Introduction
Lung cancer is one of the most prevalent types of
malignant tumor worldwide, with a five-year survival rate of ~16%
(1). Mortality among lung cancer
patients is more frequently due to metastasis rather than their
primary tumors. Therefore, it is necessary to elucidate the
molecular mechanisms of lung cancer metastasis in order to develop
effective treatment options.
Epithelial-mesenchymal transition (EMT) is a process
characterized by downregulation of epithelial markers and
upregulation of mesenchymal markers (2,3).
Previous studies have proposed that EMT may be a key step in the
progression of tumor cell metastasis (4–6).
Octamer-binding protein 4 (Oct4), a transcription
factor that belongs to the Pit-Oct-Unc (POU) family, has been
reported to be a master regulator of maintenance and
differentiation in pluripotent cells. It has been suggested that
Oct4 may be a key component of the regulation of self-renewal and
differentiation in stem cells (7–9); in
addition, Oct4 may also have a crucial role in cancer development
(10). Chen et al (11) demonstrated that Oct4 expression was
involved in the tumorigenesis and malignancy of lung cancer. The
aims of the present study were to investigate the effect of Oct4 on
the cell biology of lung cancer cells in vitro, elucidate
the underlying mechanisms associated with lung cancer metastasis
and examine the effect of Oct4 on the degradation of the
β-catenin/E-cadherin complex degradation, a process strongly
associated with EMT.
Materials and methods
Cell culture
A549 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Life
Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS;
Invitrogen Life Technologies) at 37°C in a 5% CO2
humidified atmosphere.
Tissues
Tumor and adjacent normal lung tissue specimens were
collected from ten patients with non-small cell lung cancer at
Jiangxi Provincial Chest Hospital (Nanchang, China). This study was
approved by the Ethics Committee of Jiangxi Provincial Chest
Hospital. All patients provided written informed consent in
compliance with the code of ethics of the World Medical Association
(Declaration of Helsinki; Ferney-Voltaire, France). None of the ten
patients had received chemotherapy or radiotherapy prior to
surgery. The tumor and adjacent normal tissue specimens were frozen
in liquid nitrogen directly following surgery and stored at −80°C
until further use.
Constructs and transfection
An open reading frame clone of homo Oct4 was
subcloned into enhanced green fluorescent protein plasmind-C1
(pEGFP-C1) vector (Invitrogen Life Technologies). Small hairpin RNA
(shRNA) targeting Oct4 was designed and inserted into pGPU6/GFP/Neo
vector (Invitrogen Life Technologies). The plasmids pEGFP-C1-Oct4
and shRNA-Oct4 were transfected into A549 cells using Lipofectamine
2000 (Invitrogen Life Technologies) according to the manufacturer’s
instructions.
MTT assay
Cells were seeded in 96-well plates at a density of
1×105/ml and allowed to grow at 37°C in a 5%
CO2 humidified atmosphere. 10 μl MTT reagent
(Sigma-Aldrich, St. Louis, MO, USA) was added to each well and
incubated at 37°C for 4 h. The formazan dye was solubilized in 150
μl dimethyl sulfoxide and the absorbance was measured at 570 nm
using a microplate reader (Multiskan Ascent 354; Thermo Labsystems,
Waltham, MA, USA).
Flow cytometry
Cells were dual-stained with Alexa Fluor 488-Annexin
V and propidium iodide (PI) using an Annexin V-fluorescein
isothiocyanate/PI apoptosis kit (Kaiji Biological Inc., Nanjing,
China) according to the manufacturer’s instructions. The apoptotic
rate was measured using flow cytometry (FC 500 MPL system; Beckman
Coulter Inc., Miami, FL, USA).
Immunoprecipitation
Cells were extracted with immunoprecipitation lysis
buffer (Beyotime, Shanghai, China). The cell lysates were incubated
with an anti-E-cadherin antibody or normal immunoglobulin G (IgG),
followed by a recombinant fusion of protein A and protein G agarose
(Sigma). Following centrifugation at 1000 × g for 5 min and washing
with immunoprecipitation lysis buffer (Beyotime, Shanghai, China)
five times, proteins were analyzed using SDS-PAGE.
Cell invasion assay
The cell invasion assay was carried out using a
Transwell chamber (Corning Inc., Corning, NY, USA) pre-coated with
Matrigel® (BD Biosciences, Franklin Lakes, NJ, USA).
5×104 A549 cells were added to the upper chamber of the
Transwell plates (Corning Inc., Corning, NY, USA) with serum-free
medium (DMEM; Invitrogen Life Technologies) and the lower chamber
was filled with 1 ml DMEM containing 10% FBS. Following 12 h of
incubation at 37°C, cells in the upper chamber were removed using a
cotton swab, the invaded cells were fixed using 95% ethanol
(Xinchenghuagong Inc., Guangzhou, China) for 15 min and then
stained with hematoxylin (Maixin, Fuzhou, China) for 10 min. The
invaded cells were then counted under a light microscope (TS100,
Nikon, Tokyo, Japan).
Cell adhesion assay
A 96-well-plate was precoated with fibronectin
(Sigma) for 2 h. The wells were washed with phosphate-buffered
saline (PBS; Maixin) and then blocked with 1% bovine serum albumin
(BSA; Amresco Inc., Solon, OH, USA) for 2 h. Cells were seeded in
the wells at a final concentration of 3×105 cells/ml in
serum-free medium. Following 2 h of incubation, the wells were
washed with PBS and the cells were fixed in paraformaldehyde
(Xinchenghuagong Inc., Guangzhou, China). The number of adherent
cells was quantified using the colorimetric MTT assay.
Quantitative polymerase chain reaction
(qPCR)
Total RNA from cells or tissue specimens was
isolated using Trizol reagent (Invitrogen Life Technologies).
Complementary DNA (cDNA) was synthesized by a First Strand cDNA
Synthesis kit (Fermentas, Vilnius, Lithuania) using 1 μg RNA
template. qPCR was performed in a 7300 Sequence Detection System
(Applied Biosystems, Foster City, CA, USA) using a SYBR®
Green PCR kit (Applied Biosystems). The relative expression levels
of each messenger RNA (mRNA) were calculated using comparative
computerized tomography (CT) methods (SDS Software version 1.4.1;
Applied Biosystems) with GAPDH as an internal control.
Western blot analysis
Whole cell extracts were obtained using a total
protein extraction kit (Promab, Changsha, China), β-catenin nuclear
and cytoplasmic fractions were obtained using a nuclear and
cytoplasmic extraction kit (Beyotime, Shanghai, China). 50 μg
protein was separated using 10% SDS-PAGE and transferred onto a
nitrocellulose membrane (Millipore, Billerica, MA, USA). Membranes
were blocked with 5% BSA at room temperature for 1 h. Membranes
were then washed with PBS and incubated with the corresponding
primary antibody [rabbit polyclonal to Oct4 (1:400), rabbit
polyclonal to β-catenin (1:500), mouse monoclonal to
histone(1:1000) and mouse monoclonal to GAPDH (1:800) (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA); rabbit monoclonal to
vimentin (1:1000; Abcam, Cambridge, MA, USA); rabbit polyclonal to
N-cadherin (1:400), rabbit polyclonal to E-cadherin (1:400) and
rabbit polyclonal to pan-cytokeratin (1:400) (Signalway Antibody
Inc., College Park, MD, USA)] overnight at 4°C, then incubated with
horseradish peroxidase-conjugated secondary antibodies [Goat Anti
Mouse IgG/HRP(1:40000) and Goat Anti Rabbit IgG/HRP(1:40000);Santa
Cruz Biotechnology, Inc.)] for 1 h at room temperature. Bands were
detected using an enhanced chemiluminesence detection kit (Pierce,
Rockford, IL, USA). GAPDH expression was used as an internal
control.
Immunofluorescence
Cell slides were washed with PBS three times, then
fixed in 4% paraformaldehyde solution at 4°C for 1 h. Following
fixation, slides were washed again with PBS at room temperature and
permeabilized by exposure to 0.2% Triton X-100 (Sigma-Aldrich) at
4°C for 1 h. Slides were blocked with goat serum (Maixin) at 4°C
for 1 h, then incubated with primary antibodies [mouse monoclonal
to E-cadherin (1:400) and rabbit monoclonal to β-catenin (1:400);
Abcam] for 1 h at 37°C. Slides were washed again with PBS and
secondary antibodies [Alexa Fluor 555-labeled Goat anti-Rabbit IgG
(1:200) or Alexa Fluor488 labeled Goat anti mouse IgG (1:200);
Abcam] were added, slides were then incubated at 37°C for 1 h. DAPI
(Invitrogen Life Technologies) was used to label the nucleus and
slides were visualized under a fluorescence microscope (80i,
Nikon).
Statistical analysis
SPSS 19.0 statistical software (IBM corp., Armonk,
NY, USA) was used for statistical analysis. All data represent the
mean ± standard deviation of at least three independent
experiments. Statistical differences were analyzed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
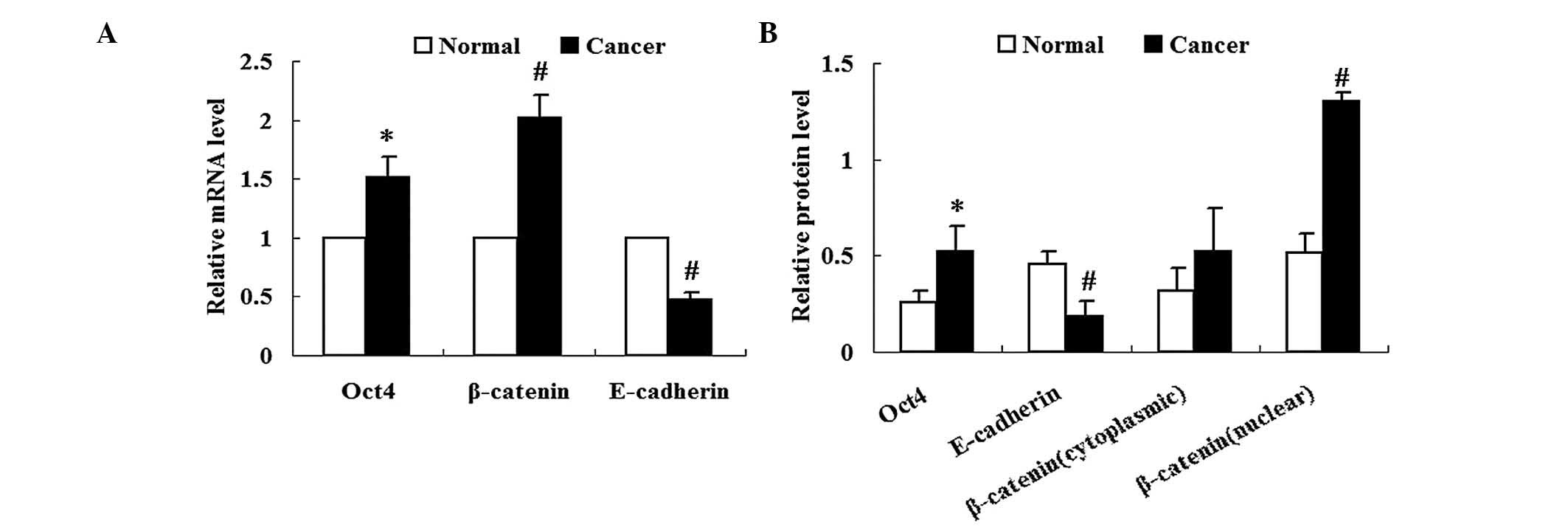
Expression of Oct4 is increased in lung
cancer tissues
Expression of Oct4 in lung cancer tissues was
analyzed using qPCR and western blot analysis. Results from qPCR
and western blot analysis revealed increased expression of Oct4
mRNA and relative protein levels, respectively, in lung cancer
tissues compared with those in the adjacent normal tissues
(P<0.05) (Fig. 1A and B).
Expression of β-catenin and E-cadherin is
altered in lung cancer tissues
As shown in Fig.
1A, β-catenin mRNA expression was significantly increased in
lung cancer tissues compared with that in adjacent normal tissues
(P<0.05). Nuclear and cytoplasmic fractions of β-catenin were
collected from the respective tissues. Western blot analysis
revealed that the protein expression levels of cytoplasmic
β-catenin in lung cancer tissues were not significantly different
from those of adjacent normal tissues (P>0.05) (Fig. 1B). By contrast, protein expression
levels of nuclear β-catenin were significantly increased in lung
cancer tissues compared with those of adjacent normal tissues
(P<0.01) (Fig. 1B).
The expression of E-cadherin was significantly
reduced in lung cancer tissues compared with that of adjacent
normal tissues at the mRNA and protein level (P<0.01) (Fig. 1).
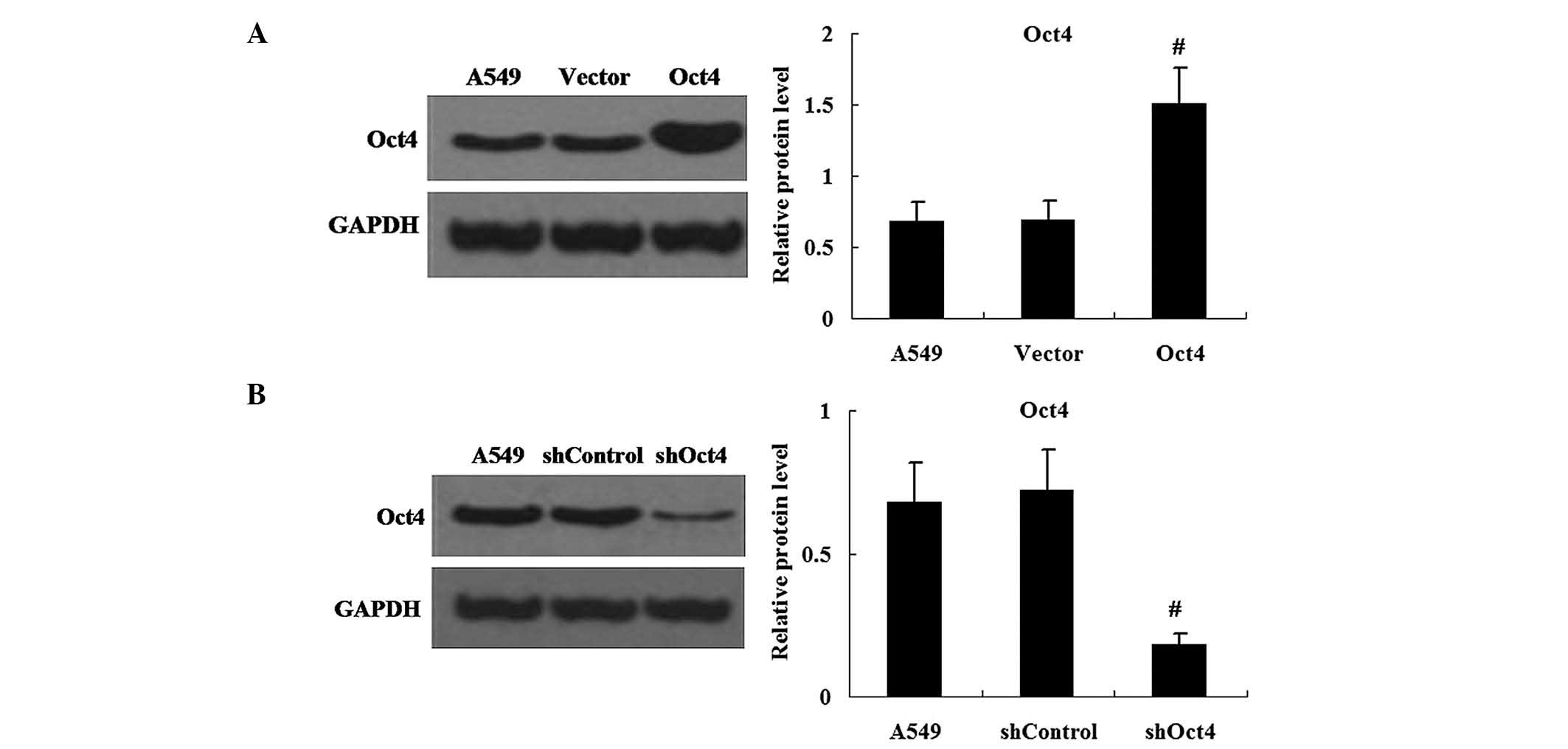
Changes in expression levels of Oct4 in
pEGFP-C1-Oct4 and shRNA-Oct4-transfected cells
In order to efficiently enhance or repress Oct4
expression, A549 cells were transfected with pEGFP-C1-Oct4 or
shRNA-Oct4. Western blot analysis was used to measure the protein
expression levels of Oct4 in pEGFP-C1-Oct4- and
shRNA-Oct4-transfected cells. The results demonstrated that the
expression levels of Oct4 protein were increased in pEGFP-C1-Oct4
transfected cells compared with those in the pEGFP-C1 vector
transfected cells (P<0.01) (Fig.
2A). As hypothesized, the shRNA-Oct4 transfected cells showed
decreased expression of Oct4 protein in comparison to that of the
shRNA-Control transfected cells (P<0.01) (Fig. 2B).
Opposite effects of overexpression and
repression of Oct4 on cell viability, apoptosis, invasion and
adhesion in vitro
The effect of Oct4 on cell biology was examined in
A549 cells using an MTT assay to analyze cell proliferation. As
shown in Fig. 3A, Oct4
overexpression significantly increased cell viability, whereas
repression of Oct4 significantly reduced cell viability in
vitro. Furthermore, the apoptotic rate in the Oct4
overexpression group was significantly decreased compared with that
of the vector control group, whereas shRNA-Oct4 had the reverse
effect (Fig. 3B). Cell invasion
assays revealed that Oct4 overexpression resulted in an increased
number of invasive cells in comparison to that of the vector
control. However, in the shRNA-Oct4 group, the number of invasive
cells was significantly decreased compared with that of the
shRNA-control group (Fig. 3C).
Cell adhesion assays revealed that the adhesive activity in the
Oct4 overexpression group was significantly increased compared with
that of the vector control group. Following transfection with
shRNA-Oct4, the adhesion activity of the A549 cells was reduced
compared with that of the shRNA-control (Fig. 3D).
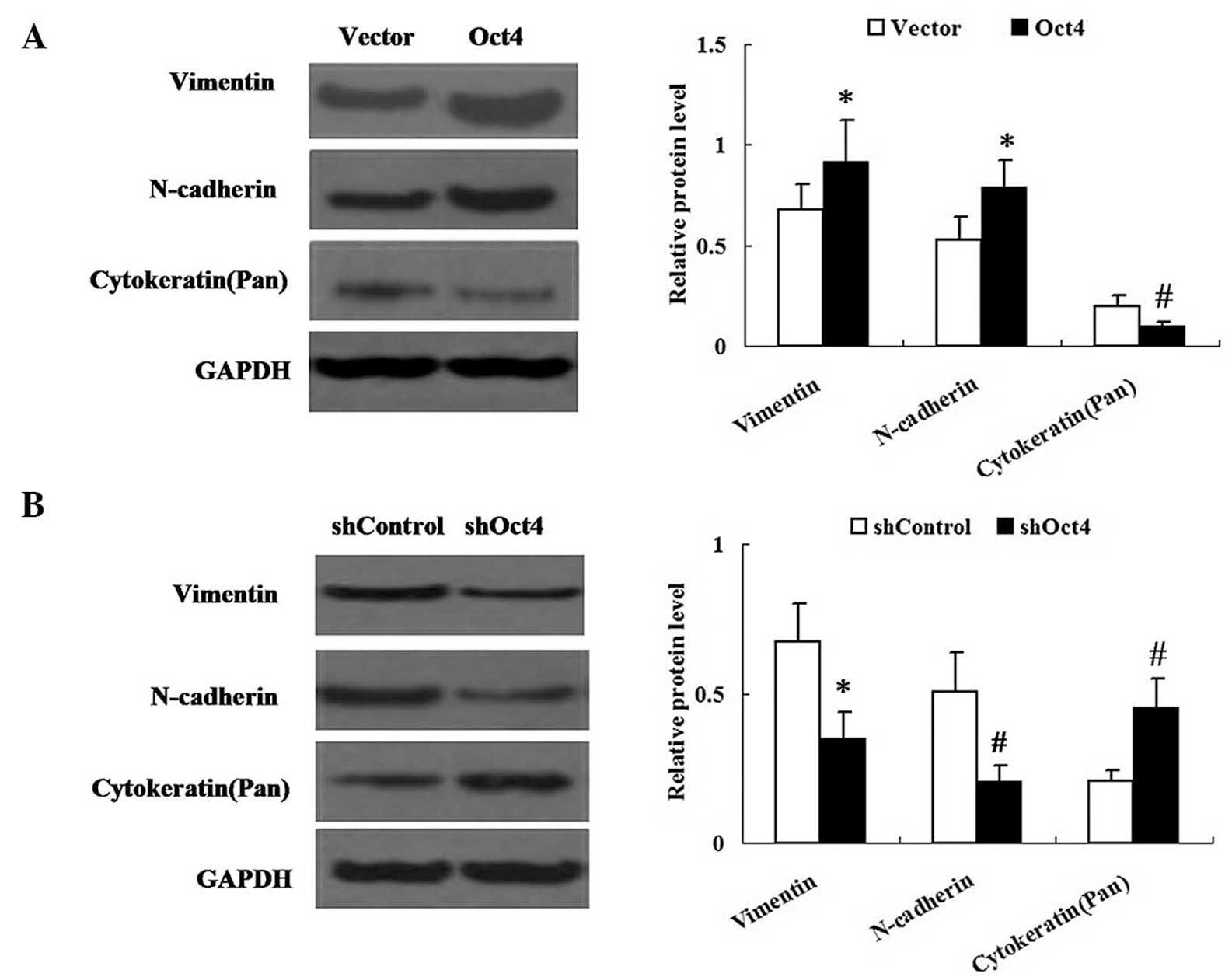
Changes in expression of Oct4 alter the
expression levels of phenotypic transition markers in A549
cells
The effect of Oct4 on EMT-like phenotypic changes in
A549 cells was examined. Western blot analysis was performed in
order to determine the expression levels of vimentin, N-cadherin
and cytokeratin in A549 cells. As shown in Fig. 4, the expression levels of vimentin
and N-cadherin were significantly increased in the
pEGFP-C1-Oct4-transfected group but decreased in the shRNA-Oct4
group. By contrast, expression of cytokeratin was decreased in the
pEGFP-C1-Oct4-transfected group, but in the shRNA-Oct4 group
cytokeratin expression was increased compared with that in the
vector control group.
Changes in expression of Oct4 alters the
expression of the β-catenin/E-cadherin complex
In order to examine the effect of Oct4 on the
association and degradation of the β-catenin/E-cadherin complex,
cell lysates were immunoprecipitated with an anti-E-cadherin
antibody and western blot analysis was performed with an
anti-β-catenin antibody. Equal protein was confirmed in each group
using a western blot with anti-E-cadherin antibody.
The co-immunoprecipitation study, shown in Fig. 5, demonstrated that the degradation
of β-catenin with E-cadherin was increased in the Oct4
overexpression group compared with that of the vector control
group. However, the association of β-catenin with E-cadherin was
enhanced in the shRNA-Oct4 group.
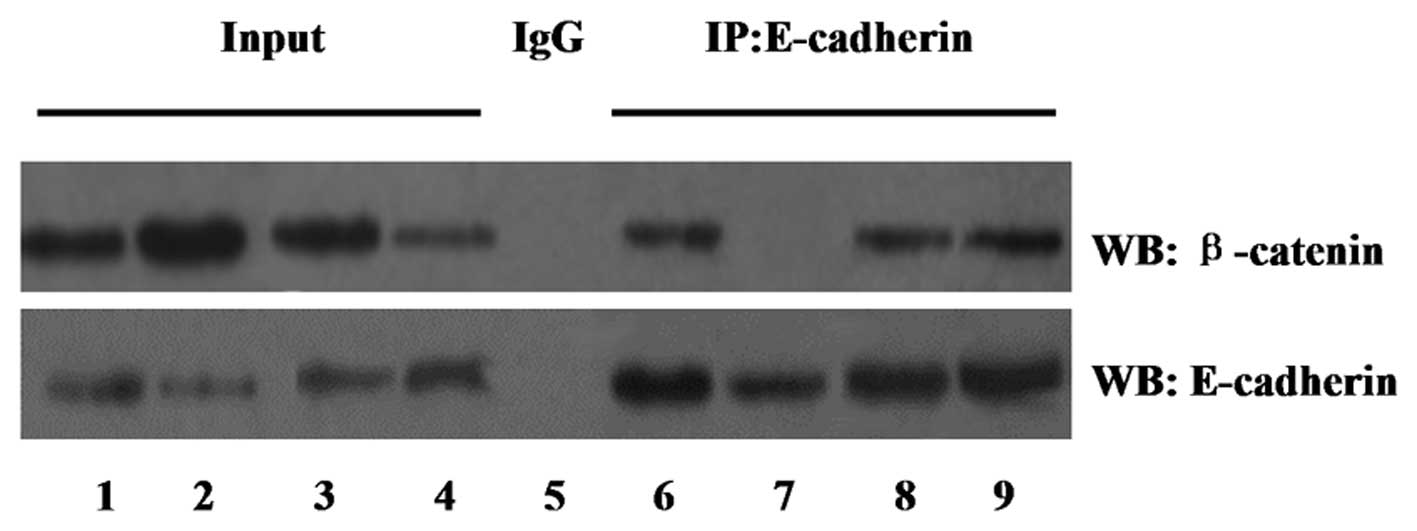 | Figure 5Effect of Oct4 on the association and
degradation of the β-catenin/E-cadherin complex determined by
co-immunoprecipitation assay. Input lanes represent the total cell
lysates. IgG was used as a control. Lanes: 1 and 6, vector group; 2
and 7, Oct4 group; 3 and 8, shRNA-control group; 4 and 9,
shRNA-Oct4 group; 5, combination of the four groups. IP,
immunoprecipitaion; WB, western blot; Oct4, octamer-binding protein
4; shRNA, small hairpin RNA; IgG, immunoglobulin G. |
As shown in Fig. 6,
relative protein levels of E-cadherin were decreased in the Oct4
overexpression group; however, they were increased in the
shRNA-Oct4 group compared with those of the control groups. Nuclear
and cytoplasmic fractions of β-catenin were obtained from A549
cells. Western blot analysis demonstrated that β-catenin nuclear
protein levels were significantly increased in the Oct4
overexpression group but decreased in the shRNA-Oct4 group. The
expression of cytoplasmic β-catenin did not alter significantly in
the Oct4 overexpression group or shRNA-Oct4 group compared with
that of the control group.
In addition, an immunofluorescence assay was used to
examine the localization of β-catenin and E-cadherin. The results
of the assay demonstrated induced nuclear localization of β-catenin
in the Oct4 overexpression group; however, in the shRNA-Oct4 group,
β-catenin was primarily localized in cytoplasm. Furthermore, the
immunofluorescence assay revealed a decrease in membrane
localization of E-cadherin in the Oct4 overexpression group
compared with that in the control group, but increased expression
of membrane E-cadherin in the shRNA-Oct4 group (Fig. 7).
Discussion
Oct4 is a transcription factor that has been shown
to be highly expressed in embryonic stem (ES) cells and essential
for the induction of somatic cell pluripotency (7). In addition, Oct4 has been implicated
in various human cancers and was reported to be associated with
tumor progression or bad prognosis (12–17).
The present study demonstrated that the mRNA and protein expression
of Oct4 was higher in human lung cancer tissues than that in
adjacent normal tissues. In addition, Oct4 was able to affect the
cell biology of lung cancer cells by inducing cell proliferation,
inhibiting apoptosis as well as promoting cell invasion and
adhesion. Numerous clinical studies have examined the expression of
Oct4 in lung cancer patients (18–20);
however, the molecular mechanisms of its oncogenic role remain to
be elucidated.
The majority of newly diagnosed lung cancer patients
have locally invasive cancer, and almost all of these patients go
on to develop metastatic disease, which accounts for most
cancer-associated mortalities worldwide (21). In order to investigate the role of
Oct4 in lung cancer cell metastasis, the present study examined
whether Oct4 could regulate cell invasion and adhesion in
vitro using A549 cells. The results indicated that increased
expression of Oct4 led to enhanced cell invasion and adhesion
abilities. Conversely, repression of Oct4 demonstrated the opposite
effect, therefore indicating that Oct4 promoted lung cancer cell
metastasis.
Epithelial-mesenchymal transition (EMT) has been
shown to be an important process for the metastatic progression of
epithelial cancer (3,22). Therefore, the present study aimed
to investigate whether Oct4 had a role in the regulation of EMT in
lung cancers. During EMT, epithelial cell-derived cancer cells lose
their epithelial properties and acquire mesenchymal properties
(23). Vimentin, a member of the
intermediate filament family, is an important canonical marker of
EMT (24), as it was reported to
induce changes in cell shape, motility and adhesion during the EMT
(25). N-cadherin was reported to
be involved in the metastasis of cancer cells indicated by the
association between abnormal N-cadherin expression, the acquisition
of the EMT phenotype and the enhanced invasive properties of lung
cancer cell lines (26). The
remodeling of the cytoskeleton has been suggested to be a hallmark
of EMT. Loss of cytokeratins leads to alterations in cell-to-cell
adhesions and changes in polarity and cell motility (27). The results of the present study
showed that Oct4 upregulated the expression of the mesenchymal
markers vimentin and N-cadherin, as well as downregulated the
expression of the epithelial marker cytokeratin in A549 cells.
These results indicated that Oct4 induced lung cancer cell
metastasis via the mechanism of EMT.
β-catenin/E-cadherin association has an essential
role in the regulation and provision of cellular adhesion (28). β-catenin interacts with E-cadherin
by binding directly to its cytoplasmic tail, therefore creating a
bridge between E-cadherin and the actin cytoskeleton, which
stabilizes the adherence junction (29). Nuclear β-catenin has been suggested
to have a pivotal role in tumor progression (30,31).
The results of the present study demonstrated the downregulation of
E-cadherin as well as the upregulation of nuclear β-catenin protein
in lung cancer tissues, therefore indicating that nuclear β-catenin
acts as an oncogenic protein in lung cancer. EMT is controlled by
several transcription factors, which may be able to suppress
E-cadherin promoter activity and repress E-cadherin expression
(32,33). It has been suggested that nuclear
β-catenin can induce Slug or Twist 1 gene expression (34), which may lead to the further
repression of E-cadherin and thereby contribute to EMT.
β-catenin/E-cadherin degradation is associated with tumor invasion
and metastasis (35). In the
present study, immunoprecipitation assays revealed that Oct4
promoted β-catenin/E-cadherin degradation in lung cancer cells
during EMT. Oct4 was shown to induce EMT of lung cancer cells while
repressing E-cadherin expression. In addition, the localization of
β-catenin was examined using western blot and immunofluorescence
assays, which demonstrated the upregulation of nuclear β-catenin
protein by Oct4.
In conclusion, the results of the present study
indicated that Oct4 affected the cell biology of lung cancer cells
in vitro, promoted lung cancer cell metastasis through EMT
and regulated the β-catenin/E-cadherin complex during the process
of EMT.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar
|
|
3
|
Drasin DJ, Robin TP and Ford HL: Breast
cancer epithelial-to-mesenchymal transition: Examining the
functional consequences of plasticity. Breast Cancer Res.
13:2262011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jechlinger M, Grünert S and Beug H:
Mechanisms in epithelial plasticity and metastasis: insights from
3D cultures and expression profiling. J Mammary Gland Biol
Neoplasia. 7:415–432. 2002. View Article : Google Scholar
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nichols J, Zevnik B, Anastassiadis K, et
al: Formation of pluripotent stem cells in the mammalian embryo
depends on the POU transcription factor Oct4. Cell. 95:379–391.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hay DC, Sutherland L, Clark J and Burdon
T: Oct-4 knockdown induces similar patterns of endoderm and
trophoblast differentiation markers in human and mouse embryonic
stem cells. Stem Cells. 22:225–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boiani M and Schöler HR: Regulatory
networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell
Biol. 6:872–884. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monk M and Holding C: Human embryonic
genes re-expressed in cancer cells. Oncogene. 20:8085–8091. 2001.
View Article : Google Scholar
|
|
11
|
Chen YC, Hsu HS, Chen YW, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim RJ and Nam JS: OCT4 expression
enhances features of cancer stem cells in a mouse model of breast
cancer. Lab Anim Res. 27:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zhang X, Wang X, et al:
Inhibition of LDH-A by lentivirus-mediated small interfering RNA
suppresses intestinal-type gastric cancer tumorigenicity through
the downregulation of Oct4. Cancer Lett. 321:45–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Y, Liu S, Wang P, et al: Expression
profile of embryonic stem cell-associated genes Oct4, Sox2 and
Nanog in human gliomas. Histopathology. 59:763–775. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79. 2012.
|
|
16
|
He W, Li K, Wang F, Qin YR and Fan QX:
Expression of OCT4 in human esophageal squamous cell carcinoma is
significantly associated with poorer prognosis. World J
Gastroenterol. 18:712–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schoenhals M, Kassambara A, De Vos J, et
al: Embryonic stem cell markers expression in cancers. Biochem
Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Han B, Huang J, Zheng B, Geng Q,
Aziz F and Dong Q: Prognostic significance of OCT4 expression in
adenocarcinoma of the lung. Jpn J Clin Oncol. 40:961–966. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moreira AL, Gonen M, Rekhtman N and Downey
RJ: Progenitor stem cell marker expression by pulmonary carcinomas.
Mod Pathol. 23:889–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Wang J, Xu Z, et al: Expression of
sox2 and oct4 and their clinical significance in human
non-small-cell lung cancer. Int J Mol Sci. 13:7663–7675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee W, Jiang Z, Liu J, et al: The mutation
spectrum revealed by paired genome sequences from a lung cancer
patient. Nature. 465:473–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brabletz T, Hlubek F, Spaderna S, et al:
Invasion and metastasis in colorectal cancer:
Epithelial-mesenchymal transition, mesen-chymal-epithelial
transition, stemcells and beta-catenin. Cells Tissues Organs.
179:56–65. 2005. View Article : Google Scholar
|
|
23
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes incell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Liu G, Kang Y, et al: N-cadherin
expression is associated with acquisition of EMT phenotype and with
enhanced invasion in erlotinib resistant lung cancer cell lines.
PLoS One. 8:e576922013. View Article : Google Scholar
|
|
27
|
König K, Meder L, Kröger C, et al: Loss of
the keratin cytoskeleton is not sufficient to induce epithelial
mesenchymal transition in a novel KRAS driven sporadic lung cancer
mouse model. PLoS One. 8:e579962013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gumbiner B, Stevenson B and Grimaldi A:
The role of the cell adhesion molecule uvomorulin in the formation
and maintenance of the epithelial junctional complex. J Cell Biol.
107:1575–1587. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Provost E and Rimm DL: Controversies at
the cytoplasmic face of the cadherin based adhesion complex. Curr
Opin Cell Biol. 11:567–572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morin PJ, Sparks AB, Korinek V, et al:
Activation of beta-catenin-Tcf signaling in colon cancer by
mutations in beta catenin or APC. Science. 275:1787–1790. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korinek V, Barker N, Morin PJ, et al:
Constitutive transcriptional activation by a beta-catenin-Tcf
complex in APC-/colon carcinoma. Science. 275:1784–1787. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bolós V, Peinado H, Pérez-Moreno MA, et
al: The transcription factor Slug represses E-cadherin expression
and induces epithelial to mesenchymal transitions: a comparison
with Snail and E47 repressors. J Cell Sci. 116:499–511. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, et al: Autoregulation of E-cadherin expression by
cadherin-cadherin interactions: The roles of beta-catenin
signaling, Slug, and MAPK. J Cell Biol. 163:847–857. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sommers CL, Gelmann EP, Kemler R, et al:
Alterations in beta-catenin phosphorylation and plakoglobin
expression in human breast cancer cells. Cancer Res. 54:3544–3552.
1994.PubMed/NCBI
|