
|
24
|
Chen DY, Yan XJ, Xu JL, et al: Lipidomic
profiling and discovery of lipid biomarkers in Stephanodiscus sp
under cold stress. Metabolomics. 9:949–959. 2013. View Article : Google Scholar
|
|
25
|
Zhang X, Choi FF, Zhou Y, et al:
Metabolite profiling of plasma and urine from rats with
TNBS-induced acute colitis using UPLC-ESI-QTOF-MS-based
metabonomics - a pilot study. FEBS J. 279:2322–2338. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu YT, Jia HM, Chang X, et al: Metabolic
pathways involved in Xin-Ke-Shu protecting against myocardial
infarction in rats using ultra high-performance liquid
chromatography coupled with quadrupole time-of-flight mass
spectrometry. J Pharm Biomed Anal. 90:35–44. 2014. View Article : Google Scholar
|
|
27
|
Harewood GC, Baron TH, Stadheim LM, et al:
Prospective, blinded assessment of factors influencing the accuracy
of biliary cytology interpretation. Am J Gastroenterol.
99:1464–1469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malhi H and Gores GJ: Review article: the
modern diagnosis and therapy of cholangiocarcinoma. Aliment
Pharmacol Ther. 23:1287–1296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Bellis M, Fogel EL, Sherman S, et al:
Influence of stricture dilation and repeat brushing on the cancer
detection rate of brush cytology in the evaluation of malignant
biliary obstruction. Gastrointest Endosc. 58:176–182. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahmoudi N, Enns R, Amar J, et al: Biliary
brush cytology: factors associated with positive yields on biliary
brush cytology. World J Gastroenterol. 14:569–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dunn WB, Bailey NJ and Johnson HE:
Measuring the metabolome: current analytical technologies. Analyst.
130:606–625. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilson ID, Plumb R, Granger J, et al:
HPLC-MS-based methods for the study of metabonomics. J Chromatogr B
Analyt Technol Biomed Life Sci. 817:67–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen H, Yoo SS, Kang J, et al: A new
NMR-based metabolomics approach for the diagnosis of biliary tract
cancer. J Hepatol. 52:228–233. 2010. View Article : Google Scholar
|
|
34
|
Sharif AW, Williams HR, Lampejo T, et al:
Metabolic profiling of bile in cholangiocarcinoma using in vitro
magnetic resonance spectroscopy. HPB (Oxford). 12:396–402. 2010.
View Article : Google Scholar
|
|
35
|
AbdAlla MSH, Taylor-Robinson SD, Sharif
AW, et al: Differences in phosphatidylcholine and bile acids in
bile from Egyptian and UK patients with and without
cholangiocarcinoma. HPB (Oxford). 13:385–390. 2011. View Article : Google Scholar
|
|
36
|
Helmberger H, Hellerhoff K, Rüll T and
Rösch T: Chronic infections of the biliary system. Radiologe.
40:530–536. 2000.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Albiin N, Smith IC, Arnelo U, et al:
Detection of cholangiocarcinoma with magnetic resonance
spectroscopy of bile in patients with and without primary
sclerosing cholangitis. Acta Radiol. 49:855–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Billah M and Anthes J: The regulation and
cellular functions of phosphatidylcholine hydrolysis. Biochem J.
269:281–291. 1990.PubMed/NCBI
|
|
39
|
van Helvoort A, Smith A, Sprong H, et al:
MDR1 P-glycoprotein is a lipid translocase of broad specificity,
while MDR3 P-glycoprotein specifically translocates
phosphatidylcholine. Cell. 87:507–517. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barrios JM and Lichtenberger LM: Role of
biliary phosphatidylcholine in bile acid protection and NSAID
injury of the ileal mucosa in rats. Gastroenterology.
118:1179–1186. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Komichi D, Tazuma S, Nishioka T, et al:
Unique inhibition of bile salt-induced apoptosis by lecithins and
cytoprotective bile salts in immortalized mouse cholangiocytes. Dig
Dis Sci. 48:2315–2322. 2003. View Article : Google Scholar
|
|
42
|
Komichi D, Tazuma S, Nishioka T, et al:
Glycochenodeoxycholate plays a carcinogenic role in immortalized
mouse cholangiocytes via oxidative DNA damage. Free Radic Biol Med.
39:1418–1427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alpini G, Invernizzi P, Gaudio E, et al:
Serotonin metabolism is dysregulated in cholangiocarcinoma, which
has implications for tumor growth. Cancer Res. 68:9184–9193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang A, Sun H, Han Y, et al: Exploratory
urinary metabolic biomarkers and pathways using UPLC-Q-TOF-HDMS
coupled with pattern recognition approach. Analyst. 137:4200–4208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang S, Minkler P and Hoppel C:
cis-3,4-Methylene-heptanoylcarnitine: characterization and
verification of the C8:1 acylcarnitine in human urine. J Chromatogr
B Analyt Technol Biomed Life Sci. 857:251–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Trauner M, Fickert P and Wagner M: MDR3
(ABCB4) defects: a paradigm for the genetics of adult cholestatic
syndromes. Semin Liver Dis. 27:77–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mauad TH, van Nieuwkerk CM, Dingemans KP,
et al: Mice with homozygous disruption of the mdr2 P-glycoprotein
gene. A novel animal model for studies of nonsuppurative
inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol.
145:1237–1245. 1994.PubMed/NCBI
|
|
48
|
Saintigny Y, Dumay A, Lambert S and Lopez
B: A novel role for the Bcl-2 protein family: specific suppression
of the RAD51 recombination pathway. EMBO J. 20:2596–2607. 2001.
View Article : Google Scholar : PubMed/NCBI
|