Introduction
The primary function of the urinary bladder is to
store urine at low intravesical pressures and expel it periodically
via a coordinated well-maintained physiological contraction. As a
dynamic smooth muscle organ, the urinary bladder is continuously
subjected to mechanical stimuli, including hydrodynamic pressure
and stretch, which have been shown to be required for the growth
and development of the urinary bladder (1). However, a pathological mechanical
environment, resulting from bladder outlet obstruction (2), neurological disease (3) and bladder overactivity, may result in
a hyperplastic or hypertrophic response in the detrusor smooth
muscle and increased extracellular matrix production, followed by a
deleterious change in bladder function. A number of studies
(4–6) have demonstrated that cyclic stretch
induces cell proliferation, and several signaling pathways,
including the phosphoinositide 3-kinase (PI3K)/Akt (7), p38 (8), signal transducer and activator of
transcription 3 (9) and
extracellular signal-regulated kinase (ERK)1/2 (10) signaling pathways, have been
investigated as possible molecular mechanisms underlying the cell
proliferation induced by cyclic stretch. However, thus far, whether
the proliferation of human bladder smooth muscle cells (HBSMCs)
resulting from cyclic stretch is mediated by muscarinic (M)
receptors has, to the best of our knowledge, not been
demonstrated.
A previous study (11) demonstrated that exposure of HBSMCs
to sustained hydrostatic pressure may result in increased
expression levels of muscarinic (M) receptor M2 and M3 subtypes in
a time- and pressure-dependent manner. This result prompted the
evaluation of the effect of cyclic stretch on M receptor expression
levels in the present study. To investigate whether the
proliferative effect occurs via M receptors, experiments were
performed at the degree of stretch that yielded maximally increased
expression levels of M receptors and this stretch was used in all
subsequent experiments. Acetylcholine has been demonstrated to
exert a mitogenic effect on numerous cell types, although studies
have focused on neuronal (12–14)
and tumor cell types (15–17). Studies analyzing the mitogenic
effect of acetylcholine on HBSMCs are limited (18). In the present study, the
proliferative effect of acetylcholine and/or cyclic stretch on
HBSMCs was assessed, along with whether the proliferative effect is
mediated by muscarinic signaling pathways. The cell cycle
distribution was also specifically quantified, and the effect of M
receptor and protein kinase C (PKC) antagonists on HBSMC
proliferation was evaluated following cell exposure to cyclic
stretch, in order to identify the possible underlying mechanisms
involved.
The present study, by evaluating the role of the M
signaling pathway on HBSMC proliferation induced by cyclic stretch,
may enrich understanding of the possible molecular mechanisms of
signaling pathways for cell proliferation resulting from cyclic
stretch, together with the cellular and molecular changes that
affect the bladder wall, and provide novel targets for specific
medications or therapies of a number of urinary bladder
diseases.
Materials and methods
HBSMC culture
HBSMCs (catalog no. 4310; ScienCell, Carlsbad, CA,
USA) were expanded in culture using low-glucose Dulbecco’s modified
Eagle’s medium (DMEM; HyClone; GE Healthcare, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS, HyClone; Thermo
Fisher Scientific), penicillin (100 U/ml) and streptomycin (100
μg/ml). These cells were plated in 75-cm2 culture flasks
and cultured at 37°C, in a humidified atmosphere of 5%
CO2/95% air. The medium in the flasks was changed every
48 h and the cells were passaged every 2–3 days. All experiments
were performed on cells between passages 3 and 7.
In vitro cyclic stretch
The HBSMCs were seeded onto silicone membranes for
24 h prior to transferal to a bioreactor (BioDynamic; Bose
Corporation, Framingham, CA, USA) chamber. The treated silicone
membrane was subjected to 0.1 Hz cyclic stretch with a 1:1
stretch/relaxation ratio sine wave stretch pattern. A control
silicone membrane was placed in the same chamber without stretch.
Initially, the effects of cyclic stretch were examined at 5, 10, 15
and 20% stretch for 6 and 12 h, respectively. The effect of cyclic
stretch on M2 and M3 mRNA expression levels, which were
subsequently determined by reverse transcription polymerase chain
reaction (RT-PCR), was maximal at 10% stretch for 6 h. Therefore,
all subsequent experiments were performed at this degree of stretch
for 6 h, to investigate whether cyclic stretch causes proliferation
of HBSMCs via M receptors.
RNA isolation and RT-PCR
Total RNA was isolated from control and treated
HBSMCs using TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. The extracted
RNA was dissolved in nuclease-free water and then quantified by
measuring the absorbance at 260 nm using a Nanodrop 2000
spectrophotometer (Thermo Fisher Scientific). cDNA was then
synthesized using an iScript cDNA Synthesis kit (Bio-Rad, Hercules,
CA, USA) according to the manufacturer’s instructions. M2 and M3
mRNA expression levels were quantified by RT-PCR analysis with
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal
standard. RT-PCR was performed using SYBR Premix EX Taq premix
reagent [Takara Biotechnology (Dalian) Co., Ltd., Dalian, China]
and a Bio-Rad iQ5 detection system (Bio-Rad). The reactions were
performed under the following conditions: 94°C for 3 min, 40 cycles
at 94°C for 5 sec, 54°C for 30 sec and 72°C for 20 sec. PCR product
quality was monitored using post-PCR melt curve analysis. The
sequences of primers used in RT-PCR were as follows: GAPDH sense,
GCTTCGCTCTCTGCTCCT; GAPDH antisense, CGCCCAATACGACCAAAT; M2 sense,
AGCAAACATGCATCAGAATTGG; M2 antisense, GTGCACAAAAGGTGTTAATGAG; M3
sense, ACCCAGCTCCGAGCAGATGGAC; and M3 antisense,
CGGCTGACTCTAGCTGGATGG.
Flow cytometric analysis of the cell
cycle profile
Once the stretch procedure was complete, the control
and treated HBSMCs were harvested. The cells were washed in cold
phosphate-buffered saline (PBS) twice and then fixed in 70% ethanol
overnight at 4°C. Following centrifugation at 25°C and 241 × g for
3 min, the cells were washed with cold PBS, and gently resuspended
in 500 μl PBS containing 100 μg/ml RNaseA and 50 μg/ml propidium
iodide for 30 min in the dark. The cells were then diluted with PBS
and flow cytometry was performed using a Cytomics FC500 flow
cytometer (Beckman Coulter, Miami, FL, USA). All samples were
assayed in triplicate and the cell apoptotic rate was calculated as
follows: Apoptotic rate (%) = (apoptotic cell number/total cell
number) × 100. In addition, cell proliferation was calculated as
follows: Proliferation index (%) = (S+G2/M)/(G0/G1+S+G2/M) ×
100.
5-Bromo-2-deoxyuridine (BrdU)
incorporation assay
HBSMCs from each group were harvested and then
suspended at a concentration of 4×105 cells/ml with
DMEM. Cell suspensions were transferred to 96-well plates (200
μl/well), 10 μM 5-bromo-2-deoxyuridine labeling solution (BrdU Cell
Proliferation ELISA kit; Roche Applied Science, Penzberg, Germany)
was added to each well and the incubation was continued for 16 h.
The cells were fixed and exposed to anti-BrdU (1:100) antibody and
substrate solutions. Proliferation was quantified by measuring the
absorbance value at 450 nm wavelength using an ELISA plate reader
(Model 680; Bio-Rad). For the acetylcholine experiments, various
concentrations of acetylcholine (10 nM-100 μM) were added following
harvesting of the HBSMCs from each group. For the M and PKC
receptor antagonist experiments, 1 μM of the M antagonists 4-DAMP
(sc-200167) for the M3 receptor antagonist, AF-DX116 (sc-223772)
for the M2 receptor antagonist and atropine for the non-selective
antagonist (All from Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) and 5 μM PKC antagonist (GF 109203X; sc-24003; Santa Cruz
Biotechnology, Inc.) were added for 1 h prior to the HBSMCs being
subjected to cyclic stretch.
Western blot analysis
The M2, M3, PKC and phosphorylated (p)-PKC protein
expression levels in HBSMCs were detected by western blot analysis
using GAPDH as an internal standard. For antigen retrieval, total
cellular protein was extracted from control and treated cells using
cell lysis buffer containing 50 mm Tris-HCl (pH 8.0), 150 mm NaCl,
100 μg/ml phenylmethanesulfonylfluoride and 1% Triton X-100.
Following removal of cell debris by centrifugation at 12,000 × g
for 5 min, 50 μg of each lysate sample was boiled for 5 min in
sample buffer, separated by 10% SDS-PAGE and transferred to a
nitrocellulose membrane (Pall Corporation, Port Washington, NY,
USA). Nonspecific reactivity was blocked by incubating the membrane
in 5% non-fat dry milk in TBST containing 10 mm Tris-HCl, pH 7.5,
150 mm NaCl and 0.05% Tween-20 for 1 h at room temperature. The
membrane was then incubated with specific primary antibodies at 4°C
overnight followed by secondary anti-rabbit IgG (Jackson
Immunoresearch Inc., West Grove, PA, USA) for 1 h. Reactive protein
was detected using an enhanced chemiluminescence system (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The antibodies used for
western blotting were anti-GAPDH, polyclonal, rabbit anti-human M2
(ab123421), polyclonal mouse anti-human M3 (ab167566) and
polyclonal rabbit anti-human PKC (ab69531) antibodies and
polyclonal rabbit anti-human p-PKC antibodies (ab195769). All
antibodies were at a diluteion of 1:1,000 and were purchased from
Abcam (Cambridge, MA, USA).
Statistical analysis
In the BrdU incorporation assays, triplicate culture
wells were used and each experiment was repeated at least three
times. All statistical tests were conducted using SPSS software,
version 11.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance tests and paired t-test were used to determine significant
differences (P<0.05) between experimental samples and controls.
Data are expressed as the mean ± standard deviation.
Results
Expression levels of M receptor subtypes
following cyclic stretch in vitro
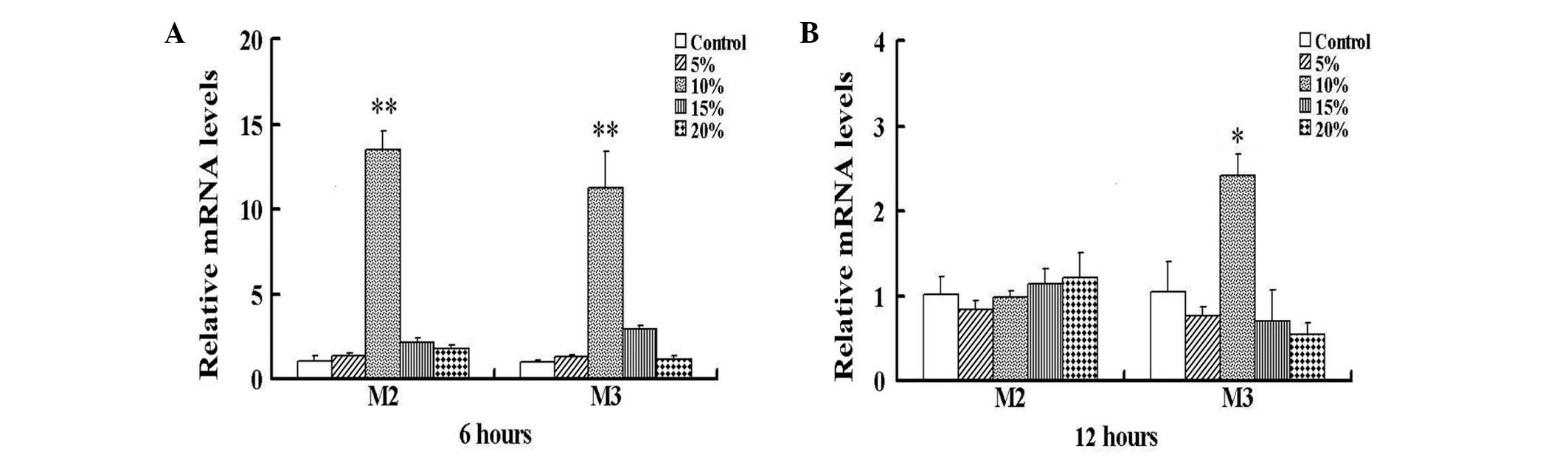
The exposure of HBSMCs to 6 h cyclic stretch in
vitro resulted in significantly increased expression levels of
M2 and M3 mRNA at 10% stretch, compared with those of the control
nonstretched HBSMCs (P<0.01; Fig.
1A). No significant differences between nonstretched and
stretched cells were identified below or over 10% stretch.
Following exposure to 12 h cyclic stretch, M2 mRNA expression in
the HBSMCs declined to the initial levels; however the M3 mRNA
expression levels remained significantly increased compared with
those of the control cells (P<0.05; Fig. 1B). As the effect of cyclic stretch
on the expression levels of M2 and M3 mRNA was maximal at 6 h 10%
stretch, all subsequent experiments were performed at this stretch.
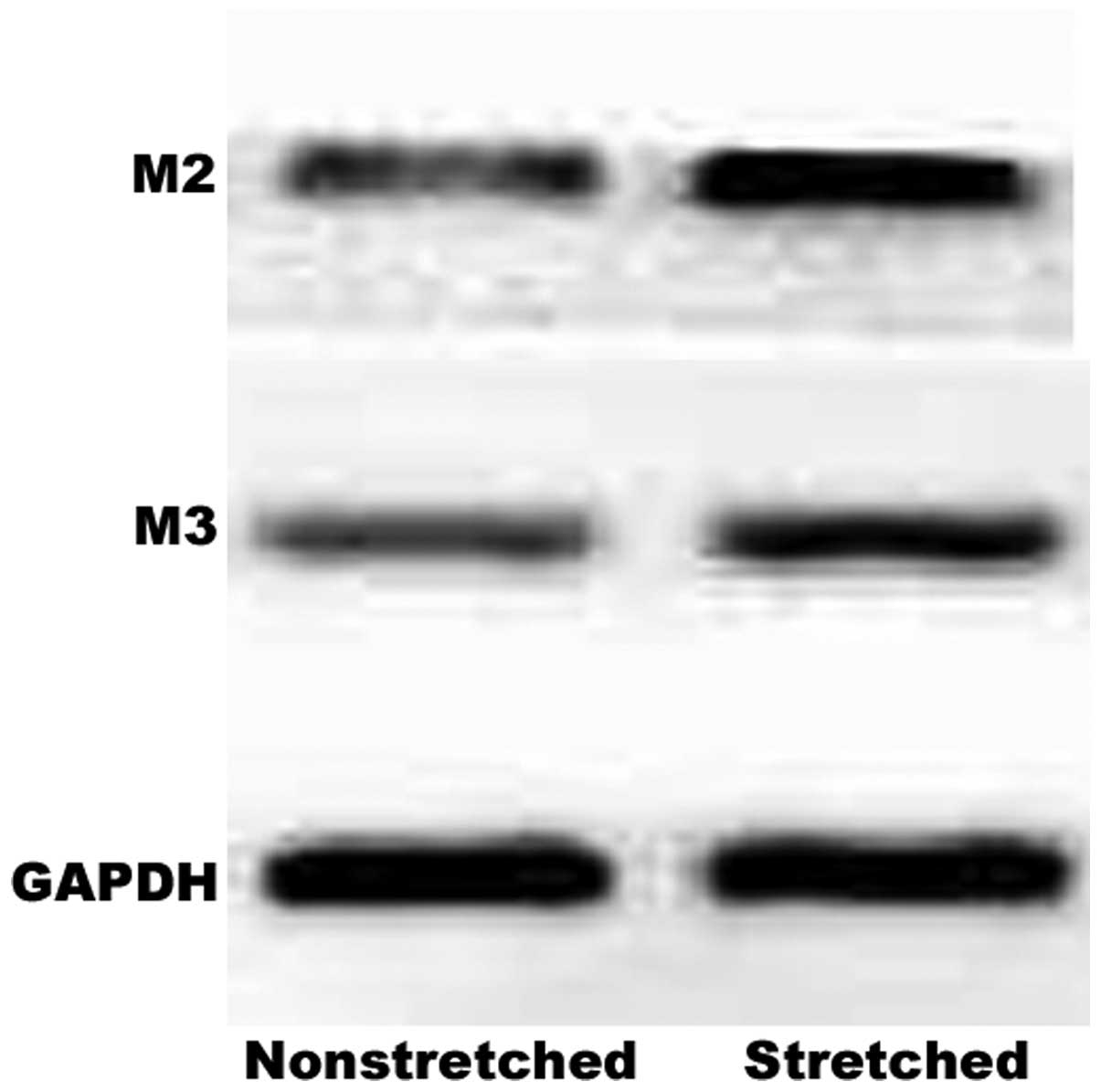
Western blot analysis also demonstrated a marked increase in M2 and
M3 receptor protein expression levels in response to this stretch
(Fig. 2).
Effect of cyclic stretch on cell cycle
and cell proliferation
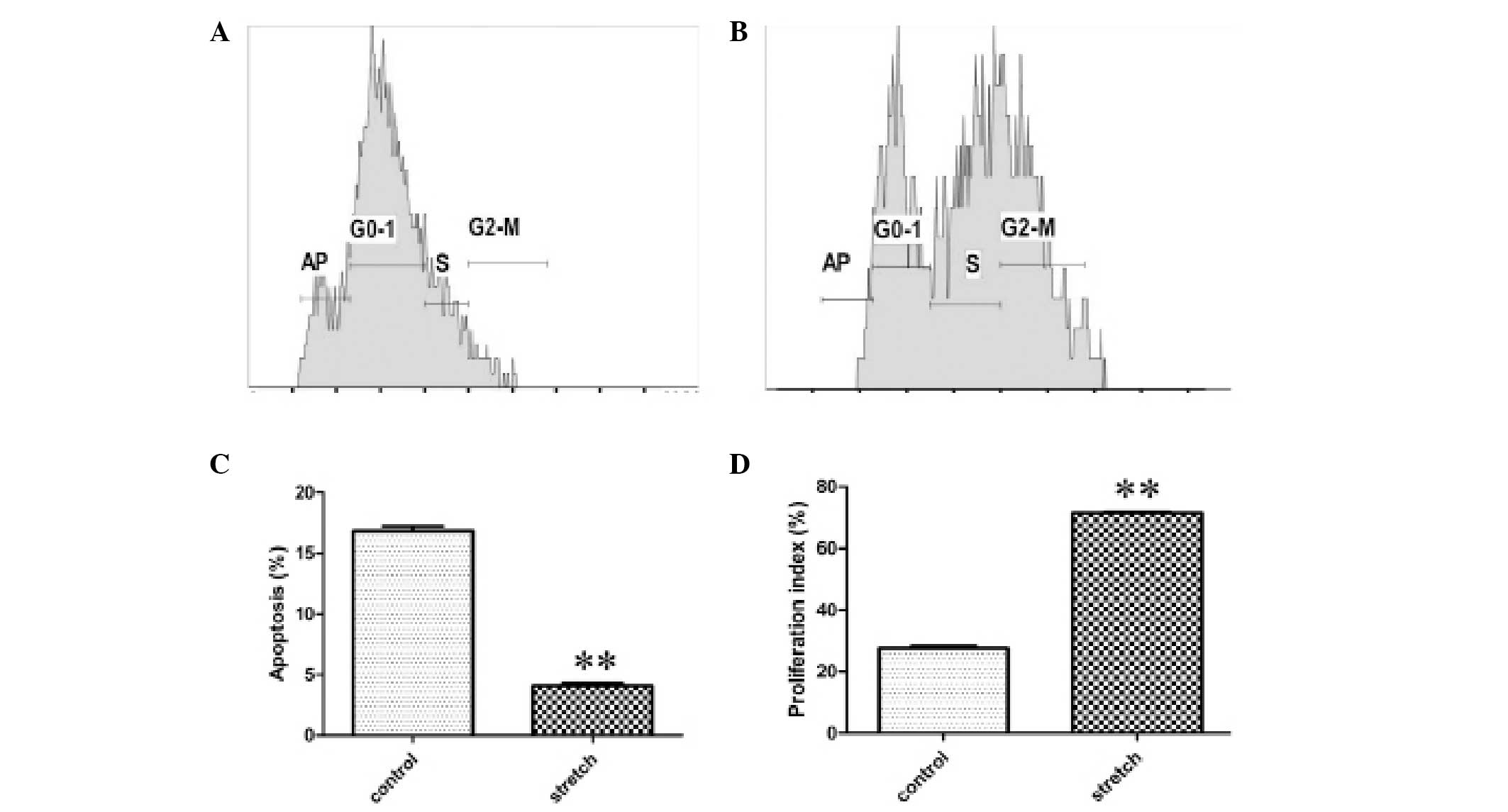
Compared with the control group, the numbers of S
and G2/M phase HBSMCs were increased in the cyclic stretch group,
as shown by representative samples of the flow cytometric data in
Fig. 3A and B. HBSMC apoptosis was
inhibited. The rate of apoptosis was reduced from 16.8±0.42% in the
control to 4.1±0.22% following cyclic stretch (P<0.01; Fig. 3C), while the cell proliferation
index was increased from 27.6±0.76% in the control to 71.5±0.31%
following cyclic stretch (P<0.01; Fig. 3D). In conclusion, the data suggest
that the exposure of HBSMCs to cyclic stretch inhibits apoptosis
and stimulates proliferation.
Effect of acetylcholine and/or cyclic
stretch on BrdU incorporation in HBSMCs
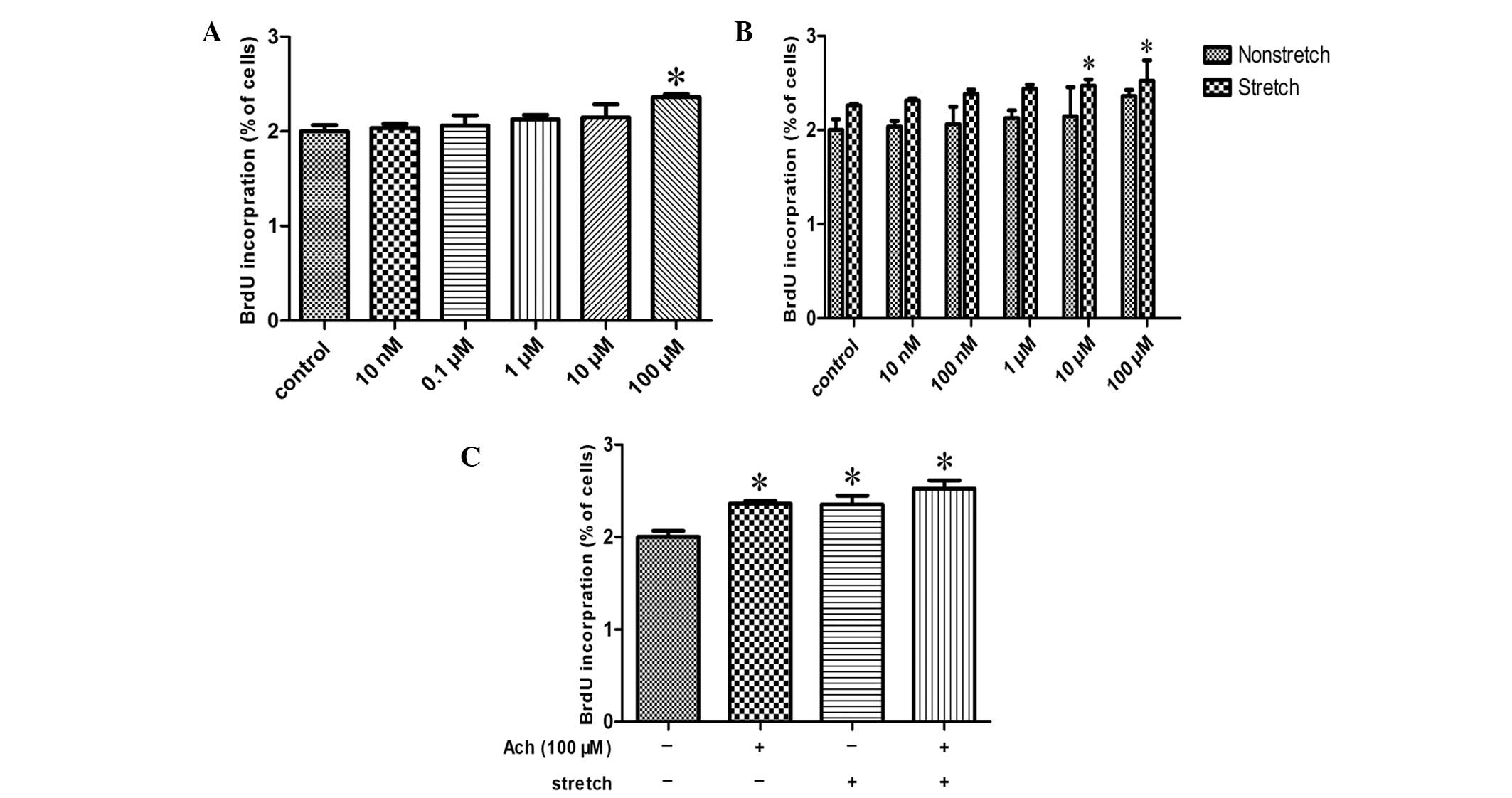
Exposure of HBSMCs to different concentrations of
acetylcholine, ranging between 10 nM and 100 μM, resulted in
increased cell proliferation, as measured by BrdU incorporation,
compared with the control in a concentration-dependent manner,
reaching significance at 100 μM (P<0.05; Fig. 4A). In addition, when 10 or 100 μM
acetylcholine was administered to stretched HBSMCs, statistically
significant increases in BrdU incorporation were detected compared
with nonstretched HBSMCs treated with the same respective
acetylcholine doses (P<0.05; Fig.
4B). Exposure of HBSMCs to 100 μM acetylcholine and/or cyclic
stretch induced significant increases in cell proliferation
compared with the control (P<0.05; Fig. 4C). BrdU incorporation following
exposure to 100 μM acetylcholine, cyclic stretch, or 100 μM
acetylcholine and cyclic stretch was increased by 17.4, 17.6 and
26.1%, respectively.
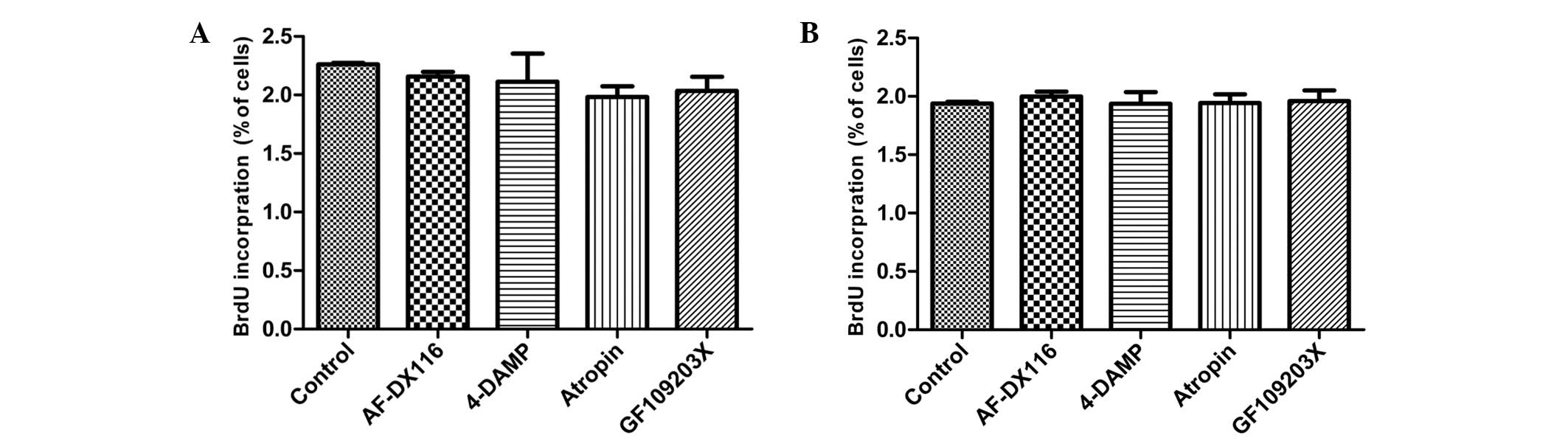
Effect of muscarinic and PKC antagonists
on HBSMCs
In order to investigate whether M receptor signaling
pathways are involved in a possible mechanosensitive mechanism for
the cell proliferation induced by cyclic stretch, 1 μM M receptor
antagonist [AF-DX16, M2 receptor antagonist;
1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP), M3
receptor antagonist; and atropine, non-selective antagonist) was
added to compare the effects of M receptor antagonists on stretched
and nonstretched HBSMCs. Stretched cells exhibited a reduction in
BrdU incorporation when exposed to M antagonists, although these
reductions failed to reach statistic significance (Fig. 5A). By contrast, M receptor
antagonists exerted no clear effect on nonstretched HBSMCs
(Fig. 5B).
As the results of the experiments involving M2 and
M3 receptor antagonists suggest that the cell proliferation induced
by cyclic stretch is caused primarily by activation of the M3
receptor, the effect of PKC, a predominant downstream effector of
M3 receptor signaling pathways, on the proliferation of HBSMCs was
analyzed. To determine the involvement of PKC, 5 μM PKC antagonist
(GF 109203X) was administered to nonstretched and stretched HBSMCs.
As shown in Fig. 5A, BrdU
incorporation following the exposure of stretched HBSMCs to GF
109203X was reduced compared with that in the control cells,
although this reduction failed to reach statistical significance.
Nonstretched cells exhibited no change in BrdU incorporation in the
presence of GF 109203X (Fig. 5B).
Following exposure to AF-DX16, 4-DAMP, atropine and GF 109203X,
BrdU incorporation was reduced by 8.4, 10.2, 15.8 and 13.6%,
respectively, in stretched cells.
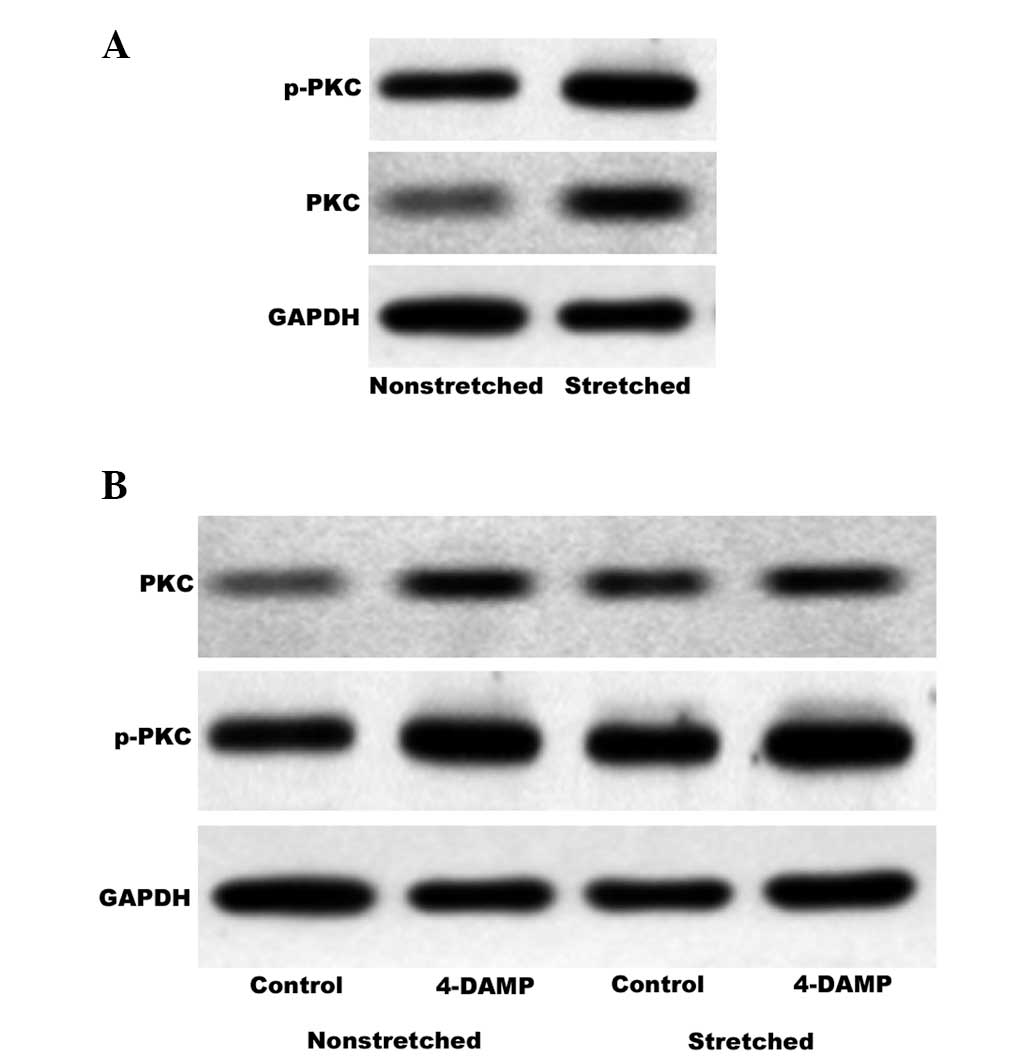
Involvement of stretch-activated PKC in
the M3 receptor signaling pathway
PKC is an important downstream effector of the M3
receptor signaling pathway and p-PKC is the activated form. PKC is
physiologically activated by diacylglycerol (DAG) in a process that
occurs subsequent to activation of the prototypical M3 receptor
signaling pathway. In bladder smooth muscle, this may contribute to
contraction and proliferation. The BrdU assay indicated that GF
109203X reduces stretch-induced HBSMC proliferation. In order to
investigate whether activation of PKC via the M3 receptor signaling
pathway is involved in the proliferative effect of cyclic stretch,
western blot analysis was used to quantify PKC and p-PKC expression
levels in nonstretched and stretched HBSMCs. During stretching, the
cells were treated in the presence or absence of 4-DAMP. As shown
in Fig. 6A, cyclic stretch
enhanced the expression and activation of PKC, Notably, the
observed increases in PKC and p-PKC expression levels induced by
stretching were not inhibited by 4-DAMP (Fig. 6B).
Discussion
Abnormal function of the urinary bladder, resulting
from outlet obstruction or neurogenic bladder, may result in high
intravesical pressure and constant abnormal stretch of the bladder
wall. The constant abnormal stretch, marked by profound molecular
and cellular level changes, may alter the mechanical and functional
properties of the bladder (19).
To achieve an improved understanding of the underlying cellular and
molecular mechanisms, the role of the muscarinic signaling pathway
on HBSMC proliferation induced by cyclic stretch was investigated.
In order to find out the most effective cyclic stretch parameters,
the impact of cyclic stretch on M2 and M3 mRNA expression levels
was examined at 0, 5, 10, 15 and 20% stretch for 6 and 12 h,
respectively. Previous studies have reported that M2 and M3
receptor density may be upregulated or downregulated in response to
an abnormal mechanical environment (unstable bladder) generated by
outlet obstruction or neurogenic bladder in different in
vivo and ex vivo models (20–22).
However, these studies simply examined M2 and M3 receptor density
at a single degree of stretch. The present study revealed dynamic
changes in M2 and M3 mRNA expression levels in response to
different cyclic stretches, and that this expression was
upregulated to the maximum extent at 10% stretch for 6 h. These
results support the hypothesis that the expression levels of M2 and
M3 receptor subtypes may signify a dynamic process in the
development of an unstable bladder, which also may be a possible
reason for the differences in the results of previous reports.
Proliferation and apoptosis are considered to be
opposing cellular processes that mediate the response of the
bladder to short-term obstructive stimuli (23). The present study, using HBSMCs
in vitro, demonstrated that mechanical deformation regulates
these two cellular processes. A significant increase in the
proliferation of HBSMCs was accompanied by a significant reduction
in the rate of apoptosis in response to cyclic stretch. These
findings are consistent with those of Galvin et al (4), who found that mechanical stretch at
12.3% significantly reduced HBSMC apoptosis in vitro as
early as 6 h after an apoptotic peak at 3 h, which was continued
following a 48-h stretch. The authors suggested that, in contrast
to the low rates of apoptosis in vivo, the high basal rate
of spontaneous apoptosis of HBSMCs in vitro produced
opposite results from those of in vivo models. Similar
results were obtained by Estrada et al (24) in an ex vivo model bladder
smooth muscle cell system, indicating that stretch may be an
antiapoptotic stimulus in bladder smooth muscle cells.
Acetylcholine has been extensively investigated with
regard to its role as the primary neurotransmitter responsible for
bladder contraction. In addition, a number of studies have
demonstrated the mitogenic effect of acetylcholine on numerous cell
types (25–27). However, to the best of our
knowledge, no study thus far has demonstrated the mitogenic effect
of acetylcholine on stretched HBSMCs in vitro. The results
from the present study show that acetylcholine exerted a mitogenic
effect on HBSMCs in a concentration-dependent manner. Following 10
or 100 μM acetylcholine treatment, the cells that were exposed to
cyclic stretch exhibited statistically significant increases in
cell proliferation compared with nonstretched HBSMCs at these
acetylcholine concentrations. Lee et al (18) observed similar results following 1
μM acetylcholine treatment. The possible reasons for this
discrepancy include different experimental methods and conditions,
and different time periods of exposure to acetylcholine. The
finding that acetylcholine and stretching exerted a greater
mitogenic effect than stretch treatment alone suggests that cyclic
stretch induces a mitogenic acetylcholinergic effect on HBSMCs.
Possible reasons for this effect include the upregulation of M2 and
M3 receptor expression and an increased number of activated M
receptor targets. Thus, the proliferative effect on HBSMCs of
acetylcholine and/or cyclic stretch may be through the activation
of M receptors.
With regard to the effect of M antagonists on BrdU
incorporation in stretched and nonstretched HBSMCs, the stretched
cells exposed to M receptor antagonists demonstrated a reduction in
BrdU incorporation compared with a negative control, while
nonstretched cells exhibited no evident changes among these groups.
These results support the hypothesis that the proliferative effect
on stretched cells was due to the activation of M receptors. As the
relative BrdU incorporation activity was reduced to the greatest
extent by 4-DAMP and atropine, stretch-induced HBSMC proliferation
was considered to be caused primarily by activation of the M3
receptor, although the contribution of the M2 receptor cannot be
ruled out. PKC is a major downstream signaling kinase of the
prototypical M3 receptor signaling pathway. BrdU incorporation in
the presence of PKC antagonist was reduced; furthermore, western
blotting results revealed that cyclic stretch significantly
upregulated PKC and p-PKC expression, demonstrating the possible
involvement of PKC in stretch-stimulated cell proliferation.
However, PKC activation was shown, through western blot analysis,
to occur in response to stretch in the presence of 4-DAMP,
indicating that PKC activation following stretching is independent
of the M3 receptor signaling pathways. This finding is notable for
two reasons. The apparent independence of the M3 receptor signaling
pathway in stretch-induced PKC activation appears inconsistent with
the prototypical M3 receptor signaling pathway. This finding also
contrasts with a previous study, which observed that PKC activation
via the M3 receptor signaling pathway induced cell proliferation in
other types of cells (12).
Another study suggested that stimulation of the M3 receptors
activates adenylate cyclase, phospholipase A2, inositol
triphosphate and DAG. Each of these molecules in turn activates
different signaling pathways (28). The PI3K and ERK intracellular
signaling pathways have also been demonstrated to regulate, at
least partially, M receptor-mediated cell proliferation (29). Combined with the results of the
present study, this suggests that cyclic stretch induced HBSMC
proliferation via the M3 receptor signaling pathway through the
activation of downstream signaling pathways other than the PKC
signaling pathway. In conclusion, these findings indicate that
stretch-stimulated HBSMC proliferation occurred via multiple
independent signaling pathways and that the M3 receptor signaling
pathway was involved in this process by PKC-independent
mechanisms.
In conclusion, cyclic stretch was demonstrated to
induce HBSMC proliferation mediated by the M receptor. Furthermore,
the signal transduction mechanism for this process primary involves
the M3 receptor signaling pathway in a PKC-independent manner.
These data suggest that the M receptor is more deeply involved in
the pathological processes of bladder outlet obstruction and
neurogenic bladder than has been previously considered. Therefore,
M receptor antagonists should not only be considered for treating
the stretch-injured bladder but also for stopping or reversing the
cellular changes that affect the bladder wall. In addition, a
highly selective M3 antagonist may be more effective than a M2
antagonist. As the PKC antagonist reduced stretch-induced HBSMC
proliferation, its molecular target may be of significance in the
treatment of such patients. The potential downstream signaling
pathways of M3 receptor activation involved in stretch-induced cell
proliferation require identification in further studies.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30872593 and 31170907), the
Programs Foundation of Ministry of Education of China (grant no.
20070610156) and the Technology Support Programs Foundation of
Science and Technology Department of Sichuan Province, China (grant
no. 2010SZ0163).
References
|
1
|
Haberstroh KM, Kaefer M, Retik AB, Freeman
MR and Bizios R: The effects of sustained hydrostatic pressure on
select bladder smooth muscle cell functions. J Urol. 162:2114–2118.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knutson T, Edlund C, Fall M and Dahlstrand
C: BPH with coexisting overactive bladder dysfunction - an everyday
urological dilemma. Neurourol Urodyn. 20:237–247. 2001. View Article : Google Scholar
|
|
3
|
Abdel-Azim M, Sullivan M and Yalla SV:
Disorders of bladder function in spinal cord disease. Neurol Clin.
9:727–740. 1991.PubMed/NCBI
|
|
4
|
Galvin DJ, Watson RW, Gillespie JI, Brady
H and Fitzpatrick JM: Mechanical stretch regulates cell survival in
human bladder smooth muscle cells in vitro. Am J Physiol Renal
Physiol. 283:F1192–F1199. 2002.PubMed/NCBI
|
|
5
|
Orsola A, Adam RM, Peters CA and Freeman
MR: The decision to undergo DNA or protein synthesis is determined
by the degree of mechanical deformation in human bladder muscle
cells. Urology. 59:779–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adam RM, Eaton SH, Estrada C, et al:
Mechanical stretch is a highly selective regulator of gene
expression in human bladder smooth muscle cells. Physiol Genomics.
20:36–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adam RM, Roth JA, Cheng HL, et al:
Signaling through PI3K/Akt mediates stretch and PDGF-BB-dependent
DNA synthesis in bladder smooth muscle cells. J Urol.
169:2388–2393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen HT, Adam RM, Bride SH, et al:
Cyclic stretch activates p38 SAPK2-, ErbB2-, and AT1-dependent
signaling in bladder smooth muscle cells. Am J Physiol Cell
Physiol. 279:C1155–C1167. 2000.PubMed/NCBI
|
|
9
|
Halachmi S, Aitken KJ, Szybowska M, et al:
Role of signal transducer and activator of transcription 3 (STAT3)
in stretch injury to bladder smooth muscle cells. Cell Tissue Res.
326:149–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aitken KJ, Block G, Lorenzo A, et al:
Mechanotransduction of extracellular signal-regulated kinases 1 and
2 mitogen-activated protein kinase activity in smooth muscle is
dependent on the extracellular matrix and regulated by matrix
metalloproteinases. Am J Pathol. 169:459–470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SD, Misseri R, Akbal C, et al:
Muscarinic receptor expression increases following exposure to
intravesical pressures of < or =40 cm-H2O: a possible
mechanism for pressure-induced cell proliferation. World J Urol.
26:387–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guizzetti M, Costa P, Peters J and Costa
LG: Acetylcholine as a mitogen: muscarinic receptor-mediated
proliferation of rat astrocytes and human astrocytoma cells. Eur J
Pharmacol. 297:265–273. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma W, Maric D, Li BS, et al: Acetylcholine
stimulates cortical precursor cell proliferation in vitro via
muscarinic receptor activation and MAP kinase phosphorylation. Eur
J Neurosci. 12:1227–1240. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashkenazi A, Ramachandran J and Capon DJ:
Acetylcholine analogue stimulates DNA synthesis in brain-derived
cells via specific muscarinic receptor subtypes. Nature.
340:146–150. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Resende RR, Alves AS, Britto LR and Ulrich
H: Role of acetylcholine receptors in proliferation and
differentiation of P19 embryonal carcinoma cells. Exp Cell Res.
314:1429–1443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Español AJ and Sales ME: Different
muscarinic receptors are involved in the proliferation of murine
mammary adenocarcinoma cell lines. Int J Mol Med. 13:311–317.
2004.
|
|
17
|
Jiménez E and Montiel M: Activation of MAP
kinase by muscarinic cholinergic receptors induces cell
proliferation and protein synthesis in human breast cancer cells. J
Cell Physiol. 204:678–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SD, Akbal C, Jung C and Kaefer M:
Intravesical pressure induces hyperplasia and hypertrophy of human
bladder smooth muscle cells mediated by muscarinic receptors. J
Pediatr Urol. 2:271–276. 2006. View Article : Google Scholar
|
|
19
|
Halachmi S: The molecular pathways behind
bladder stretch injury. J Pediatr Urol. 5:13–16. 2009. View Article : Google Scholar
|
|
20
|
Braverman AS, Luthin GR and Ruggieri MR:
M2 muscarinic receptor contributes to contraction of the denervated
rat urinary bladder. Am J Physiol. 275:R1654–R1660. 1998.PubMed/NCBI
|
|
21
|
Tong YC, Chin WT and Cheng JT: Alterations
in urinary bladder M2 muscarinic receptor protein and mRNA in
2-week steptozotocin-induced diabetic rats. Neurosci Lett.
277:173–176. 1999. View Article : Google Scholar
|
|
22
|
Tong YC and Cheng JT: Alteration of M(3)
subtype muscarinic receptors in the diabetic rat urinary bladder.
Pharmacology. 64:148–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santarosa R, Colombel MC, Kaplan S, et al:
Hyperplasia and apoptosis. Opposing cellular processes that
regulate the response of the rabbit bladder to transient outlet
obstruction. Lab Invest. 70:503–510. 1994.PubMed/NCBI
|
|
24
|
Estrada CR, Adam RM, Eaton SH, Bägli DJ
and Freeman MR: Inhibition of EGFR signaling abrogates smooth
muscle proliferation resulting from sustained distension of the
urinary bladder. Lab Invest. 86:1293–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cohen RI, Molina-Holgado E and Almazan G:
Carbachol stimulates c-fos expression and proliferation in
oligodendrocyte progenitors. Brain Mol Brain Res. 43:193–201. 1996.
View Article : Google Scholar
|
|
26
|
Fucile S, Napolitano M and Mattei E:
Cholinergic stimulation of human microcytoma cell line H69. Biochem
Biophys Res Commun. 230:501–504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rayford W, Noble MJ, Austenfeld MA, et al:
Muscarinic cholinergic receptors promote growth of human prostate
cancer cells. Prostate. 30:160–166. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Resende RR and Adhikari A: Cholinergic
receptor pathways involved in apoptosis, cell proliferation and
neuronal differentiation. Cell Commun Signal. 7:202009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arrighi N, Bodei S, Zani D, et al:
Different muscarinic receptor subtypes modulate proliferation of
primary human detrusor smooth muscle cells via Akt/PI3K and MAP
kinases. Pharmacol Res. 74:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|