Introduction
Gastric carcinoma is a common malignant tumor of the
digestive system. The morbidity and mortality of gastric cancer are
the highest of any type of cancer in China (1). Surgery, chemotherapy, radiotherapy
and immunotherapy are the primary methods of treatment for gastric
cancer. However, the overall outcome of these therapies remains
unsatisfactory (1,2). With the rapid development of tumor
molecular biology and genetic engineering technology, the
development of antitumor drugs has become a focus of interest in
the treatment of a variety of types of cancer.
Bile acids are synthesized in the liver and secreted
with the bile into the duodenum, where they perform essential
functions, including the digestion and absorption of dietary
lipids, and the regulation of gene expression (3–6).
Previous studies have demonstrated a variety of roles for different
bile acids in the treatment of cancer, depending on the nature of
their chemical structures. For instance, it has been reported that
certain components of bile, such as tauroursodeoxycholic acid,
ursodeoxycholic acid and deoxycholic acid possess different
capacities to induce apoptosis in tumor cell lines (7–10).
Deoxycholic acid is a free bile acid derivative, which is composed
of metabolized bile acids, and is known to promote bile secretion
and to produce anti-inflammatory effects. In recent years, DCA has
gained increasing attention as a potential anticarcinogenic agent.
DCA has been shown to rapidly induce apoptosis in the HCT116 colon
tumor cancer cell line (11,12).
As a result of these studies, it was postulated that DCA may
inhibit cell proliferation and induce apoptosis, and may thus be
amenable to development as an antitumor agent. However, although
DCA has been shown to possess anticancer properties, the mechanisms
underlying these effects remain unclear. The present study aimed to
assess the effects of DCA on the growth of gastric carcinoma cells
by determining the ability of this compound to induce apoptosis and
to explore the possible mechanisms of action.
Materials and methods
Reagents
Deoxycholic acid was obtained from Sigma-Aldrich
(St. Louis, MO, USA). DCA was maintained as 100 mM stock solutions
in ethanol and were stored at −20°C. DCA stock solutions were
diluted to the final concentration in RPMI-1640 medium (Gibco Life
Technologies, Grand Island, NY, USA), as required.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT), bisbenzimide (Hoechst 33258), acridine orange (AO), ethidium
bromide (EB) and propidium iodide (PI), were purchased from
Sigma-Aldrich. RPMI-1640 medium and fetal calf serum were purchased
from Gibco Life Technologies (Grand Island, NY, USA). Mouse
monoclonal antibodies against human p53, Bax, Bcl-2, cyclin D1,
cyclin E1 and cyclin-dependent kinase (CDK)2 were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All other
chemicals used were of the highest purity grade available.
Cell lines and cell culture
The BGC-823 gastric carcinoma cell line, which was
provided by the China Center for Type Culture Collection (Shanghai,
China), were maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml
streptomycin and 50 μg/ml kanamycin (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C in a 5% CO2 in air atmosphere. Following
seeding for 24 h, cells were treated with culture medium containing
various concentrations of DCA (0.2, 0.4, 0.6 or 0.8 mM).
MTT cytotoxicity assay
The cytotoxicity of DCA to the BGC823 cell line was
evaluated by an MTT assay (13).
Briefly, cells were arrayed in a 96-well plate at a density of
1×105/ml. Following overnight growth, cells were treated
with DCA at various concentrations for 24, 48 or 72 h. Following
treatment with DCA, 20 μl MTT (5 g/l) was added to each well and
cells were continually cultured for another 4 h at 37°C. The medium
was then removed and 150 μl dimethyl sulfoxide (DMSO;
Sigma-Aldrich) was added to each well. The color intensity was
measured with an ELISA microplate reader (Molecular Devices,
Sunnyvale, CA, USA) at a wavelength of 490 nm.
Giemsa staining
BGC-823 cells from the control group (treated with
RPMI-1640, including 0.5% DMSO) and the group treated with 0.4 mM
DCA for 36 h were seeded onto cover slips and grown for 24 h. Cells
were washed with phosphate-buffered saline (PBS) three times,
stained with Giemsa staining solution (Sangon Biotech Co., Ltd,
Shanghai, China) for 10 min and observed under a light
microscope.
Hoechst 33258 and AO/EB staining
Cells that had been treated with 0.4 mM DCA for 36 h
were harvested and fixed with a mixture of glacial acetic acid and
methanol (1:3, v/v; Sangon Biotech Co., Ltd) for 5 min and then
washed twice with PBS. Cells were resuspended in Hoechst 33258
solution (5 μg/ml) and incubated at room temperature for 10 min.
Following three washes with PBS, the cells were thoroughly dried
naturally at room temperature and observed under a fluorescence
microscope (Eclipse Te2000-E; Nikon Corp., Tokyo, Japan). For AO/EB
staining, the cells were harvested and washed twice with PBS. Cells
were then incubated with 100 μl PBS plus 4 μl AO/EB solution (100
μg/ml AO and 100 μg/ml EB in PBS) for 3 min at room temperature in
darkness and immediately observed under a fluorescence
microscope.
Flow cytometry
Following treatment with DCA at different
concentrations (0.2, 0.4, 0.6 and 0.8 mM) for 36 h, the BGC-823
cells were harvested, washed twice with PBS and fixed with 70%
ethanol at 4°C overnight. Cells were then centrifuged at 800 × g
for 5 min, resuspended in 100 μg/ml RNase A (Sigma-Aldrich) at 37°C
for 30 min and stained with 50 μg/ml propidium iodide at 4°C for 30
min in darkness. Cells were analyzed by flow cytometry (Guava
easyCyte 8; EMD Millipore, Billerica, MA, USA) at 488 nm, and the
data were analyzed with CellFit software (Guava InCyte; EMD
Millipore).
Analysis of mitochondrial transmembrane
potential (ΔΨm)
Following treatment with various concentrations of
DCA (0.2, 0.3, 0.4 and 0.5 mM) for 48 h, the BGC-823 cells were
incubated with Rh123 (1 mg/ml in DMSO; Sigma-Aldrich) at 37°C for
30 min and washed three times with PBS. Cells were then harvested
and analyzed by flow cytometry at an excitation wavelength of 488
nm and an emission wavelength of 530 nm.
Western blotting analysis
Western blotting analysis was performed as
previously described (13).
Briefly, cell lysates were prepared, separated with 12% SDS-PAGE
and transferred onto polyvinylidene fluoride membranes (Amersham
Biosciences, Piscataway, NJ, USA). Non-specific reactivity was
blocked by incubating the membranes for 1 h in 5% nonfat milk at
room temperature. The membranes were incubated with primary
antibody overnight at 4°C. After three washes for 10 min with
phosphate-buffered saline Tween-20 (PBST), the membranes were
incubated at 37 °C for 1 h with the appropriate secondary antibody
(1:5,000; Sigma-Aldrich) and washed three times with PBST. Reactive
proteins were detected with an enhanced chemiluminescence detection
system (Pierce Biotechnology Inc., Rockford, IL, USA). β-actin was
used as an internal control.
Statistical analysis
Data were analyzed using SPSS software, version 13.0
(SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ±
standard error of the mean of separate experiments (n≥3). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of DCA on the proliferation of
BGC-823 cells
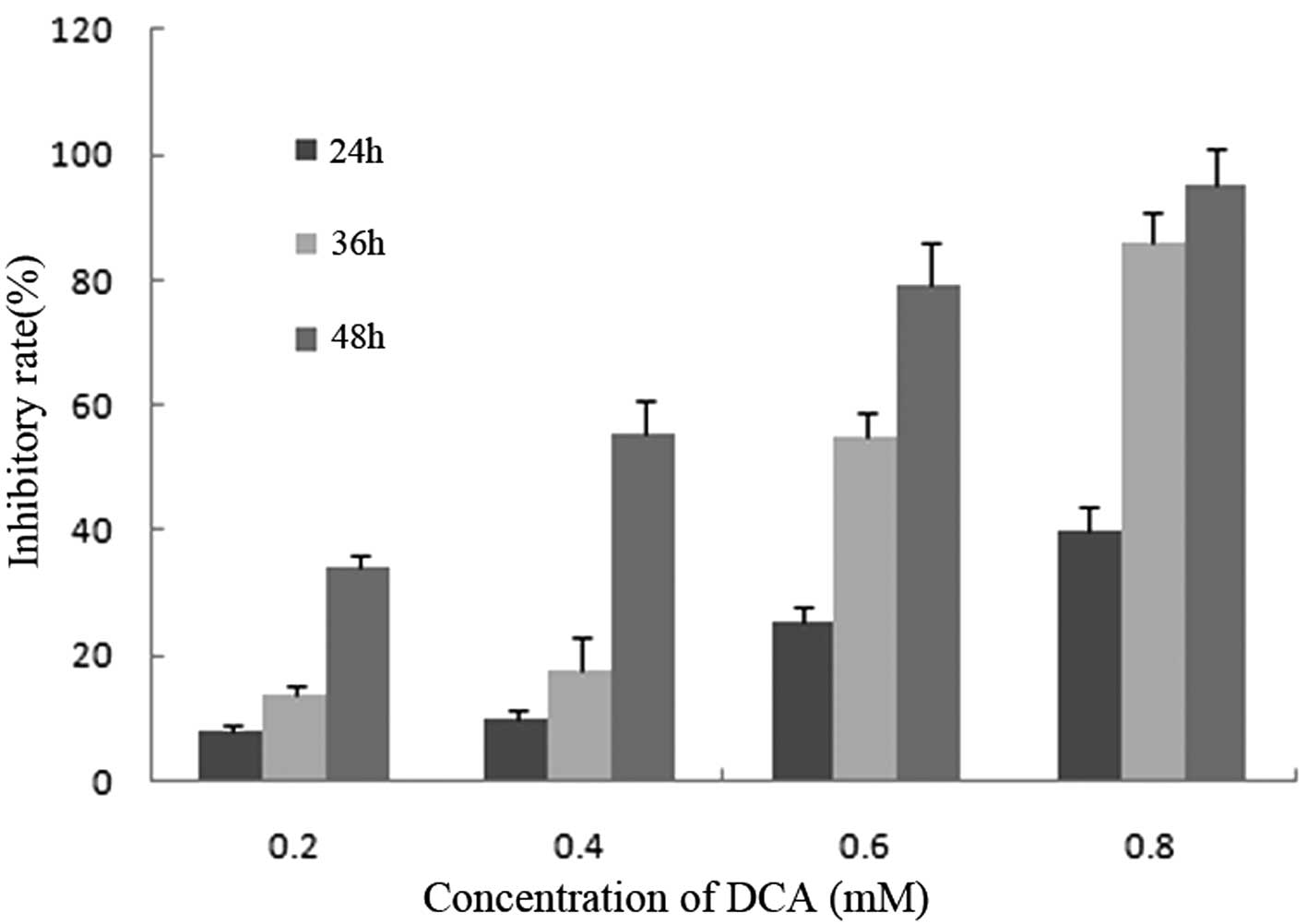
With DCA concentrations of 0.2, 0.4, 0.6 and 0.8 mM,
the proliferation of BGC-823 cells was significantly inhibited in a
dose- and time-dependent manner. Following treatment with various
concentrations of DCA the percentage of inhibition at 24 h were
7.89, 10.12, 25.56 and 40.12%, and at 48 h were 34.09, 55.22, 79.21
and 95.02%, with doses of 0.2, 04.4, 0.6 and 0.8 mM DCA,
respectively (Fig. 1).
Morphological changes of BGC-823 cells
following DCA treatment
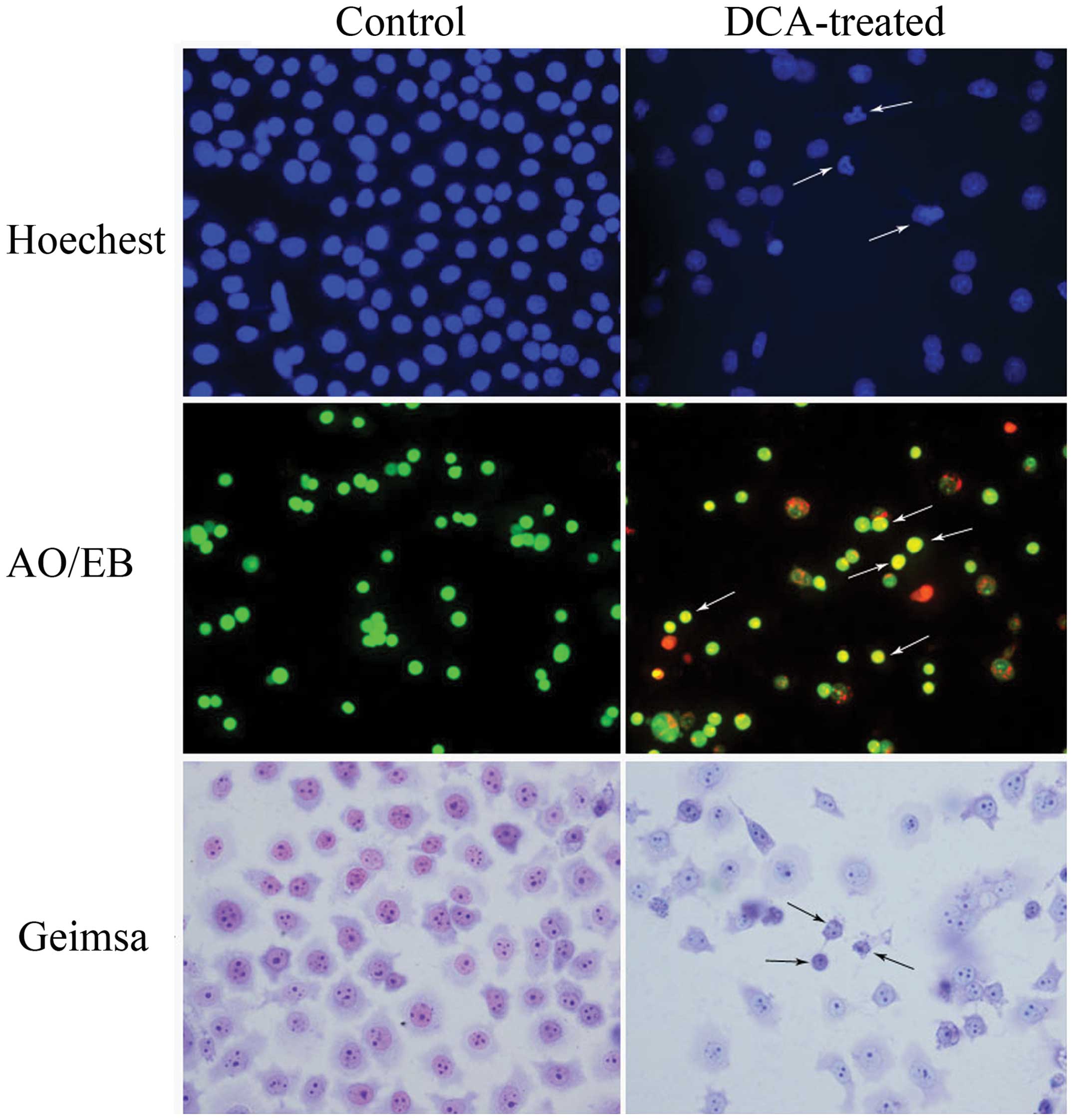
Hoechst 33258 staining showed that the nuclei of
cells in the control group exhibited a uniform dispersion of
low-intensity fluorescence and had an integral structure. However,
following treatment with 0.3 mM DCA, the nuclei exhibited pyknosis
or a granular distribution of fluorescence. In these cells, the
intensity of fluorescence was uneven and the nuclei appeared
deformed and horseshoe-shaped, in addition to other typical
characteristics of apoptosis, including condensed chromatin,
gradual disintegration of the nuclear membrane, and pyknotic
(shrunken and dark) nuclei (Fig.
2). The results of the AO/EB staining demonstrated that the
control BGC-823 cells appeared uniformly green, and contained
nuclei with apparently normal structures. However, the DCA-treated
cells appeared orange or red, the nuclei exhibited pyknosis and
there was cataclastic scattering (non-uniform fluorescence
distribution) in the cytoplasm.
Following Giemsa staining, BGC-823 cells exhibited
various morphological forms, such as epithelioid, round, and
irregular. When treated with a concentration of 0.3 mM DCA, BGC-823
cells were observed to undergo a significant morphological change
and appeared shrunken, with a small nucleus and chromatin
agglutination. Apoptotic cells were observed singly or in
groups.
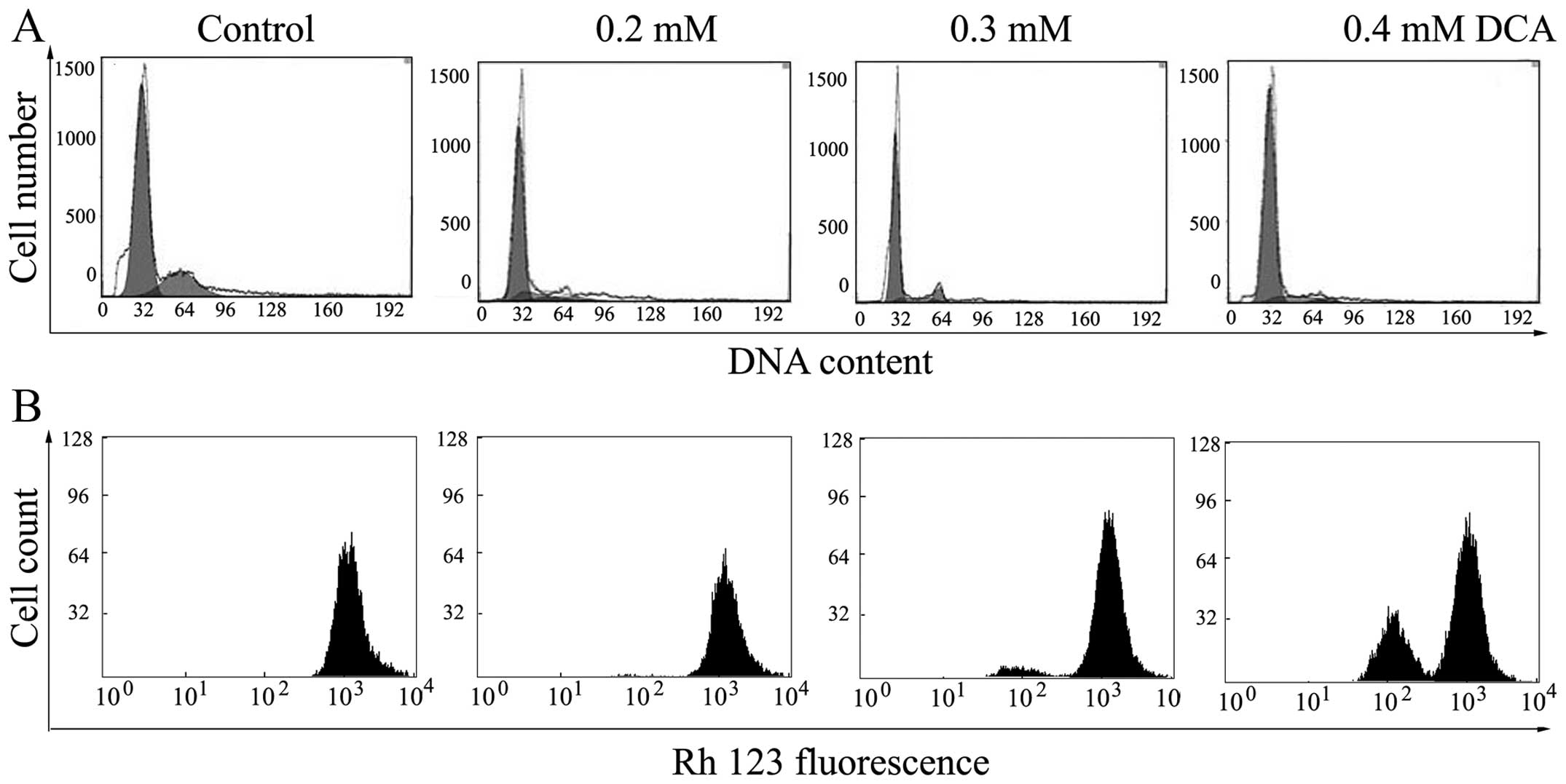
Flow cytometric analysis
Cell cycle progression in the BGC-823 cells was
analyzed using flow cytometry. The results showed that the cell
cycle distribution of BGC-823 cells changed markedly following
treatment with various concentrations of DCA (0.2, 0.3, 0.4 and 0.5
mM). The proportion of cells in G0/G1 phase
in the control group was 60.12, which increased to 88.35% following
treatment with 0.3 mM DCA, while the proportion of cells at
G2 phase decreased significantly from 25.16 to 4.98%.
These results indicate that the cell cycle was arrested in
G0/G1 phase following treatment with DCA.
Analysis of ΔΨm
In order to explore whether mitochondrial damage is
involved in DCA-induced apoptosis, the increase of Rh123
hyperfluorescence that occurs with a decrease in membrane
potential, was used to investigate the changes in ΔΨm following
treatment with DCA. As shown in Fig.
3B, a significant dose-dependent increase in the mean
fluorescence intensity of the cells, associated with collapse of
the ΔΨm, were observed in the BGC-823 cells following 48 h of
treatment with DCA.
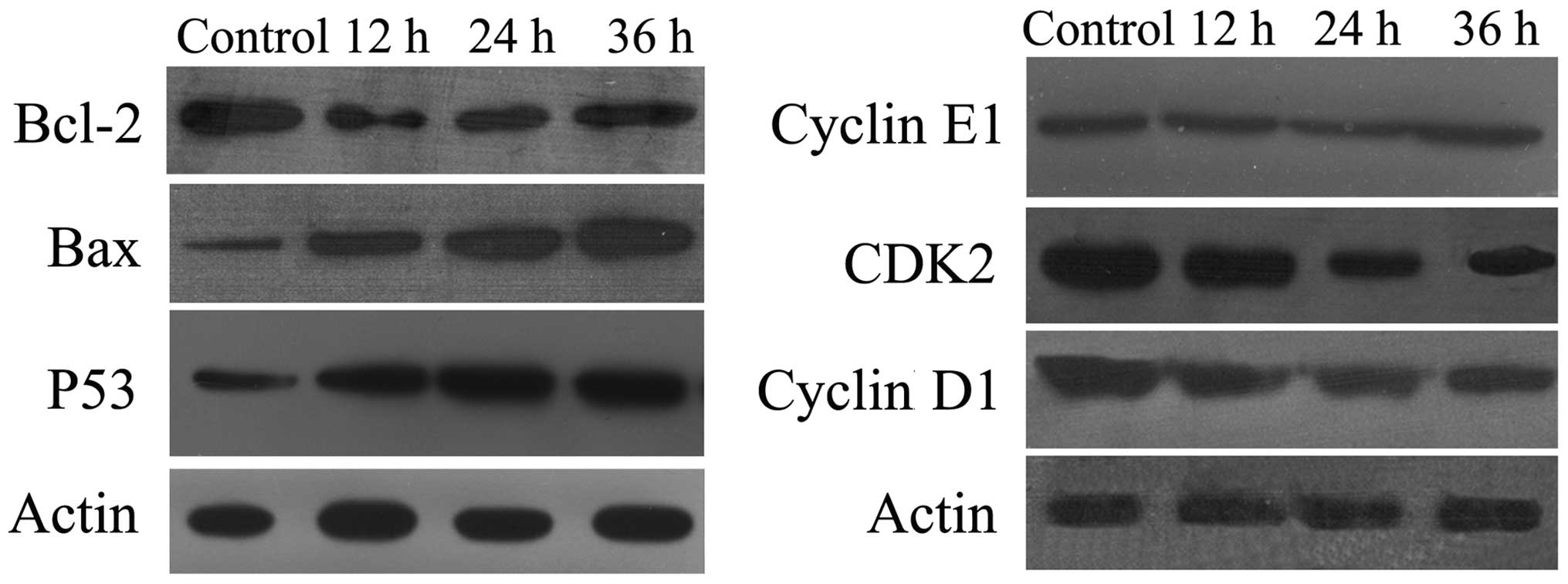
Western blot analysis
To investigate the mechanisms underlying the
induction of apoptosis in BGC-823 cells by DCA, the levels of
expression of apoptosis-related proteins, including p53, Bax,
Bcl-2, CKD2, Cyclin D1 and Cyclin E1 were measured. The
tumor-suppressor protein, p53, regulates apoptosis through the
transcriptional activation of its target genes, and acts as a key
facilitator of cross-talk between numerous pathways. Following
treatment with DCA, the level of the p53 protein was significantly
increased (Fig. 4A). As the
proteins of the Bcl-2 family are known to regulate the
mitochondrial pathway by controlling the permeability of the outer
mitochondrial membrane during apoptosis, the effect of DCA on the
expression of Bcl-2 and Bax was investigated. As shown in Fig. 4A, following exposure to DCA for 48
h, Bcl-2 expression was diminished while Bax expression was
elevated. Therefore, the Bcl-2:Bax ratio was markedly decreased in
DCA-treated BGC-823 cells. Furthermore, the levels of cyclin D1 and
CDK2 decreased in DCA-treated BGC-823 cells in a dose-dependent
manner (Fig. 4B). These results
suggest that p53 is involved in the induction of apoptosis by
DCA.
Discussion
Over recent years, it has been shown that DCA
induces apoptosis in a number of types of human malignancies
(14,15). The results from the present study
indicated that DCA significantly inhibits the proliferation of
BGC-823 cells in a dose-dependent manner and induces apoptosis via
activation of the mitochondrial pathway. p53 was also shown to be
involved in the DCA-mediated apoptosis of BGC-823 cells.
Apoptosis was initially described according to its
morphological characteristics, and morphology remains a relevant
experimental method to demonstrate the occurrence of this process.
Morphological features characteristic of apoptosis were observed in
BGC-823 cells following exposure to DCA, including cell shrinkage,
chromatin agglutination, marginalization, nuclear fragmentation and
apoptotic body formation. An important feature of apoptosis is the
permeabilization of mitochondria. Mitochondria are known to be an
important part of apoptotic signaling. DCA treatment led to a
concentration-dependent reduction in mitochondrial membrane
potential. This drop in ΔΨm produces changes in mitochondrial
biogenesis and activity, decreasing the numbers of mitochondria and
influencing the stability of mitochondrial function.
There are several checkpoints during the cell cycle.
The most important points are the G1/S phase transition
and the G2/M phase transition. Healthy cells replicate
during S phase and reduce in number during G1 phase. By
contrast, tumor cells are able to bypass the checkpoint of the
G1/S and G2/M phase transition points,
allowing cells to proliferate without restraint. Therefore, the
genes controlling the G1/S or G2/M phase
transitions are potential targets for the development of antitumor
drugs (16). To determine whether
the toxicity of DCA in BGC-823 cells is due to the induction of
cell cycle arrest, the cell cycle phase distribution of the treated
cells was examined by flow cytometry. The results showed that
BGC-823 cells were arrested at the G1 phase when treated
with DCA. The ratio of cells in the G1 phase was
positively correlated with the duration and concentration of DCA
administered, which indicates that the inhibition of gastric cancer
cell growth by DCA may be due to G1 phase arrest.
Furthermore, following treatment of BGC-823 cells with DCA, the
expression of Cyclin D1 and CDK2 proteins was downregulated.
The mechanism underlying DCA-induced apoptosis in
BGC-823 cells is unclear. The intrinsic apoptosis pathway is a
mitochondrial-mediated cell death process, which is regulated by
the Bcl-2 family of proteins that comprises proapoptotic proteins,
such as Bax, Bak, Bad and Bid, and antiapoptotic proteins, such as
Bcl-2 and Bcl-XL. Activation of the intrinsic pathway is often
associated with a change in the level of Bax:Bcl-2 expression or
the ratio of these proteins (17).
In the present study, the expression of Bax was shown to be
upregulated, while the expression of Bcl-2 was downregulated. The
ratio of Bax:Bcl-2 was increased. This rise in the Bax:Bcl-2 ratio
suggested a commitment of BGC-823 cells to apoptosis via activation
of the mitochondria-dependent pathway.
A study demonstrated that almost 50% of human tumors
carry p53 mutations, and that additional mechanisms are able to
disrupt wild-type p53 function (18). Although a number of studies have
shown that apoptosis may be induced in a p53-independent manner
(19–26), other studies have reported that p53
is actively required for apoptosis to occur (24,27,28).
The action of p53 has been shown to operate predominantly through
induction of apoptosis in cells via the intrinsic pathway (29,30).
Therefore, it is important to further investigate this process and
to identify whether the activation of p53 is involved in the
mechanisms underlying the induction of apoptosis in BGC-823 cells.
The results from the current study demonstrated that p53 was active
during DCA-induced apoptosis, and suggested that P53 may have a
central role in the apoptosis of BGC-823 cells.
In conclusion, the present study demonstrated that
DCA significantly inhibited proliferation and arrested cell cycle
progression at the G0/G1 phase in BGC-823
cells. The results indicated that DCA-induced apoptosis of BGC-823
cells occurs via activation of the mitochondrial pathway, in which
p53 is involved. An improved understanding of the way in which DCA
regulates cell death may provide insight into potential anticancer
mechanisms, and aid in the selection of novel natural compounds for
screening and ultimately the development of cancer treatments.
Acknowledgements
This work was supported by grants from National
Natural Science Foundation of China (81402309); Foundation of Henan
Educational Committee (13A180044); and Henan Planning Project of
Science and Technology (132102310118).
References
|
1
|
Baan R, Grosse Y, Straif K, et al: A
review of human carcinogens-part F: chemical agents and related
occupations. Lancet Oncol. 10:1143–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El Ghissassi F, Baan R, Straif K, et al: A
review of human carcinogens-part D: radiation. Lancet Oncol.
10:751–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai HC, Lin FC, Chen YC and Chang SC: The
role of total bile acid in oral secretions in ventilator-associated
pneumonia. J Crit Care. 27:526 e1–e6. 2012. View Article : Google Scholar
|
|
4
|
Charach G, Rabinovich A, Argov O,
Weintraub M and Rabinovich P: The role of bile acid excretion in
atherosclerotic coronary artery disease. Int J Vasc Med.
2012:9496722012.
|
|
5
|
Park WI, Park MJ, An JK, et al: Bile acid
regulates c-jun expression through the orphan nuclear receptor SHP
induction in gastric cells. Biochem Biophys Res Commun.
369:437–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keitel V, Donner M, Winandy S, Kubitz R
and Haussinger D: Expression and function of the bile acid receptor
TGR5 in kupffer cells. Biochem Biophys Res Commun. 372:78–84. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krishna-Subramanian S, Hanski ML,
Loddenkemper C, et al: UDCA slows down intestinal cell
proliferation by inducing high and sustained ERK phosphorylation.
Int J Cancer. 130:2771–2782. 2012. View Article : Google Scholar
|
|
8
|
Dosa PI, Ward T, Castro RE, Rodrigues CM
and Steer CJ: Synthesis and evaluation of water-soluble prodrugs of
ursodeoxycholic acid (UDCA), an anti-apoptotic bile acid. Chem Med
Chem. 8:1002–1011. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng MF, Shen SY and Huang WD: DCA
increases the antitumor effects of capecitabine in a mouse B16
melanoma allograft and a human non-small cell lung cancer A549
xenograft. Cancer Chemother Pharmacol. 72:1031–1041. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha YH and Park DG: Effects of DCA on cell
cycle proteins in colonocytes. J Korean Soc Coloproctol.
26:254–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng H, Botnen JH and Briske-Anderson M:
Deoxycholic acid and selenium metabolite methylselenol exert common
and distinct effects on cell cycle, apoptosis, and MAP kinase
pathway in HCT116 human colon cancer cells. Nutr Cancer. 62:85–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yui S, Kanamoto R and Saeki T: Deoxycholic
acid can induce apoptosis in the human colon cancer cell line
HCT116 in the absence of Bax. Nutr Cancer. 60:91–96. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song W, Shen DY, Kang JH, et al: Apoptosis
of human cholangiocarcinoma cells induced by ESC-3 from Crocodylus
siamensis bile. World J Gastroenterol. 18:704–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ignacio Barrasa J, Olmo N, Perez-Ramos P,
et al: Deoxycholic and chenodeoxycholic bile acids induce apoptosis
via oxidative stress in human colon adenocarcinoma cells.
Apoptosis. 16:1054–1067. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higuchi H, Bronk SF, Takikawa Y, et al:
The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5
expression and apoptosis. J Biol Chem. 276:38610–38618. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Yang W, Shi C, et al: The G1 phase
arrest and apoptosis by intrinsic pathway induced by valproic acid
inhibit proliferation of BGC-823 gastric carcinoma cells. Tumour
Biol. 32:335–346. 2011. View Article : Google Scholar
|
|
17
|
Gao LW, Zhang J, Yang WH, Wang B and Wang
JW: Glaucocalyxin A induces apoptosis in human leukemia HL-60 cells
through mitochondria-mediated death pathway. Toxicology in vitro.
25:51–63. 2011. View Article : Google Scholar
|
|
18
|
Wiman KG: Strategies for therapeutic
targeting of the p53 pathway in cancer. Cell Death Differ.
13:921–926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Jiang Y and Yang J:
p53-independent signaling pathway in DNA damage-induced cell
apoptosis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 42:217–223. 2013.(In
Chinese). PubMed/NCBI
|
|
20
|
Yerlikaya A, Okur E and Ulukaya E: The
p53-independent induction of apoptosis in breast cancer cells in
response to proteasome inhibitor bortezomib. Tumour Biol.
33:1385–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abeysinghe RD, Greene BT, Haynes R, et al:
p53-independent apoptosis mediated by tachpyridine, an anti-cancer
iron chelator. Carcinogenesis. 22:1607–1614. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arita D, Kambe M, Ishioka C and Kanamaru
R: Induction of p53-independent apoptosis associated with G2M
arrest following DNA damage in human colon cancer cell lines. Jpn J
Cancer Res. 88:39–43. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cattaneo-Pangrazzi RM, Schott H,
Wunderli-Allenspach H, Rothen-Rutishauser B, Guenthert M and
Schwendener RA: Cell-cycle arrest and p53-independent induction of
apoptosis in vitro by the new anticancer drugs 5-FdUrd-P-FdCydOct
and dCydPam-P-FdUrd in DU-145 human prostate cancer cells. J Cancer
Res Clin Oncol. 126:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyake N, Chikumi H, Takata M, Nakamoto M,
Igishi T and Shimizu E: Rapamycin induces p53-independent apoptosis
through the mitochondrial pathway in non-small cell lung cancer
cells. Oncol Rep. 28:848–854. 2012.PubMed/NCBI
|
|
25
|
Aimola P, Carmignani M, Volpe AR, et al:
Cadmium induces p53-dependent apoptosis in human prostate
epithelial cells. PLoS One. 7:e336472012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watson JL, Hill R, Yaffe PB, et al:
Curcumin causes superoxide anion production and p53-independent
apoptosis in human colon cancer cells. Cancer Lett. 297:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saha S, Hossain DM, Mukherjee S, et al:
Calcarea carbonica induces apoptosis in cancer cells in
p53-dependent manner via an immuno-modulatory circuit. BMC
Complement Altern Med. 13:2302013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoeferlin LA, Fekry B, Ogretmen B,
Krupenko SA and Krupenko NI: Folate stress induces apoptosis via
p53-dependent de novo ceramide synthesis and up-regulation of
ceramide synthase 6. J Biol Chem. 288:12880–12890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sznarkowska A, Olszewski R and
Zawacka-Pankau J: Pharmacological activation of tumor suppressor,
wild-type p53 as a promising strategy to fight cancer. Postepy Hig
Med Dosw (Online). 64:396–407. 2010.(In Polish).
|
|
30
|
Jedrych M, Wawryk-Gawda E,
Jodlowska-Jedrych B, Chylinska-Wrzos P and Jasinski L:
Immunohistochemical evaluation of cell proliferation and apoptosis
markers in ovarian surface epithelial cells of cladribine-treated
rats. Protoplasm. 250:1025–1034. 2013. View Article : Google Scholar
|