Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignant tumor worldwide, and is the third leading
cause of cancer-associated mortality, after lung and colon cancer
(1–4). It is one of the most aggressive human
malignancies and previous data have shown that the five-year
survival rate is poor (5). The
most effective therapy for HCC is usually surgical resection or
liver transplantation; however, in ~70% of patients surgery is
unsuitable due to tumor metastasis or liver cirrhosis (6). The standard therapy for HCC is
currently chemotherapy, particularly when surgical resection is not
suitable (7). However, the current
therapeutic options for HCC are not effective, since resistance and
tumor relapse eventually develop (8). Novel therapeutic strategies, such as
effective chemotherapy with low toxicity, are required, in order to
decrease the incidence and improve the prognosis of patients with
HCC.
Flavonoids are plant polyphenols that are well-known
for their analgesic, physiological antipyretic and
anti-inflammatory activities. Flavonoids have recently gained
attention due to their antitumor activity (9–11).
Icaritin is a prenylflavonoid and is a hydrolytic product of
icaritin, derived from Herba Epimedii. Icaritin exhibits
various pharmacological and biological activities, including
antirheumatic and antidepressant effects, stimulation of cardiac
and neuronal differentiation (12–13),
prevention of steroid-associated osteonecrosis (14), and inhibition of growth and
induction of apoptosis of PC-3 human prostate carcinoma and MCF-7
breast cancer cells (15–16).
It has recently been demonstrated that icaritin
induces apoptosis of breast and endometrial cancer cells, through
the mitochondrial pathway (17,18).
However, whether icaritin may trigger the extrinsic pathway, which
is mediated by death receptors, remains unclear. The present study
aimed to investigate the anticancer activities of icaritin in
SMMC-7721 cells, and to identify the underlying mechanisms.
Materials and methods
Drugs and reagents
Icaritin (purity, >98%; Fig. 1A) was purchased from Yousi
Biotechnology Co., Ltd. (Shanghai, China). Dulbecco’s modified
Eagle’s medium (DMEM), RPMI-1640 medium and newborn calf serum were
purchased from Gibco Life Technologies (Carlsbad, CA, USA). An
Annexin V-fluorscein isothiocyanate (FITC) Apoptosis Detection kit
was purchased from Bipec Biopharma Inc. (Cambridge, MA, USA); and a
Bicinchoninic Acid (BCA) Protein Quantitation kit was purchased
from GENMED Scientifics Inc., USA (Shanghai, China). Primary
antibodies targeting β-actin (rabbit polyclonal antibody, 4967S),
cleaved caspase-3 (rabbit monoclonal antibody, Asp175) and cleaved
caspase-8 (mouse monoclonal antibody, Asp387) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Primary
antibodies targeting Bax (mouse monoclonal antibody, sc-7480) and
extrinsic signal-regulated Fas (rabbit polyclonal antibody,
sc-7886) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). All of the other chemicals and solvents used in
the present study were of the highest commercially available
grade.
Cell culture and icaritin treatment
L02 human liver cells and SMMC-7721 human HCC cells
were purchased from Nanjing KeyGen Biotech., Co., Ltd. (Nanjing,
China). The cells were cultured in DMEM supplemented with 10%
newborn calf serum at 37°C in a humidified atmosphere containing 5%
CO2.
Stock solution of icaritin, which could be further
diluted with cell culture medium, was prepared in dimethyl
sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) to a
concentration of 10 mM, and maintained at −20°C. The final
concentration of DMSO was <0.1% in culture.
Cell proliferation assay
Cell proliferation was analyzed by an 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
(Sigma-Aldrich). The L02 and SMMC-7721 cells were seeded into
96-well plates, at a density of 5×103 cells/200 μl/well
and incubated at 37°C in a humidified 5% CO2 incubator.
The cells were then treated with 0, 5, 10, 20, 40 or 60 μmol/l
icaritin for 12, 24 or 48 h, and the plates were returned to
standard tissue incubator conditions for an additional 4 h. The
media was removed and the cells were solubilized in 150 μl DMSO.
The color intensity of formazan was measured at a wavelength of 490
nm, using an automated microplate spectrophotometer (iMark,
Bio-Rad, Hercules, CA, USA). The survival rate of the cells was
calculated using the following formula: (OD value of the treated
group/OD value of untreated group) × 100%. Where OD is the optical
density. The assays were performed in triplicate, in three
independent experiments.
Cell apoptosis assay by flow cytometry
(FCM)
The cells were stained with Annexin V-FITC/propidium
iodide (PI) and measured using a FACSCalibur™ flow cytometer (BD
FACSCanto™ II Flow Cytometer, BD Biosciences, Franklin Lakes, NJ
USA). The samples were washed twice, and adjusted to a
concentration of 1×106 cells/ml, with phosphate-buffered
saline (PBS). A total of 200 μl of cell suspension was added to
each tube, 5 μl Annexin V-FITC and 10 μl PI were added to the
labeled tubes, and the samples were incubated for 15 min at room
temperature in the dark. Subsequently, 200 μl PBS binding buffer
was added to each tube and the samples were analyzed by FCM
analysis (BD Biosciences) as soon as possible. The results were
analyzed using FlowJo software (version 6.1.3, FlowJo, LLC,
Ashland, OR, USA). The rate of apoptosis was calculated as the
relative number of apoptotic cells, as compared with the total
number of cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cells were frozen in liquid nitrogen and stored
at −80°C, until further use. Bcl-2, Bax and Fas mRNA expression
levels were quantified by RT-qPCR using a Reverse Transcription
system (Promega Corporation, Madison, WI, USA). The reaction was
conducted at 42°C for 60 min.
Total cellular RNA was extracted using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA), according to the manufacturer’s instructions. The primer
sequences used were as follows: Sense: 5′-CCACCAAGAAAGCAGGAAAC-3′
and antisense: 5′-GCAGGATAGCAGCACAGGA-3′ for Bcl-2; sense:
5′-CTGAGCTGACCTTGGAGC-3′ and antisense: 5′-GACTCCAGCCACAAAGATG-3′
for Bax; sense: 5′-AGCTTGGTCTAGAGTGAAAA-3′ and antisense:
5′-GAGGCAGAATCATGAGATAT-3′ for Fas; and sense
5′-CCTCTATGCCAACACAGTGC-3′ and antisense 5′-GTACTCCTGCTTGCTGATCC-3′
for β-actin. The mRNA expression levels of Bcl-2 and Bax were
analyzed using one-step RT-qPCR, with RNA-direct™ SYBR®
Green Realtime PCR Master mix (Toyobo Co., Ltd., Osaka, Japan),
according to the manufacturer’s instructions. RNA (2 μl) was
amplified under the following conditions: 95°C for 5 min for
denaturation followed by 40 cycles of 95°C for 30 sec, 57°C for 45
sec and 72°C for 1 min. The amplification was monitored on an ABI
Prism 7500 Real-Time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA).
Western blot analysis
The SMMC-7721 cells were incubated with 8, 16 or 32
μM icaritin for 24 h. The cells were then lysed with ice-cold lysis
buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl, 1 mM MgCl2,
100 μg/ml PMSF and 1% Triton X-100) for 30 min on ice. The total
proteins were contained within the supernatant following
centrifugation at 13,225 × g for 5 min at 4°C, and the protein
concentrations were measured using a BCA assay (GENMED Scientifics
Inc.). Equal quantities (40 μg) of lysate protein were separated on
10% SDS-PAGE gels, and electrophoretically transferred onto
polyvinylidene fluoride membranes (Pall Corporation, East Hills,
NY, USA). The membranes were then blocked with 5% non-fat dry milk
in TBST buffer (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.05%
Tween®-20) for 2 h at room temperature, and probed with
1:1,000 dilutions of the primary antibodies described above, at 4°C
overnight. Subsequently, the membranes were incubated with 1:5,000
dilutions of horseradish peroxidase-conjugated secondary antibody
to mouse (Sigma-Aldrich, for the detection of Bax or cleaved
caspase-8) or rabbit (Santa Cruz Biotechnology, Inc., for the
detection of cleaved caspase-3, Fas and β-actin). Protein bands
were visualized using an Enhanced Chemiluminescence Detection
system (ChemiDoc, Bio-Rad). Band intensity was quantified using
BandScan 5.0 software (Glyko, Hayward, CA, USA). All of the western
blots were performed at least three times.
Statistical analysis
Statistical analyses were performed using SPSS
version 16.0 software (SPSS Inc., Chicago, IL, USA). Each assay was
performed at least three times. Data were expressed as the mean ±
standard deviation. Student’s t-test and one-way analysis of
variance were used to determine statistically significant
differences between the data. P<0.05 was considered to indicate
a statistically significant difference.
Results
Icaritin exhibits growth inhibitory
activity on hepatoma cells, whereas the same dose causes little
adverse effect on normal human liver cells
Treatment with icaritin significantly inhibited the
growth of SMMC-7721 cells. The half maximal inhibitory
concentration (IC50) of icaritin in the SMMC-7721 cells,
which underwent treatment for 24 h, was 9.6 μM (Table I). The rate of survival of the
SMMC-7721 cells decreased in response to an increasing icaritin
concentration (0–60 μM), and this effect was time- and
dose-dependent (Fig. 1B).
Treatment with the same concentration of icaritin caused little
effect on the survival of the L02 normal human liver cells
(Fig. 1C). These results indicate
that icaritin exerts a growth inhibitory activity on hepatoma
cells, whereas the same dose did not have the same effect on normal
human liver cells.
 | Table IHalf maximal inhibitory concentration
(IC50) of icaritin in different cell lines. |
Table I
Half maximal inhibitory concentration
(IC50) of icaritin in different cell lines.
| Cell line | IC50 of
icaritin (24 h) |
|---|
| LO2 | 71.6±4.4 |
| SMMC-7721 | 9.6±0.8 |
Icaritin induces significant apoptosis of
SMMC-7721 cells in a time- and dose-dependent manner
Treatment with icaritin markedly induced apoptosis
in the SMMC-7721 human hepatoma cells (Fig. 2A). The rate of apoptosis of the
SMMC-7721 cells was 3.0±0.3, 7.8±0.9 and 56.5±6.5%, in response to
treatment with 8, 16 and 32 μM icaritin, respectively. However,
0–32 μM icaritin did not trigger significant levels of apoptosis in
the L02 human normal hepatocyte cells (Fig. 2B and C). In addition, icaritin
induced an increased rate of apoptosis in a dose-dependent manner,
when the time of treatment was fixed (Fig. 2D). These results suggest that
treatment with icaritin markedly induced apoptosis of SMMC-7721 HCC
cells in a time- and dose-dependent manner.
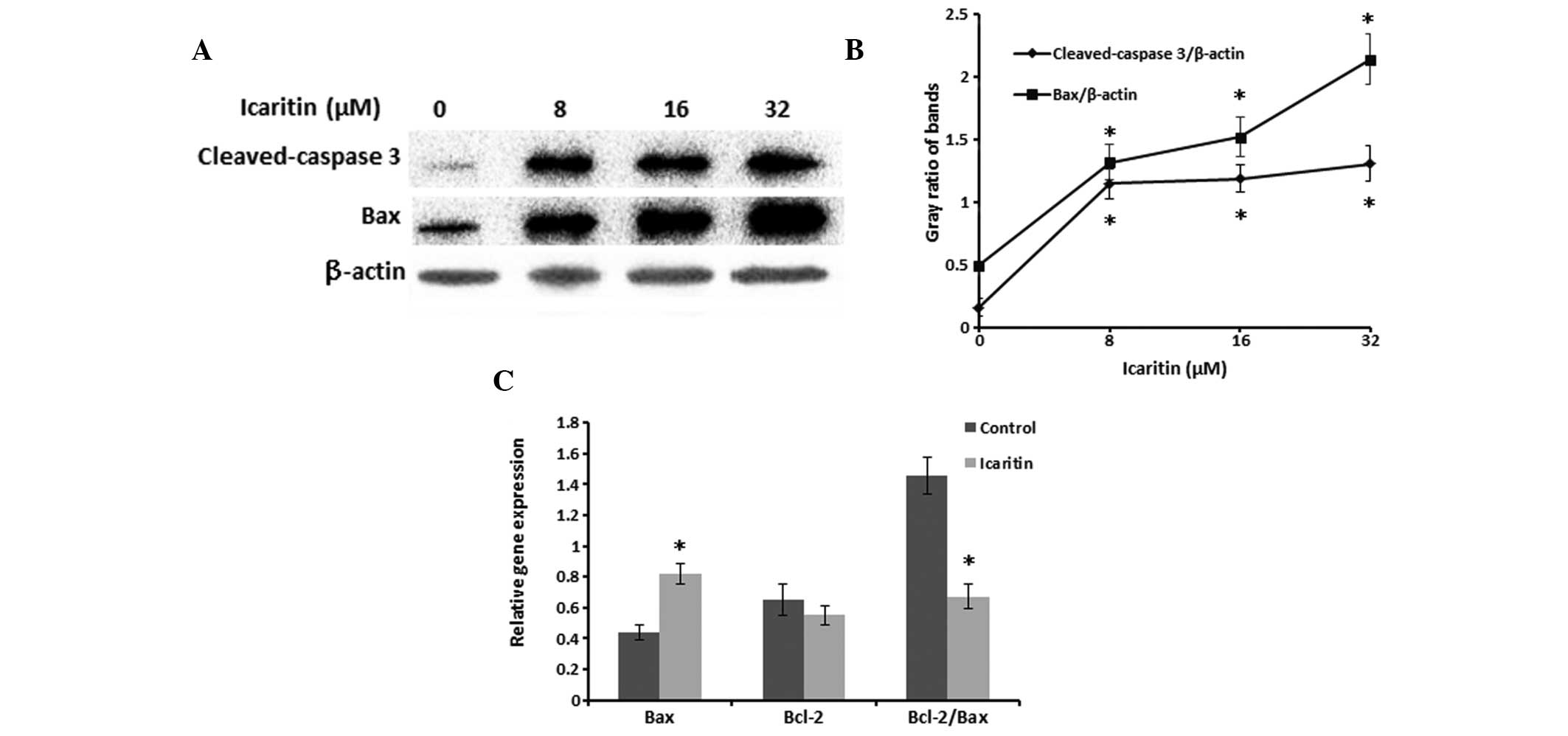
Icaritin upregulates the protein
expression levels of Bax and cleaved caspase-3 and effects gene
expression in SMMC-7721 cells
Treatment of the SMMC-7721 cells with various
concentrations of icaritin for 24 h significantly increased the
protein expression levels of Bax and cleaved caspase-3 (Fig. 3A). The protein expression levels of
Bax and cleaved caspase-3, at the mitochondrial membrane, were
significantly increased in response to treatment with icaritin, in
a dose-dependent manner (Fig. 3B).
Furthermore, treatment with icaritin decreased Bcl-2 and increased
Bax mRNA expression levels, resulting in a decreased Bcl-2/Bax
ratio (Fig. 3C).
Effects of icaritin on SMMC-7721
apoptosis, by the extrinsic pathway
The results of the present study indicate that
icaritin may trigger the mitochondrial pathway of apoptosis in
SMMC-7721 cells (Fig. 3). The
present study also aimed to determine whether icaritin could induce
apoptosis through the death receptor pathway. The protein
expression levels of Fas were increased following treatment with
icaritin (Fig. 4A). Treatment of
the SMMC-7721 cells with 32 μM icaritin for 24 h also induced
cleaved caspase-8 protein expression. Icaritin also upregulated the
mRNA expression levels of Fas in the SMMC-7721 cells (Fig. 4B).
Discussion
HCC is the third most common cause of
cancer-associated mortality, with ~600,000 fatalities worldwide
(19). The most effective therapy
for HCC is surgical resection; however, <15% of patients can
benefit from this treatment, due to the presence of numerous tumor
nodules. Until recently, chemotherapy for patients with HCC was
toxic and not particularly effective. Thus emphasizing the
requirement for further research into the molecular mechanisms of
HCC development, and identification of non-cytotoxic and effective
antineoplastic compounds for chemoprevention and treatment.
Recently, more traditional herbal medicines are being considered
for use as anticancer treatments (20–22).
However, few anticancer agents with low toxicity have been
identified to be safe and effective for the treatment of HCC. It
has previously been demonstrated that certain Chinese natural
ingredients, or herbal formulas, have preventive and therapeutic
effects against cancer. In particular, the development of icaritin
has gained attention as an antifibrotic and antineoplastic monomer,
purified from the herb Epimedium (23–25).
The present study demonstrated that icaritin may inhibit growth and
significantly induce apoptosis in SMMC-7721 cells, with an
effective concentration range between 8 and 32 μM. The
icaritin-induced growth inhibition and apoptosis were time- and
dose-dependent. Furthermore, icaritin at the concentrations
mentioned above, hardly affected the growth and apoptosis of L02
non-tumor human hepatocyte cells. These results suggest that
icaritin may possess a selective antitumor action.
The present study also investigated the underlying
mechanisms of icaritin-induced apoptosis of HCC cells. The
expression of a number of genes, including Bcl-2, Bax and Caspase-3
have been revealed to be altered following treatment with icaritin,
but the study, which demonstrated this was focussed on the
mitochondrial pathway (26). Bax
and Bcl-2 family members are critical regulators of mitochondrial
function (27–30). They may activate or inhibit the
release of cytochrome c, which leads to the activation of
caspase-3 in the process of apoptosis. Bax exerts pro-apoptotic
activity by translocation from the cytosol to the mitochondria, and
induces the release of cytochrome c; whereas Bcl-2 exerts
anti-apoptotic activity by inhibiting the translocation of Bax to
the mitochondria (31). The
Bcl-2/Bax ratio is correlated with the extent of apoptosis, and is
a crucial factor in the induction of apoptosis (32). In the present study, treatment with
icaritin resulted in increased Bax and decreased Bcl-2 expression
levels, leading to a decreased Bcl-2/Bax ratio. Caspase-3 protein
was cleaved and cleaved caspase-3 expression levels were shown to
be increased. The alterations to the Bcl-2/Bax ratio and the
expression of cleaved caspase-3, in response to treatment with
icaritin, were dose-dependent.
Mitochondria have a pivotal role in the signal
transduction of apoptosis (33);
however, activation of the mitochondrial pathway is not the only
mechanism by which icaritin may induce the apoptosis of HCC cells.
The extrinsic pathway is mediated by death receptors, and involves
Fas and the binding and activation of caspase-8 (34–36).
It has previously been reported that the engagement of Fas may
promote the formation of the death-inducing signaling complex,
resulting in activated T cell apoptosis (37). The Fas ligand activates caspase-8,
following interaction with the Fas receptor (38). Activation of caspase-8 may lead to
the activation of downstream effector proteases, such as caspase-3,
resulting in apoptosis (39–40).
The present study showed that the expression levels of Fas were
significantly increased following treatment with icaritin and the
protein expression levels of cleaved caspase-8 were detected
following treatment with 32 μM icaritin. These results demonstrate
that increased apoptosis may be achieved through the extrinsic
pathway in SMMC-7721 cells treated with icaritin.
The results of the present study indicate that
icaritin may inhibit growth and induce apoptosis in SMMC-7721
cells. These effects were dose-dependent and not observed in L02
normal hepatocyte cells. The underlying mechanisms of
icaritin-induced apoptosis were also examined. The results of the
present study indicate that icaritin-induced apoptosis involves the
intrinsic mitochondria and extrinsic death receptor pathways. Fas
activation was shown to be required for icaritin-induced apoptosis.
These data suggest that icaritin may be developed as a promising
anticancer agent against human HCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81472831), the Medical Key
Talent Foundation of Jiangsu Province (grant no. RC2011081), the
Medical Key Science and Technology Development Projects of Nanjing
(grant no. ZKX11176) and the Science and Technology Development
Foundation of Nanjing Medical University of China (grant no.
2011NJMU150).
References
|
1
|
McKillop IH, Moran DM, Jin X and Koniaris
LG: Molecular pathogenesis of hepatocellular carcinoma. J Surg Res.
136:125–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trevisani F, Cantarini MC, Wands JR and
Bernardi M: Recent advances in the natural history of
hepatocellular carcinoma. Carcinogenesis. 29:1299–1305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kusakabe A, Tanaka Y, Orito E, et al: A
weak association between occult HBV infection and non-B non-c
hepatocellular carcinoma in Japan. J Gastroenterol. 42:298–305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Serag HB, Siegel AB, Davila JA, Shaib
YH, Cayton-Woody M, McBride R and McGlynn KA: Treatment and
outcomes of treating of hepatocellular carcinoma among Medicare
recipients in the United States: a population-based study. J
Hepatol. 44:158–166. 2006. View Article : Google Scholar
|
|
6
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plataniotis G and Castiglione M; ESMO
Guidelines Working Group. Endometrial cancer : ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 2:v41–v45. 2010. View Article : Google Scholar
|
|
8
|
Gehrig PA and Bae-Jump VL: Promising novel
therapies for the treatment of endometrial cancer. Gynecol Oncol.
116:187–194. 2010. View Article : Google Scholar
|
|
9
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
10
|
Li YL, Gan GP, Zhang HZ, Wu HZ, Li CL,
Huang YP, Liu YW and Liu JW: A flavonoid glycoside isolated from
Smilax china L. rhizome in vitro anticancer effects on human cancer
cell lines. J Ethnopharmacol. 113:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Díaz JG, Carmona AJ, Torres F, Quintana J,
Estévez F and Herz W: Cytotoxic activities of flavonoid glycoside
acetates from Consolida oliveriana. Planta Med. 74:171–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Wang H, Wu J, Zhu D, Zhang X, Ou
L, Yu Y and Lou Y: Enhanced co-expression of beta-tubulin III and
choline acetyltransferase in neurons from mouse embryonic stem
cells promoted by icaritin in an estrogen receptor-independent
manner. Chem Biol Interact. 179:375–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wo YB, Zhu DY, Hu Y, Wang ZQ, Liu J and
Lou YJ: Reactive oxygen species involved in prenylflavonoids,
icariin and icaritin, initiating cardiac differentiation of mouse
embryonic stem cells. J Cell Biochem. 103:1536–1550. 2008.
View Article : Google Scholar
|
|
14
|
Zhang G, Qin L, Sheng H, Wang XL, Wang YX,
Yeung DK, Griffith JF, Yao XS, Xie XH, Li ZR, Lee KM and Leung KS:
A novel semisynthesized small molecule icaritin reduces incidence
of steroid-associated osteonecrosis with inhibition of both
thrombosis and lipid-deposition in a dose-dependent manner. Bone.
44:345–356. 2009. View Article : Google Scholar
|
|
15
|
Huang X, Zhu D and Lou Y: A novel
anticancer agent, icaritin, induced cell growth inhibition, Gl
arrest and mitochondrial transmenbrane potential drop in human
prostate carcinoma PC-3 cells. Eur J Pharmacol. 564:26–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang ZQ and Lou YJ:
Proliferation-stimulating effects of icaritin and desmethylicaritin
in MCF-7 cells. Eur J Pharmacol. 504:147–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Y, Zhang X, Meng J and Wang ZY: An
anticancer agent icaritin induces sustained activation of the
extracellular signal-regulated kinase (ERK) pathway and inhibits
growth of breast cancer cells. Eur J Pharmacol. 658:114–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong JS, Zhang QH, Huang X, Fu XQ, Qi ST,
Wang YP, Hou Y, Sheng J and Sun QY: Icaritin causes sustained
ERK1/2 activation and induces apoptosis in human endometrial cancer
cells. PLoS One. 6:e167812011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou NN, Tang J, Chen WD, Feng GK, Xie BF,
Liu ZC, Yang D and Zhu XF: Houttuyninum, an active constituent of
Chinese herbal medicine, inhibits phosphorylation of HER2/neu
receptor tyrosine kinase and the tumor growth of
HER2/neu-overexpressing cancer cells. Life Sci. 90:770–775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang CZ, Calway T and Yuan CS: Herbal
medicines as adjuvants for cancer therapeutics. Am J Chin Med.
40:657–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ben-Arye E, Schiff E, Hassan E, Mutafoglu
K, Lev-Ari S, Steiner M, Lavie O, Polliack A, Silbermann M and Lev
E: Integrative oncology in the Middle East: from traditional herbal
knowledge to contemporary cancer care. Ann Oncol. 23:211–221. 2012.
View Article : Google Scholar
|
|
23
|
Li J, Liu P, Zhang R, Cao L, Qian H, Liao
J, Xu W, Wu M and Yin Z: Icaritin induces cell death in activated
hepatic stellate cells through mitochondrial activated apoptosis
and ameliorates the development of liver fibrosis in rats. J
Ethnopharmacol. 137:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Wu J, Chen X, Fortenbery N,
Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY and Wei S: Icariin
and its derivative, ICT, exert anti-inflammatory, anti-tumor
effects, and modulate myeloid derived suppressive cells (MDSCs)
functions. Int Immunopharmacol. 11:890–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Yuan L, Wang X, Zhang TL and Wang
K: Icaritin and its glycosides enhance osteoblastic, but suppress
osteoclastic, differentiation and activity in vitro. Life Sci.
81:832–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He J, Wang Y, Duan F, Jiang H, Chen MF and
Tang SY: Icaritin induces apoptosis of HepG2 cells via the JNK1
signaling pathway independent of the estrogen receptor. Planta Med.
76:1834–1839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Golbano JM, Lóppez-Aparicio P, Recio MN
and Pérez-Albarsanz MA: Finasteride induces apoptosis via Bcl-2,
Bcl-xL, Bax and caspase-3 proteins in LNCaP human prostate cancer
cell line. Int J Oncol. 32:919–924. 2008.PubMed/NCBI
|
|
28
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Loo G, Saelens X, van Gurp M,
MacFarlane M, Martin SJ and Vandenabeele P: The role of
mitochondrial factors in apoptosis: a Russian roulette with more
than one bullet. Cell Death Differ. 9:1031–1042. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Llambi F, Moldoveanu T, Tait SW,
Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP and Green DR:
A unified model of mammalian BCL-2 protein family interactions at
the mitochondria. Mol Cell. 44:517–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
32
|
Susnow N, Zeng L, Margineantu D and
Hockenbery DM: Bcl-2 family proteins as regulators of oxidative
stress. Semin Cancer Biol. 19:42–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tait SW and Green DR: Mitochondria and
cell death: outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamaguchi Y, Shiraki K, Fuke H, Inoue T,
Miyashita K, Yamanaka Y and Nakano T: Adenovirus-mediated
transfection of caspase-8 sensitizes hepatocellular carcinoma to
TRAIL- and chemotherapeutic agent-induced cell death. Biochim
Biophys Acta. 1763:844–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang SH, Chen LM, Yang WK and Lee JD:
Increased extrinsic apoptotic pathway activity in patients with
hepatocellular carcinoma following transarterial embolization.
World J Gastroenterol. 17:4675–4681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muppidi JR and Siegel RM:
Ligand-independent redistribution of Fas (CD95) into lipid rafts
mediates clonotypic T cell death. Nat Immunol. 5:182–189. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abd El-Ghany RM, Sharaf NM, Kassem LA,
Mahran LG and Heikal OA: Thymoquinone triggers anti-apoptotic
signaling targeting death ligand and apoptotic regulators in a
model of hepatic ischemia reperfusion injury. Drug Discov Ther.
3:296–306. 2009.PubMed/NCBI
|
|
39
|
Hyer ML, Shi R, Krajewska M, Meyer C,
Lebedeva IV, Fisher PB and Reed JC: Apoptotic activity and
mechanism of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic-acid and
related synthetic triterpenoids in prostate cancer. Cancer Res.
68:2927–2933. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Zhang Y, Zhang L, Yang X and Lv
Z: Lupeol, a dietary triterpene, inhibited growth, and induced
apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer
Invest. 27:163–170. 2009. View Article : Google Scholar : PubMed/NCBI
|