Introduction
Pancreatic cancer is a highly aggressive and
devastating disease, which is fatal in 95% of patients within six
months of diagnosis (1). Previous
studies have made a degree of progress in utilizing improved
diagnostic methods and developing novel targeted therapies,
however, despite this the overall survival rate has not increased
in >10 years (2). As such,
pancreatic cancer remains the fourth highest cause of
cancer-related mortalities in females and males (3). Therefore, it is necessary to
investigate the underlying molecular mechanisms involved in the
progression of pancreatic cancer, in order to develop novel
therapeutic strategies to treat it.
It has been established that the development of
pancreatic cancer is associated with a number of marked metabolic
changes, including an increase in glucose consumption and lactate
production, even in an oxygen rich environment. Warburg determined
that this phenotype occurred in response to local hypoxia (4); however, it was revealed that
persistent or cyclical hypoxia caused certain selection pressures,
which leads to the upregulation of glycolysis even when oxygen is
present, a phenomenon that is known as aerobic glycolysis (5). In this process, pyruvate kinase
limits the rate of the glycolytic pathway, catalyzing the transfer
of a high-energy phosphate group from phosphoenolpyruvate to
generate pyruvate and ATP (6).
This metabolic pathway drives the cellular growth and survival.
While the Warburg effect has been studied thoroughly, novel
mechanisms are constantly being found by researchers. As the
rate-limiting enzyme in glycolysis, pyruvate kinase M2 (PKM2), has
only been found to be present in embryonic, proliferating, and
tumor cells, and it has been determined to be critical for the
metabolism and growth of tumor cells (7).
Sun et al (7) determined that the
PI3K/AKT/mechanistic target of rapamycin (mTOR) signaling pathway
was a major positive regulator of aerobic glycolysis, and that the
receptor tyrosine kinase/PI3K/AKT/mTOR (RTK/PI3K/AKT/mTOR)
signaling pathway had an important role in the regulation of cell
metabolism, growth and survival (8,9). It
has been determined that mTOR upregulates the expression of PKM2
via the hypoxia-inducible factor 1α (HIF1α)-mediated activation of
transcription and the c-Myc-heterogeneous nuclear ribonucleoprotein
(hnRNP)-dependent regulation of PKM2 gene splicing. Therefore, PKM2
was determined to be downstream of HIF1α and c-Myc-hnRNPs of mTOR
signaling, and the mTOR/HIF1α/Myc-hnRNPs/PKM2 signaling cascade was
revealed to have an essential role in tumorigenesis (7). In summary, PKM2 has an important role
in the development of tumors.
In addition, pancreatic cancer is characterized by
the constitutive activation of the mitogen-activated protein
kinases (MAPKs). The activation of MAPKs upregulates certain genes
that are implicated in the proliferation and survival of pancreatic
cancer cells (10). Consequently,
the activation of the Ras/MAPK signaling pathway is another
aberrantly activated pathway in tumor cells. There are three
predominant distinct MAPK pathways that have been established,
including the extracellular signal-regulated kinases (ERK 1/2 or
p44/p42), the c-Jun N-terminal kinases (JNKs or stress activated
protein kinases), and the CSBP/RK/Mpk2 (or p38) kinase (11). Wang et al (12) have previously reported that
inactivation of the tumor suppressor gene Spry2 accelerated
AKT-induced hepatocarcinogenesis via activation of the MAPK and
PKM2 pathways (12). However,
there was little data to confirm that PKM2 was associated with the
MAPK signaling pathway in this study. We hypothesized that an
association between the MAPK and PKM2 pathways existed in the
proliferation, migration, invasion and anti-apoptosis of pancreatic
cancer cells.
The aim of the present study, was to verify whether
PKM2 is associated with MAPK in the induction of the progression of
pancreatic cancer. By using siRNA to downregulate the expression
levels of PKM2, the subsequent proliferation, invasion, migration
and apoptosis capabilities of Panc-1 and Sw1990 cells may be
determined. Additionally, the protein expression levels in three
major MAPK pathways were investigated following PKM2 knockdown of
the two cell lines. Hence, this study aims to investigate the
potential association between the expression level of PKM2 and the
three predominant MAPK pathways to uncover the possible underlying
mechanism of the malignant progression of pancreatic cancer
cells.
Materials and methods
Pancreatic cancer cell specimens
Tumor specimens and the paired normal pancreatic
ductal tissue specimens taken from a site distant from the
cancerous lesion were obtained from five patients. All of the
patients provided written informed consent. This study was approved
by the Medical Ethics Committee of Yixing People’s Hospital
(Yixing, China). None of the patients received radiotherapy or
chemotherapy prior to surgery.
Cell culture
Panc-1 and Sw1990 human pancreatic cancer cells
(American Type Culture Collection, Manassas, VA, USA) were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone
Laboratories, Inc., Logan, UT, USA) or L15 (HyClone Laboratories,
Inc.) supplemented with 10% fetal bovine serum (FBS, Hangzhou
Sijiqing Biological Engineering Materials Co. Ltd., Hangzhou,
China), 100 u/ml penicillin and 100 mg/l streptomycin (Beyotime
Institute of Biotechnology, Haimen, China). The cells were cultured
in a humidified incubator containing 5% CO2 at 37°C.
Immunohistochemistry
Primary pancreatic cancer tissues near the margin of
the tumor and the matched normal tissues were used to assess PKM2
expression. Sections (5 μm) of the specimens were incubated with
goat anti-human PKM2 antibody (Santa Cruz Biotechnology Inc,
Dallas, TX, USA) overnight at 4°C, followed by incubation with
horseradish peroxidase-conjugated donkey anti-goat antibody (Santa
Cruz Biotechnology Inc.) for 1 h at 37°C. Immunodetection was
performed using the EnVision™ Kit (Dako North America, Inc.
Carpinteria, CA, USA), using diami-nobenzidine as the
chromogen.
PKM2 siRNA transfection
PKM2 siRNA was purchased from Santa Cruz
Biotechnology Inc. (sc-60820), and siRNA transfection was executed
using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA). The optimum concentration of siRNA was 150 nm after a
period of 6 h. Cells were collected after 48 h, nonsense siRNA was
used as the negative control and blank control.
MTT assay
Cell proliferation was measured using an MTT assay.
Cells were collected 5 h after transfection, and plated onto
96-well plates at a density of 2×104 cells/well in DMEM
and L15 containing 10% FBS. Five duplicate wells were set up for
each group, including the negative control and untreated (Panc-1
and Sw1990) groups, and the test was repeated three times. After 48
h, 20 μl MTT (M2128; Sigma-Aldrich, St. Louis, MO, USA) in PBS (5
mg/ml) was added to each well, and the cells left for 4 h. An
Infinite F50 Microplate Reader (TECAN, Maenndorf, Switzerland) was
used to read absorbance of each well at a wavelength of 570 nm. The
optical density (OD) was used to plot proliferation curves and
compare the growth of the two cell lines prior and subsequent to
transfection.
Transwell® assay
Cell migration and invasion were determined using a
Transwell® (Costar, Corning Incorporated, Corning, New
York, NY, USA) with a pore size of 0.8 μm. 100 μl of Matrigel™ (BD
Biosciences, Franklin Lakes, NJ, USA) was placed into a 24-well
Transwell® plate for the cell migration assay, and a
Matrigel™-coated plate was used for the cell invasion assay. Cells
(2×105/ml) were seeded in the upper chamber, while DMEM
with 10% FBS was added to the lower chamber. Following a 24-h
incubation at 37°C, cells in the upper chamber were carefully
removed with a cotton swab and the cells that had traversed to
reverse face of the membrane were fixed in methanol, stained with
Giemsa (Sangon, Shanghai, China), and counted.
Western blot analysis
The proteins were extracted from the two cell lines
at 72-h post-transfection using RIPA lysate (Beyotime), and then
equal amounts (40 μg) were added to each well and separated by
SDS-PAGE. Following transfer to a Hybond ECL nitrocellulose
membrane (GE Healthcare Life Sciences, Shanghai, China), the
membrane was sealed with 5% skim milk powder at room temperature
(15–25°C) for 1 h, and incubated at 4°C overnight (15–17 h) with
rabbit polyclonal B-cell lymphoma2 (Bcl-2), Bcl-2-associated X
protein (BAX) (Abcam), MEK, ERK1/2, p38, JNK,
phosphorylated-(p-)MEK, p-ERK1/2, p-p38 and p-JNK (Cell Signaling
Technology, Inc. Danvers, MA, USA) antibodies and mouse anti-human
GAPDH monoclonal antibody (Beyotime) respectively.
The nitrocellulose membrane was washed after 15–17
h, incubated in the immunoglobulin G (IgG) secondary antibody
(Merck & Co., Inc., Whitehouse Station, NJ, USA), marked by
alkaline phosphatase and stained with enhanced chemiluminescence
(Lumi-Phos WB; 34150; Thermo Fisher Scientific, Tewksbury, MA, USA)
at room temperature. The membrane was scanned for the relative
value of protein expression. Protein levels were quantified
relative to tubulin, and the software used for was Gel-Pro analyzer
(version 4.0; Media Cybernetics Inc., Rockville, MD, USA).
Statistical analysis
Statistical significance was tested using SPSS
version 14.0 software (SPSS, Inc. Chicago, IL, USA). Data are
presented as the mean ± standard deviation (χ̄ ± s), using
student t tests or one-way ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
Pancreatic cancer tissues and cell lines
expressed high levels of PKM2
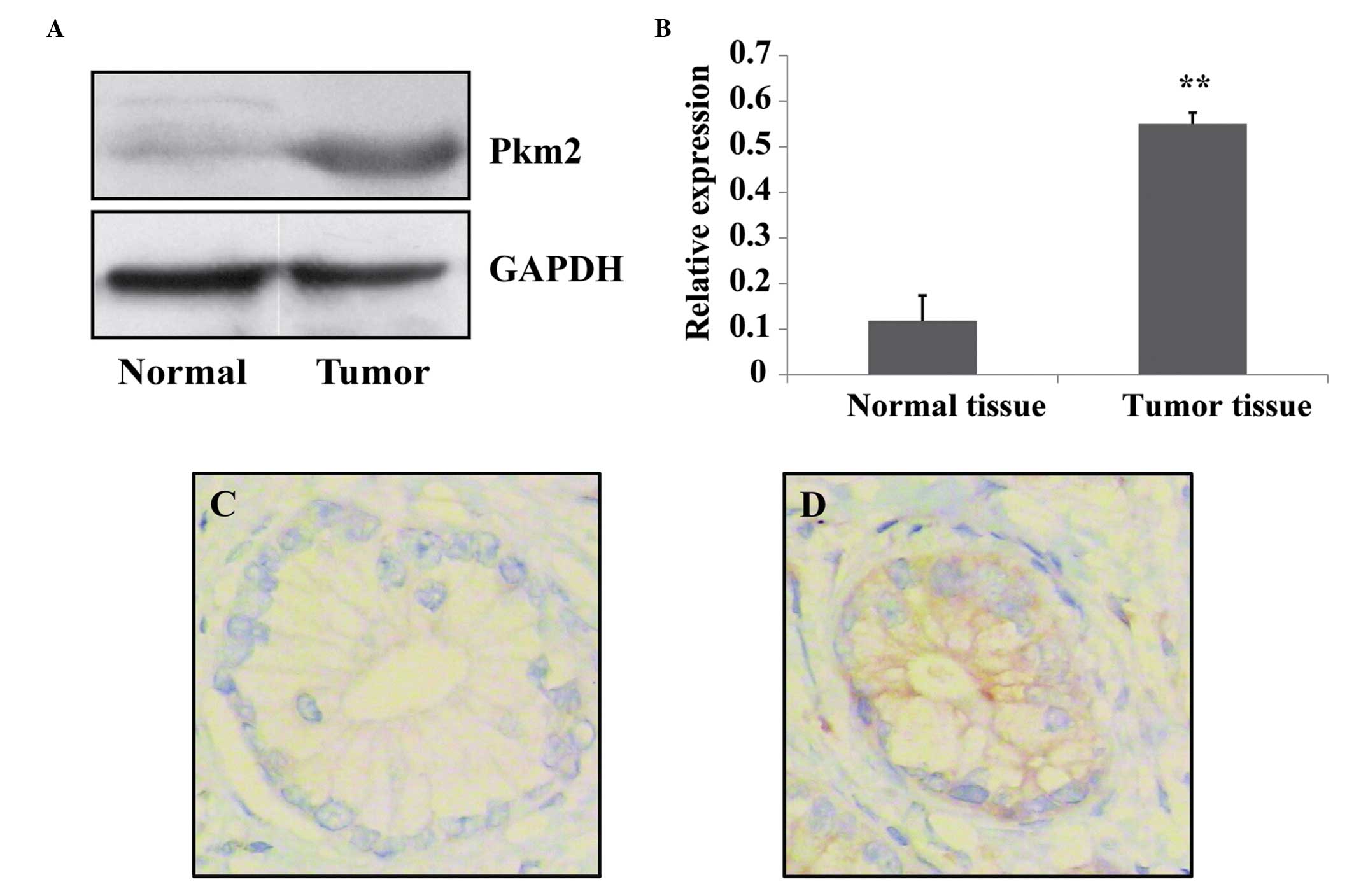
Harris et al (6) reported that PKM2 was highly expressed
in pancreatic cancer cells and promoted the proliferation and
survival in tumor cells. In the present study, the expression of
PKM2 was measured in pancreatic tumor tissue and normal tissue
using western blotting analysis. It was determined that PKM2
expression levels were significantly higher in tumor tissues
compared with those of normal tissues (Fig. 1A and B). In addition, the
expression level of PKM2 was evaluated in tumor tissue and the
matched normal pancreatic tissues via immunohistochemistry. It was
determined that the expression levels of PKM2 in pancreatic cancer
cells were increased compared with those of normal cells (Fig. 1C and D). These data suggest that
pancreatic cancer cells have the characteristic of high expression
levels of PKM2.
siRNA downregulates the expression of
PKM2 in Panc-1 and Sw1990 cells
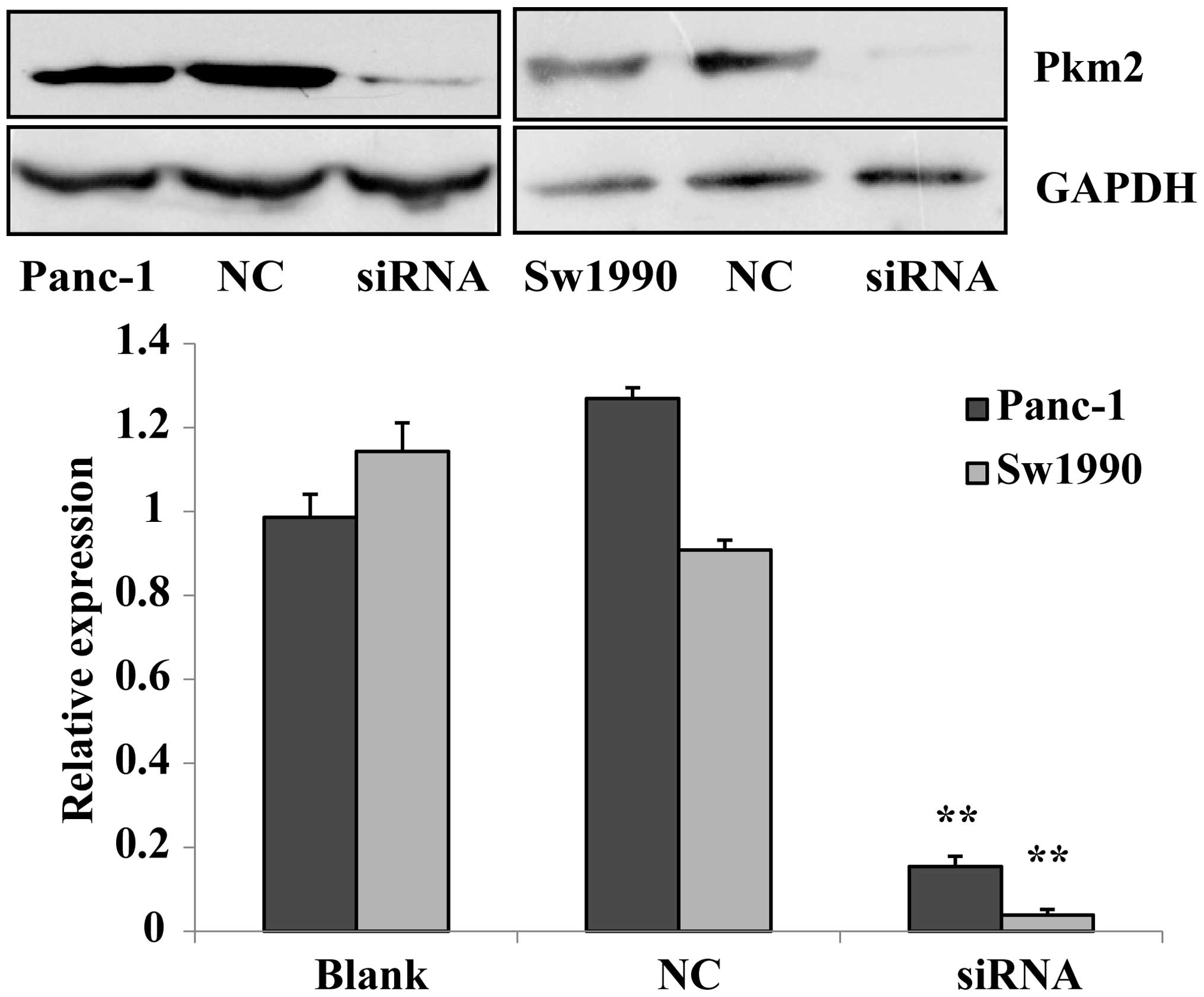
Previous experiments confirmed that PKM2 in
pancreatic cancer cells was highly expressed. PKM2 siRNA was
prepared to be transfected into Panc-1 and Sw1990 cells, and in
addition a negative control group and a blank control group were
established. The expression level of PKM2 in each group was
measured with a western blotting assay. The results revealed that
the expression of PKM2 in siRNA-transfected Panc-1 and Sw1990 cells
were significantly lower than those of the negative and blank
controls (P<0.01). This indicates that siRNA silenced the
expression of PKM2, and that the inhibitory effect on PKM2 in
Sw1990 cells was stronger than that in Panc-1 cells (Fig. 2). The transfection of siRNA to
Panc-1 and Sw1990 pancreatic cancer cells as subject matter in our
experiment.
Knocking down PKM2 decreases the
proliferation of pancreatic cancer cells
A previous study discovered that the overexpression
of PKM2 promoted cell proliferation in colon cancer cells, while
the knockdown of PKM2 inhibited their proliferation (13). In order to determine whether PKM2
had the same effect in pancreatic cells, the current study
investigated the effects of siRNA on PKM2 expression using an MTT
assay in the Panc-1 and Sw1990 cell lines. The suppressive effects
of PKM2 knockdown on Panc-1 and Sw1990 cell proliferation were
clear after 24 h, compared with that of the negative and blank
controls. The cell proliferation of the two cell lines transfected
with PKM2 siRNA was observed after 48 h and no significant
difference was found between 24 and 48 h in either cell line
(Fig. 3). The result indicates
that PKM2 knockdown downregulated the proliferation of pancreatic
cancer cells, and siRNA transfection has no significant
time-dependent effect on cell proliferation.
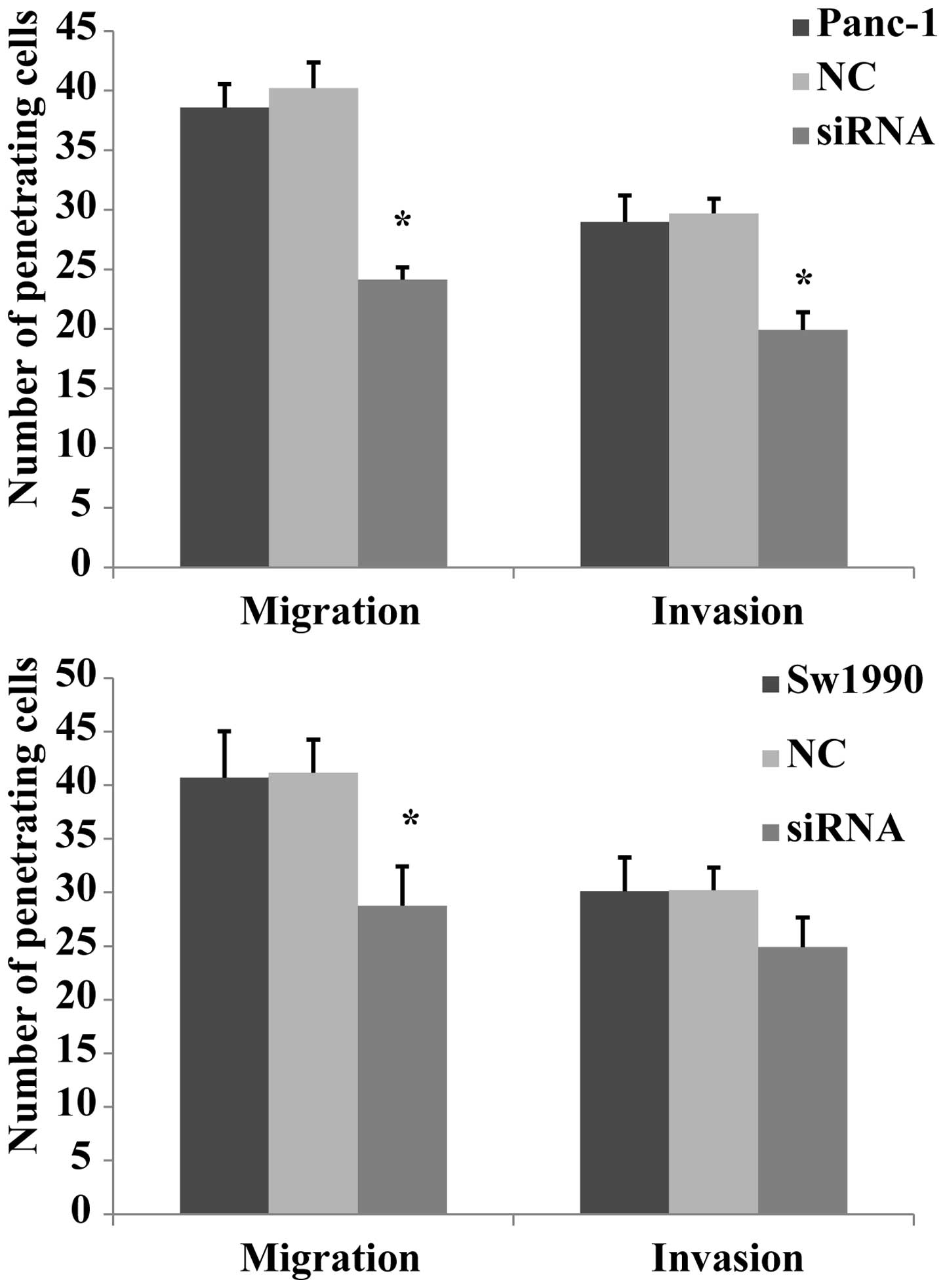
The effect of migration and invasion
following PKM2 knockdown in pancreatic cancer cells
In a previous study, it was reported that PKM2
promoted cell migration and invasion in the AGS undifferentiated
gastric carcinoma cell line, which lacks E-cadherin expression
(14). Another study reported that
PKM2 expression is high in colorectal cancer cells, and that the
knockdown of PKM2 reduced the proliferation and migration of RKO
colon cancer cells (13). In the
current experiment, the migration and invasion capabilities of
Panc-1 and Sw1990 cells was measured using a Transwell®
assay. It was revealed that the Panc-1 cell migratory and invasive
abilities were markedly reduced after siRNA transfection compared
with that of the negative and blank control groups. In Sw1990
cells, the migration of the siRNA group was significantly decreased
compared with that of the other two groups (P<0.01), however,
the invasion showed no significantly changes (Fig. 4). These results indicate that PKM2
transfection reduces the of migration and invasion capabilities of
Panc-1 cells and the migratory ability of Sw1990 cells, while
having no clear effect on the invasion of Sw1990 cells.
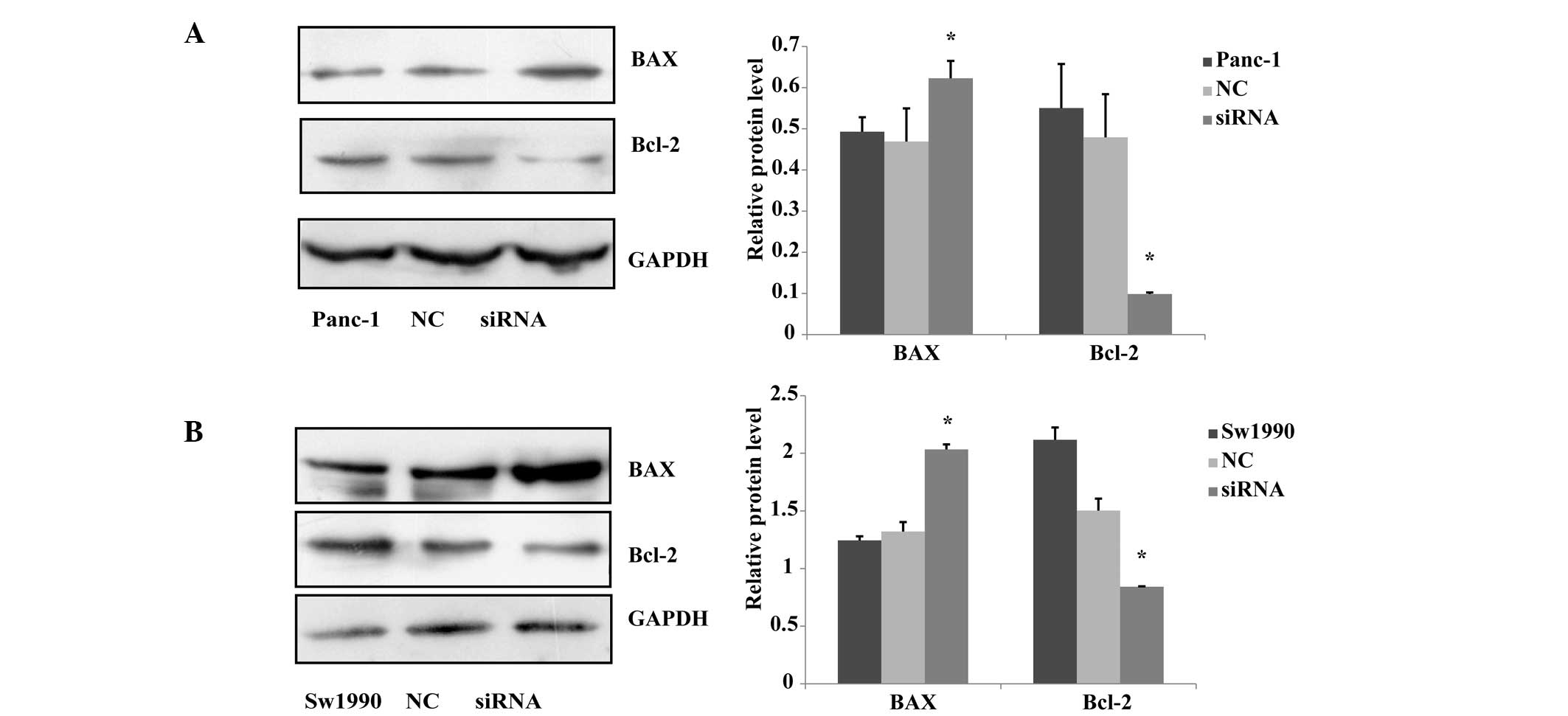
PKM2 knockown affects the levels of
apoptosis in Panc-1 and Sw1990 cells
A previous study revealed that pyruvate kinase
M2-specifc siRNA reduces the viability and increases apoptosis in
multiple cancer cell lines, but less so in normal fibroblasts or
endothelial cells (15). It has
been reported that BAX and Bcl-2 can independently regulate
apoptosis. The Bcl-2 protein, as one member of the Bcl-2 family,
inhibits cell death, while BAX protein promotes cell apoptosis
(16). In the current study, the
effect of downregulating the expression of PKM2 on apoptosis in
Panc-1 and Sw1990 cells was investigated. The expression levels of
BAX and Bcl-2 were measured using a western blot assay, and the
results showed that the level of BAX protein expression in the
siRNA-treated group was significantly upregulated compared with
that of the negative and blank control groups, and Bcl-2 protein
expression in siRNA group was significantly downregulated compared
with that of the other two groups (Fig. 5). The results showed that
downregulating the expression level of PKM2 promotes the
downregulation of the expression of BAX protein and the
upregulation of the expression of Bcl-2 protein.
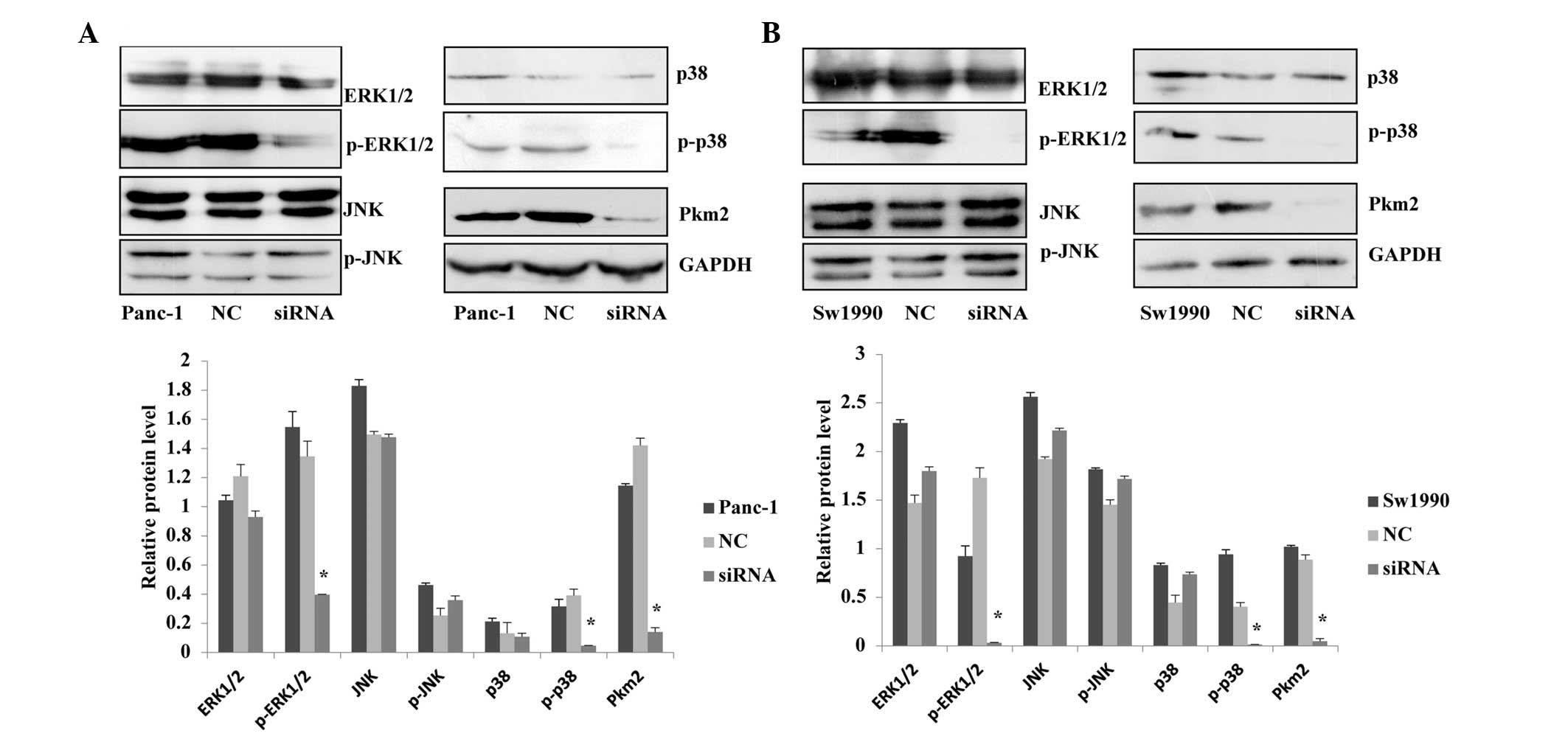
Association between PKM2 expression level
and the MAPK signaling pathway
Through immunohistochemical analyses, Wang et
al (14) have shown that the
levels of E-cadherin expression, ERK1/2 phosphorylation and
cytoplasmic PKM2 expression are correlated with each other.
Furthermore, they demonstrated a high level of ERK1/2
phosphorylation without E-cadherin expression but with a high level
of PKM2 expression in the nucleus of gastric cancer cells (14). Based on the results of previous
studies, the current study investigated whether the expression
level of PKM2 would have an effect on the MAPK signaling pathway in
pancreatic cancer, using western blotting to measure the
phosphorylated and non-phosphorylated proteins of the MAPK in
Panc-1 and Sw1990 cells treated with siRNA. The results revealed
that when the expression level of PKM2 in Panc-1 and Sw1990 cells
was reduced, p-ERK1/2 and p-p38 expression levels were
significantly reduced in the MAPK signaling pathway, while the
ERK1/2, p38, JNK, p-JNK expression were not significantly changed.
The results indicated that knockdown of PKM2 inhibited the MEK-ERK
and p38 signaling pathways, however, it had no significant effect
on the JNK pathway (Fig. 6).
Therefore, we suspected that PKM2 regulated certain biological
functions associated with of progression of pancreatic cancer cells
through MAPK pathway.
Discussion
Pancreatic cancer is a common malignancy around the
world, the development of which is a multi-step process whereby
multiple pathways are deregulated. One of the primary hallmarks of
pancreatic cancer is its early systemic dissemination and its
accelerated local tumor progression. However, these characteristics
closely correlate with poor clinical prognosis and represent a
formidable barrier to successful treatment.
In a previous study, researchers discovered that the
PI3K/AKT/mTOR signaling pathway is a major positive regulator of
the Warburg effect, a hallmark of cancer metabolism. This signaling
pathway has an important role in the regulation of the progression
of tumors (17). PKM2 is a major
effector of mTOR signaling, downstream of HIF1α and the
c-Myc-hnRNPs. The mTOR/HIF1α/Myc-hnRNPs/PKM2 signaling cascades are
critical for oncogenic mTOR-mediated tumorigenesis (7). In the current study, siRNA was
utilized to knockdown PKM2 in human pancreatic cancer cells, in
order to observe their proliferation, apoptosis, migration and
invasion capabilities. Subsequently, it was investigated whether
the expression level of PKM2 was associated with the activation of
the MAPK signal pathway.
A previous study suggested that overexpression of
PKM2 promotes the proliferation and migration of colon cancer cells
(14). At the same time,
researchers have demonstrated that the knockdown of PKM2 using
specific siRNA inhibited the proliferation and invasion of cancer
cell in vitro and the formation of xenograft tumors in
vivo (18,19). In the present study, it was
determined that in Panc-1 and Sw1990 cells, PKM2 knock-down
downregulates the proliferation of pancreatic cancer cells compared
to that of the normal pancreatic tissue, however, there was no
significant time-dependent effect on cell proliferation in either
cell line. Subsequently, the migration and invasion capabilities of
the siRNA groups was determined in the two cell lines. In Panc-1
cells, the migration and invasion abilities were significantly
reduced after siRNA transfection. Notably, in Sw1990 cells, the
migration of the experimental group was significantly reduced,
however, the invasion showed no significantly changes. This
phenomenon is worth researching and discussing in the future.
Kwon et al have previously reported that the
apoptotic pathway is involved in the reduced cell growth after PKM2
knockdown in gastric cancer cells (20). The current study utilized siRNA
transfection to downregulate the expression of PKM2 in pancreatic
cancer cells. It was revealed that the expression level of
apoptosis-associated protein BAX increased markedly, and the
expression level of Bcl-2 was reduced significantly. Therefore, it
is indicated that the low expression of PKM2 may promote the
apoptosis of tumor cells, but has no significant effect on normal
pancreatic tissue.
Wang et al have documented that activation of
the MAPK and PKM2 pathways may accelerate hepatocarcinogenesis, and
PKM2 activation is independent of MAPK or AKT/mTOR cascades
(12). Another study demonstrated
that a high level of ERK1/2 phosphorylation was associated with a
high level of PKM2 expression in gastric cancer cells (14). In order to determine the
association between PKM2 and MAPK in the present study, the protein
expression levels were detected in three major pathways of the MAPK
signaling pathway after PKM2 knockdown in pancreatic cancer. In
addition, it was determined that the expression level of p-ERK1/2
was reduced significantly in the experimental group, but the levels
of non-phosphorylated ERK1/2 had no significant change. Similarly,
significant changes appeared in the P38 signaling pathway. It was
revealed that the level of p-p38 protein was reduced in the siRNA
group following PKM2 knockdown, but the levels of
non-phosphorylated p38 had no significant change. However, the
expression levels of non-phosphorylated JNK and p-JNK did not
change in Panc-1 and Sw1990 cells after siRNA PKM2 infection. PKM2
knockdown did reduce the levels of phosphorylation of ERK1/2 and
p38. Therefore, we hypothesized that PKM2 has an important role in
regulating certsin proteins in the MEK-ERK1/2 and p38 signaling
pathways in the two cell lines.
In conclusion, the glycolytic rate-limiting enzyme
PKM2 has an essential role in the proliferation, apoptosis,
migration and invasion of Panc-1 and Sw1990 pancreatic cancer
cells. The downregulation of PKM2 expression inhibits the
proliferation, migration and invasion and promotes the apoptosis of
pancreatic cancer cells. This phenomenon may be realized via the
MEK-ERK1/2 and p38 pathways in the MAPK signaling pathways. These
findings provide novel scientific evidence for the tumorigenesis of
pancreatic cancer.
Acknowledgements
This study was supported in part by grants from the
Natural Science Foundation of Jiangsu Province (BK2012563), and the
Medical Research Project of Health Department of Jiangsu Province
(Z201218).
References
|
1
|
Botta GP, Reginato MJ, Reichert M, Rustgi
AK and Lelkes PI: Constitutive K-RasG12D activation of ERK2
specifically regulates 3D invasion of human pancreatic cancer cells
via MMP-1. Mol Cancer Res. 10:183–196. 2012. View Article : Google Scholar :
|
|
2
|
Long J, Zhang Y, Yu X, et al: Overcoming
drug resistance in pancreatic cancer. Expert Opin Ther Targets.
15:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamada M, Suematsu M and Saya H: Pyruvate
kinase M2: multiple faces for conferring benefits on cancer cells.
Clin Cancer Res. 18:5554–5561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar Y, Mazurek S, Yang S, et al: In vivo
factors influencing tumour M2-pyruvate kinase level in human
pancreatic cancer cell lines. Tumour Biol. 31:69–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris I, McCracken S and Mak TW: PKM2: a
gatekeeper between growth and survival. Cell Res. 22:447–449. 2012.
View Article : Google Scholar :
|
|
7
|
Sun Q, Chen X, Ma J, et al: Mammalian
target of rapamycin up-regulation of pyruvate kinase isoenzyme type
M2 is critical for aerobic glycolysis and tumor growth. Proc Natl
Acad Sci USA. 108:4129–4134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moritz A, Li Y, Guo A, et al: Akt-RSK-S6
kinase signaling networks activated by oncogenic receptor tyrosine
kinases. Sci Signal. 3:ra642010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Govindarajan B, Sligh JE, Vincent BJ, et
al: Overexpression of Akt converts radial growth melanoma to
vertical growth melanoma. J Clin Invest. 117:719–729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furukawa T, Tanji E, Kuboki Y, et al:
Targeting of MAPK-associated molecules identifies SON as a prime
target to attenuate the proliferation and tumorigenicity of
pancreatic cancer cells. Mol Cancer. 11:882012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Delogu S, Ho C, et al:
Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis
via activation of MAPK and PKM2 pathways. J Hepatol. 57:577–583.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou CF, Li XB, Sun H, et al: Pyruvate
kinase type M2 is upregulated in colorectal cancer and promotes
proliferation and migration of colon cancer cells. IUBMB Life.
64:775–782. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang LY, Liu YP, Chen LG, et al: Pyruvate
kinase M2 plays a dual role on regulation of the EGF/EGFR signaling
via E-cadherin-dependent manner in gastric cancer cells. PLoS One.
8:e675422013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldberg MS and Sharp PA: Pyruvate kinase
M2-specific siRNA induces apoptosis and tumor regression. J Exp
Med. 209:217–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma J, Meng Y, Kwiatkowski DJ, et al:
Mammalian target of rapamycin regulates murine and human cell
differentiation through STAT3/p63/Jagged/Notch cascade. J Clin
Invest. 120:103–114. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kefas B, Comeau L, Erdle N, et al:
Pyruvate kinase M2 is a target of the tumor-suppressive
microRNA-326 and regulates the survival of glioma cells. Neuro
Oncol. 12:1102–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldberg MS and Sharp PA: Pyruvate kinase
M2-specific siRNA induces apoptosis and tumor regression. J Exp
Med. 209:217–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon OH, Kang TW, Kim JH, et al: Pyruvate
kinase M2 promotes the growth of gastric cancer cells via
regulation of Bcl-xL expression at transcriptional level. Biochem
Biophys Res Commun. 423:38–44. 2012. View Article : Google Scholar : PubMed/NCBI
|