Introduction
As reported by the World Health Organization (WHO),
>50% of the global population use traditional medicines for the
prevention and treatment of diseases (1). This is primarily due to the high cost
of western medicines. Traditional medicine largely consists of the
use of plant extracts. The discovery of numerous effective
anticancer agents from plants may be credited, directly or
indirectly, to a history of use of the relevant plants in
traditional medicine. The first plant-derived agents to advance
into clinical use, the vinca alkaloids vinblastine and vincristine,
were isolated from the Madagascar periwinkle (Catharanthus
roseus G.Don), and are used in various cultures. Natural
products, of which the majority are plant-derived molecules, have
become an increasingly vital source of potent anticancer agents,
which have been demonstrated to be more effective and/or less toxic
than synthetic alternatives (2).
Natural products may become vital in the future for anticancer drug
discovery, as they are a potential source of compounds of vast
structural diversity and have been previously comprehensively
explored in the field of drug discovery, leading to great
successes. The majority of these were in the field of cancer
therapeutics, in which >60% of the approved drugs discovered in
recent decades have been obtained from a natural origin. Numerous
commonly applied anticancer agents, such as vincristine,
irinotecan, etoposide and paclitaxel, which represent a range of
structurally diverse anticancer drugs, are all plant-derived and
are vital components of chemotherapy (1–6).
Cancer is the second most common cause of mortality
worldwide, with a yearly increasing mortality rate despite
extensive research dedicated to the development of novel treatment
and prevention strategies (9).
Cancer develops through a multistep carcinogenesis process that
encompasses various cellular physiological systems, such as cell
signalling and apoptosis, thus making it a very complex disease.
The majority of currently used anticancer drugs, which have been
obtained by synthesis of novel compounds or are from natural
sources, are toxic to normal cells in addition to cancer cells and
thus have substantial harmful side effects. There is therefore a
continuous search for innovative chemotherapeutic drugs that act as
‘magic bullets’, specifically targeting cancer cells with minimal
damage to normal cells (10). This
ideal situation may be achievable by the induction of apoptosis in
cancer cells. Thus, apoptosis modulation may be a key factor in the
prevention and treatment of cancer. Recently, apoptosis induction
in cancer cells has been the focus for an innovative mechanism upon
which to base drug discovery (11–13).
Apoptosis is a mechanism of programmed cell death that is
characterized by highly organized biochemical processes that
eradicate injured or abnormal cells. Apoptosis serves a key
function as a protective mechanism against cancer, by removing
genetically damaged cells, or cells that have become cancerous.
When apoptosis is triggered in response to certain physiological
signals, a proteolytic cascade involving different caspases is
initiated in the suicidal cell. This cascade this leads to
activation of nucleases that initiate the degradation of
chromosomal DNA. This type of DNA fragmentation is considered a
hallmark of the apoptotic process (14,15).
Artemisia indica Willd. (of the family
Asteraceae) is a perennial herb that is indigenous to the western
Himalayas and China. It has traditionally been employed to
ameliorate chronic fever, dyspepsia and hepatobiliary ailments. The
leaves and flowering stems of A. indica have been reported
to be antihelminthic, antiseptic and antispasmodic. A previous
phytochemical study has led to the isolation of antimalarial
phytoconstituents from the crude methanol extract of A.
indica, including exiguaflavanone-A, exiguaflavanone-B,
maacklain and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxy benzofuran
(16,17).
Materials and methods
Plant material, preparation of extracts
and chromatography
The shade-dried aerial section of the Artemisia
indica plant (3.0 kg) collected from a local region of Gansu,
China (Specimen number: GNS-762) was subjected to chloroform
extraction three times. The solvent was evaporated in vacuo
to obtain a crude extract of 300 g. The obtained extract was
subjected to column chromatography over a silica-gel to obtain
compounds a (700 mg), b (2.1 g) and c (100 mg) using hexane-EtOAc
(Guoyao Chemical Co., Ltd., Shanghai, China) as an eluent with
increasing polarity of 20, 25 and 35%, respectively. Similarly, the
root section (1.5 kg) was shade-dried and macerated with
CHCl3 (Guoyao Chemical Co., Ltd.) to yield 100 g crude
extract, which upon fractionation, produced fraction I (7.4 g), II
(13.2 g) and III (17.3 g) with 10, 20 and 50% EtOAc, respectively.
Repeated column chromatography of fraction I yielded three more
compounds: D (50 mg), e (65 mg) and f (2.5 g), fraction II and III
yielded c (200 mg; also isolated from the shoot) and g (10.0 mg),
respectively. A number of the compounds were purified by
re-crystallization. The isolated compounds were characterized by
spectral techniques, such as 1nuclear magnetic resonance
(NMR), 13Carbon (C)-NMR, distortionless enhancement by
polarization transfer (DEPT)-NMR (all using a Bruker AV III NM
Spectrometer; Bruker Scientific Technology Co., Ltd., Beijing,
China) and liquid chromatography–mass spectrometry (LC-MS) using an
Agilent 1200 system (Agilent Technologies, Waldbronn, Germany)
coupled with a Bruker micro QTOF mass spectrometer (Bruker
Daltonics, Ettlingen, Germany).
Chemicals used for cytotoxicity
assay
Growth medium RPMI-1640, minimum essential medium
and fetal calf serum (FCS) were obtained from Gibco-BRL (Carlsbad,
CA, USA) and trypsin, penicillin, MTT, streptomycin,
dimethylsulfoxide (DMSO) and phosphate-buffered saline (PBS) were
obtained from Tianjin Hanyang Biologicals Technology Co. Ltd.
(Tianjin, China).
Cell lines
MCF-7 human breast cancer, BHY human oral squamous
carcinoma, Miapaca-2 human pancreatic cancer, Colo-205 human colon
cancer, A-549 human lung cancer cell lines and NIH-3T3 mouse
embryonic fibroblasts were procured from the Institute of Cancer
Research (Gansu, China). All cells were grown in a humidified
incubator with 5% CO2 atmosphere at 37°C and cultured in
RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 100
IU/ml penicillin and 100 μg/ml streptomycin.
Antiproliferative assay
Serial dilutions of the compounds (10–100 μM) were
prepared by dissolving them in DMSO. The cell cytotoxicity was
determined in MCF-7, BHY, Miapaca-2, Colo-205, A-549 and BHY cells
using MTT assay, and the the half maximal inhibitory concentration
values (IC50) were calculated. The cells were plated in
96-well plates and treated with different concentrations of the
seven compounds. Subsequent to incubation for 24, 48 and 72 h at
37°C in a humidified incubator, MTT (5 mg/ml) was added to each
well followed by incubation for a further 4 h. The medium was
carefully removed, then 0.2 ml DMSO was added to each well and the
plates were agitated to allow for mixing. The absorbance was
measured at 545 nm. The inhibitory effect of the compounds on cell
growth was assessed as the percentage cell cytotoxicity, where
vehicle-treated cells were considered to be 100% viable. The final
concentration of DMSO was 0.5% in all treatment protocols.
Flow cytometric analysis
Cell cycle analysis
MCF-7 cells (5×105) were seeded in 60-mm
dishes and treated with two concentrations (25 and 50 μM) of
compounds b and d, for 48 h. Floating and adherent cells were
collected by trypsinization and washed once with PBS. Cells were
incubated in 70% ethanol at −20°C overnight, treated with 20 μg/ml
RNase A, then stained with 1.0 μg/ml propidium iodide (PI). The
stained cells were analyzed by flow cytometry using a FACS Calibur
instrument (BD Biosciences, San Jose, CA, USA) at a wavelength of
488 nm. The data were acquired using CellQuest Pro Acquisition
software version 3.3 (BD Biosciences).
Measurement of mitochondrial membrane
potential (ΛΨm)
ΛΨm was measured by Rhodamine-123 (Rh-123) dye
(Tianjin Hanyang Biologicals Technology Co., Ltd.). Briefly,
5×105/ml MCF-7 cells were treated with different
concentrations (25 and 50 μM) of compound b and d and ΛΨm was
measured by flow cytometry. Rh-123 (1 mM) was added 1 h prior to
the termination of experiment. The cells were collected, washed in
PBS and incubated with PI (5 μg/ml) for 20 min. The reduction in
fluorescence intensity, as a result of ΛΨm loss was analyzed in the
FL-1 channel.
Fluorescence microscopy
Fluorescence microscopy was performed to evaluate
the morphological alterations on the cells following drug
treatment. Cells (1×106 cells/ml) were seeded in 6-well
plates and treated with the tested compounds at 25 and 50 μM. After
24 h, cells were spun at 1000 × g for 5 min. The pellet was
resuspended in PBS. Cells were stained with DAPI (Guoyao Chemical
Co., Ltd.). Cells were then observed, and images were captured,
under a phase contrast microscope (Nikon TMS, Nikon Corporation,
Tokyo Japan) for morphological analysis. Fluorescence-based dyes
were employed for staining the cellular components.
Statistical analysis
IC50 values were calculated by non-linear
regression analysis with Graph Pad Prism, version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Values are expressed as the
means ± standard error of at least three independent experiments. A
P<0.05 was considered to indicate a statistically significant
difference.
Results
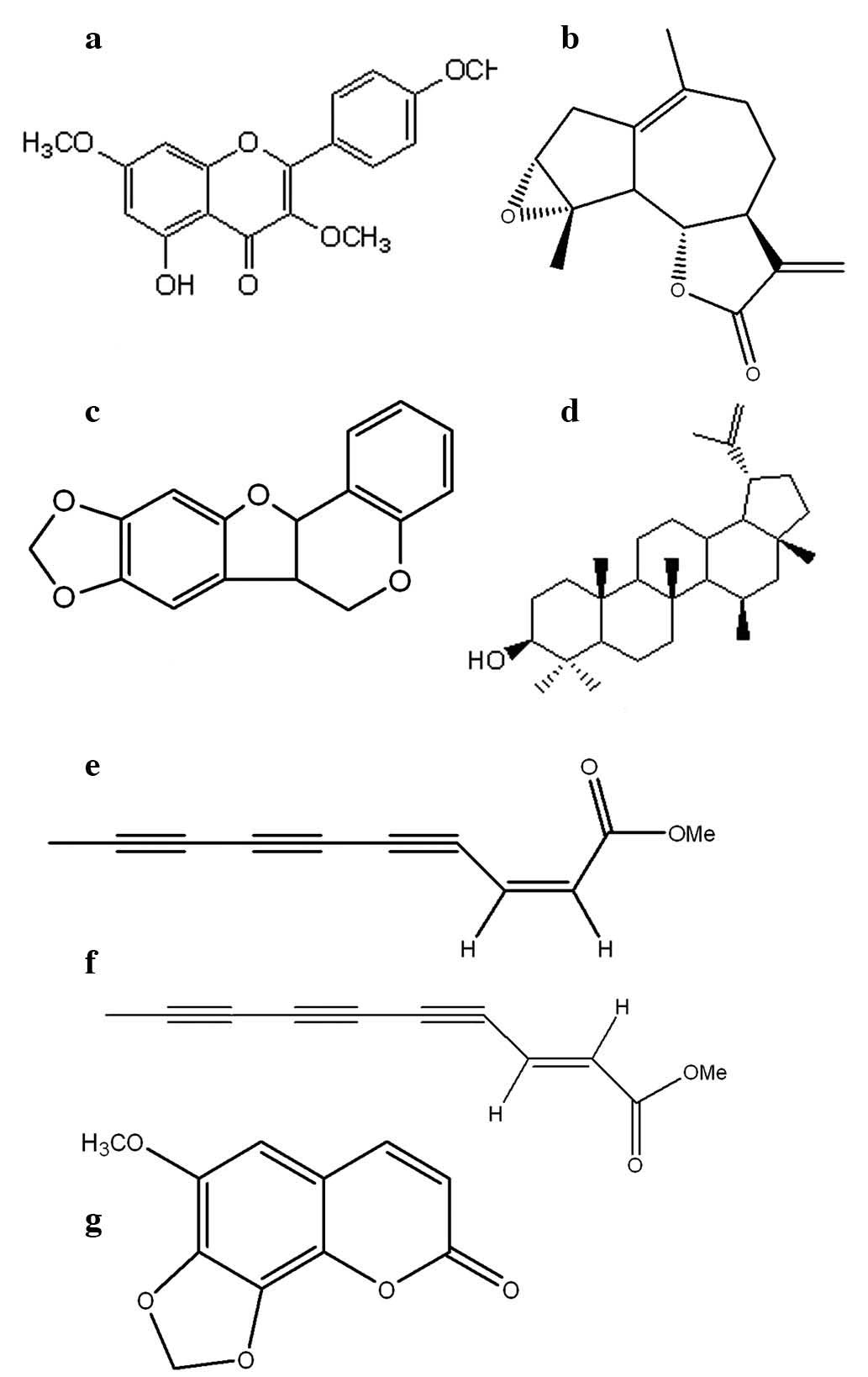
Isolation and structure elucidation of
the compounds
The seven compounds (a–g) were obtained from shoot
and root extract of A. indica. Their structures are
presented in Fig. 1, and the
molecules were identified as (a)
5-hydroxy-3,7,4′-trimethoxyflavone, (b) ludartin, (c) maackiain,
(d) lupeol, (e) cis-matricaria ester, (f)
trans-matricaria ester and (g) 6-methoxy-7,8-methylenedioxy
coumarin, by comparison of spectroscopic [1D-NMR, 2D-NMR, infrared
spectroscopy (IR), and high resolution (HR)-MS] and analytical data
comparison with previous literature (18–28).
The majority of the compounds were isolated from A. indica
for the first time in the current study, to the best of our
knowledge, with the exception of maackiain, which has been reported
previously (16). A number of the
isolated compounds have previously only been isolated from other
species of the genus Artemisia (29–32).
Antitumor activity
The isolated compounds (a–g) were evaluated for
antiproliferative activity by MTT assay against the human cancer
cell lines, MCF-7, BHY, Miapaca-2, Colo-205 and A-549, and a normal
mouse embryonic fibroblast cell line NIH-3T3 at 24, 48 and 72 h
following treatment with the compounds. All of the compounds
exhibited cytotoxic activity in a dose-dependent manner at
different time intervals with a maximum effect at 72 h. The
antiproliferative effect obtained as a result of 72-h treatment and
the IC50 data are summarized in Table I. Among the seven compounds,
compounds b and d exhibited the greatest antiproliferative effect
against all the cell lines. The IC50 values were 25.18
and 28.09 (MCF-7); 28.05 and 32.31 (BHY); 31.21 and 36.45
(Miapaca-2); and 34.33 and 39.01 μM (Colo-205) for compounds b and
d, respectively. The MCF-7 cell line was the most susceptible to
these two compounds. Furthermore, the cytotoxic effects of the
compounds (a–g) were evaluated against NIH-3T3 mouse embryonic
fibroblasts. This was performed in order to provide further insight
into whether these compounds are specific or non-specific to cancer
cells. The normal cells were treated with the IC50
equivalent concentrations of the compounds. Table II lists the cytotoxicity data of
compounds a–g in the normal cell line. The data shows that these
compounds also exhibit cytotoxic effects towards the normal cell
lines, which in this case are NIH 3T3 mouse embryonic fibroblasts.
The results demonstrate that compounds b and d exhibited lower
toxicity towards the normal cell lines. This implies that these
compounds show specific cytotoxic effect towards cancer cell
lines.
 | Table ICytotoxic effects of the extract and
the isolated compounds (a–g) of A. indica against the four
cell lines. |
Table I
Cytotoxic effects of the extract and
the isolated compounds (a–g) of A. indica against the four
cell lines.
| IC50
values (μM) |
|---|
|
|
|---|
| Cell line/Sample | Extract | a | b | c | d | e | f | g |
|---|
| MCF-7 | 67.09±0.9 | 42.24±1.2 | 25.18±1.3 | 38.12±1.2 | 28.08±0.9 | 77.12±0.9 | 55.22±0.7 | 44.32±1.1 |
| BHY | 84.17±0.7 | 48.31±0.9 | 28.05±0.8 | 47.06±1.4 | 32.31±0.5 | 65.12±0.5 | 71.12±0.9 | 43.54±0.9 |
| Miapaca-2 | 72.12±0.7 | 52.10±0.3 | 31.21±0.5 | 56.21±2.1 | 36.45±1.2 | 71.21±2.6 | 77.33±1.2 | 72.11±0.4 |
| Colo-205 | 92.33±2.2 | 64.09±1.2 | 34.33±0.7 | 65.42±2.3 | 39.01±1.1 | 51.54±1.4 | 88.12±1.5 | 71.09±1.3 |
 | Table IICytotoxic effect of the compounds
(a–g) on NIH-3T3 cells. |
Table II
Cytotoxic effect of the compounds
(a–g) on NIH-3T3 cells.
| Compound | Concentration
(μM) | Cytotoxicity
(%) |
|---|
| a | 50 | 67.21±1.6 |
| b | 25 | 22.13±1.4 |
| 50 | 28.21±0.7 |
| c | 50 | 44.45±0.7 |
| d | 25 | 28.31±0.6 |
| 50 | 34.67±0.7 |
| e | 50 | 42.32±1.8 |
| f | 50 | 66.05±0.9 |
| g | 50 | 76.45±1.3 |
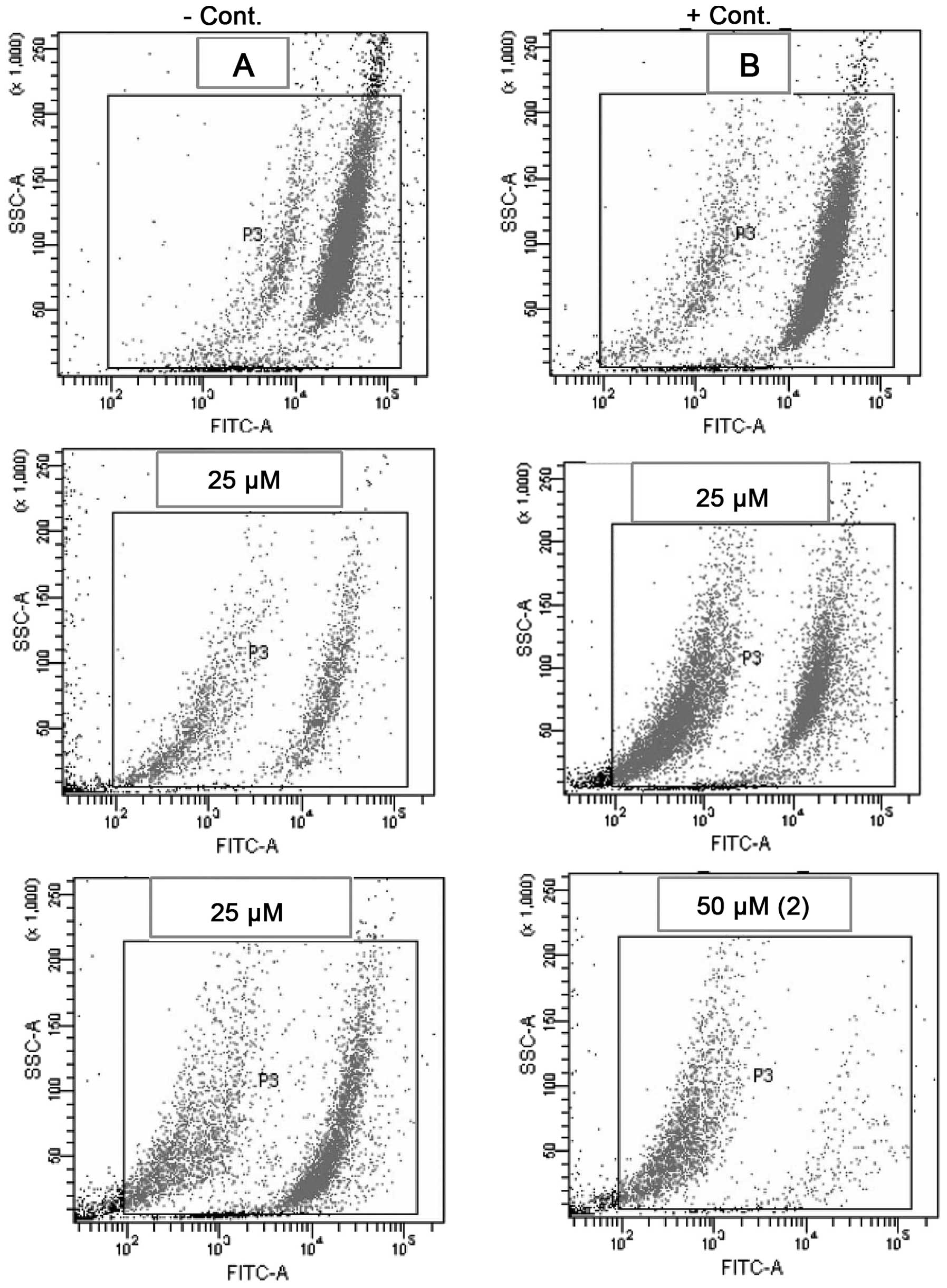
Effect of compounds b and d on DNA damage
and cell cycle phase distribution
The effect of compounds b and d on cell cycle phase
distribution was analyzed by flow cytometry. The MCF-7 cells were
stained with PI and then treated with different concentrations (25
and 50 μM) of the compounds for 72 h. Significant DNA damage was
indicated by the fluorescence patterns obtained from the flow
cytometer (Fig. 2). Apoptotic
cells were designated as shrunken cells with degraded chromatin,
high side scatter and low forward scatter properties. The rise in
the sub-G1 cell population (hypodiploid DNA content) may
be due to DNA fragmentation, which results in apoptotic cell death.
The inhibition of cell cycle progression may be one of the
molecular events concomitant with the cytotoxic activity of these
compounds.
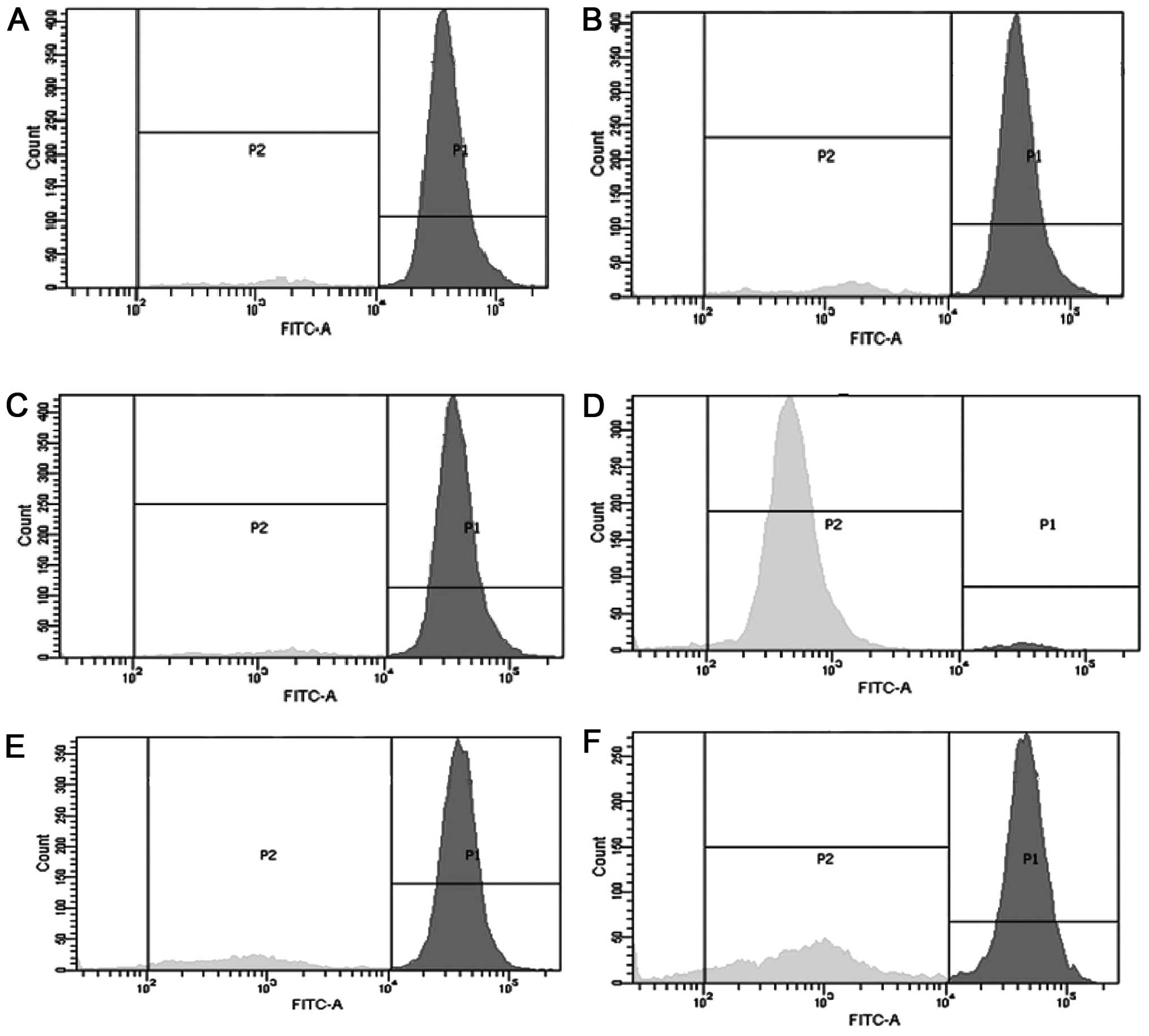
Effect of compounds b and d on ΛΨm
The effect of compounds b and d were further studied
by evaluating their effects on the ΛΨm. MCF-7 cells were treated
with 25 and 50 μM compound b and d and ΛΨm was measured by flow
cytometry. The untreated control cells had intact mitochondria, and
the majority of the cells were bioenergetically active, as
evidenced by high Rh-123 uptake as compared with the positive
control, which exhibited damaged mitochondria. The two compounds
induced a significant increase in mitochondrial membrane potential
loss. Ludartin (compound d) produced a more potent effect on the
mitochondrial membrane potential loss as compared with lupeol
(compound b) (Fig. 3).
Mitochondria have a key function in the induction of apoptosis, as
they are involved at an early period in the apoptotic pathway.
Mitochondrial membrane potential is crucial in the regulation of
apoptosis, and any reduction leads to increased mitochondrial
penetrability and the opening of the permeability transition pore,
which is a key stage in apoptosis (33). The opening of the permeability
transition pore permits the release of factors such as cytochrome c
and apoptotic inducing factor, which trigger the finishing and
degradative stage of apoptosis.
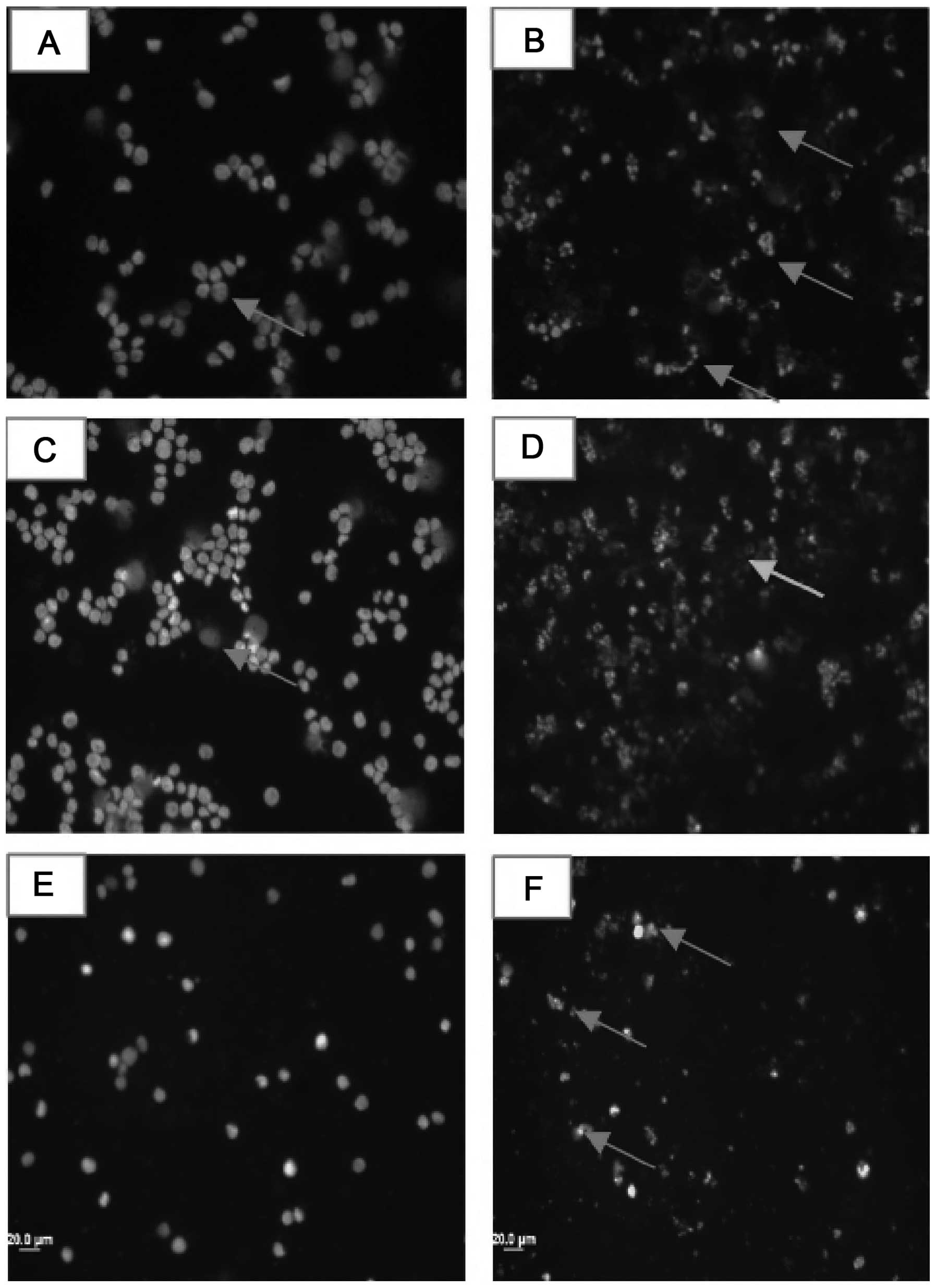
Fluorescence microscopy
The MCF-7 cells were treated with compound b and d
for 24 h, stained with DAPI and visualized to determine the
resulting morphological alterations. The untreated cells displayed
a normal nuclear morphology, but ludartin- and lupeol-treated cells
presented apoptotic bodies. The number of apoptotic bodies was
greater at a higher dose. Camptothecin, which is a known apoptotic
inducer, was used as a positive control and also led to the
appearance of typical apoptotic bodies (Fig. 4).
Discussion
Cancer is a multi-factorial disease that commonly
presents numerous complications, and requires a holistic approach
to treatment, control and prevention. Cancer develops via a
multistep carcinogenesis progression involving a number of cellular
physiological systems, such as cell signaling and apoptosis, making
it a very complex disease (11).
Cancer is the second most prominent cause of mortality worldwide.
According to global cancer statistics from 2011, cancer rates are
increasing at an alarming rate (13). Plant extracts have often been used
for the prevention and treatment of human diseases, including
cancer. The Artemisia species have well-established chemical
and pharmacological properties, and have been used traditionally
for the treatment of hepatitis, cancer, inflammation and fungi,
bacteria and virus infections. Numerous bioactive compounds that
exhibit antimalarial and anticancer (34) activity against tumor cells, such as
artemisinin, have been reported to be of the Artemisia
genus, and arglabin, another bioactive molecule is used for
treating certain types of cancer (35,36).
The present study, is the first study on the antiproliferative and
apoptotic effects of the chemical constituents of A. indica,
to the best of our knowledge.
The effects of the isolated compounds a–g on MCF-7,
BHY, Miapaca-2, Colo-205 and A-549 cell lines were investigated by
MTT assay. As indicated in Table
I, of the seven tested compounds, compounds b and d were
identified to have the strongest antiproliferative activities
against all of the cell lines. The IC50 values were
25.18 and 28.01 (MCF-7); 28.05 and 32.31 (BHY); 31.21 and 36.45
(Miapaca-2); and 35.12 and 39.01 μM (Colo-205) for compounds b and
d, respectively. On the basis of the IC50 values and
cytotoxicity data (Table I and
II), the effect of compounds b
and d on DNA damage and mitochondrial membrane potential loss in
MCF-7 cells was investigated. The aim was to determine the
mechanisms by which these compounds exert their cytotoxic effects.
Flow cytometry was used to verify apoptosis induction in MCF-7
cells following 48-h incubation with compounds b and d at their
IC50 concentrations. As presented in the figures, the
two compounds induced significant DNA damage and reduction of ΛΨm
at 25 and 50 μM concentrations.
In summary, the current bioactivity-guided
isolations led to the conclusion that the strong inhibitory effect
of the ethyl acetate extract of A. indica on the
proliferation of the cultured human tumor cell lines MCF-7, BHY,
Miapaca-2, Colo-205 and A-549 may be attributed to ludartin
(4) and lupeol (6). However, a favorable interaction
between the chemicals may be responsible for the overall
antiproliferative action of the extract. The present study also
demonstrated that the antiproliferative effects of compounds b and
d as anticancer agents may be due to the significant DNA damage and
mitochondrial membrane potential loss induced by these compounds.
Further studies are required to fully evaluate these underlying
mechanisms of action and the toxicity of the compounds, prior to
further development as potent anticancer agents.
Acknowledgements
The authors would like to acknowledge the funding
from Guangdong Hospital Pharmacy Research Foundation (nos. 2011A17
and 2013YY02).
References
|
1
|
World Health Organization. Promoting the
role of traditional medicine in health systems: a strategy for the
African region 2001–2010. Harare: World Health Organization;
2000
|
|
2
|
Li JW and Vederas JC: Drug discovery and
natural products: end of an era or an endless frontier? Science.
325:161–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma X and Wang Z: Anticancer drug discovery
in the future: an evolutionary perspective. Drug Discov Today.
14:1136–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madhuri S and Pandey G: Some anticancer
medicinal plants of foreign origin. Curr Sci India. 96:779–783.
2009.
|
|
6
|
Harvey AL: Medicines from nature: are
natural products still relevant to drug discovery? Trends Pharmacol
Sci. 20:196–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar
|
|
9
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar
|
|
10
|
Saleem K, Wani W, Haque A, Milhotra A and
Ali I: Nanodrugs: magic bullets in cancer chemotherapy. Topics in
Anti-Cancer Research. 2. Bentham Science Publishers; pp.
437–494
|
|
11
|
Reichert JM and Wenger JB: Development
trends for new cancer therapeutics and vaccines. Drug Discov Today.
13:30–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carnesecchi S, Schneider Y, Ceraline J, et
al: Geraniol, a component of plant essential oils, inhibits growth
and polyamine biosynthesis in human colon cancer cells. J Pharmacol
Exp Ther. 298:197–200. 2001.PubMed/NCBI
|
|
13
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gibbs JB: Mechanism-based target
identification and drug discovery in cancer research. Science.
287:1969–1973. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Debatin KM: Apoptosis pathways in cancer
and cancer therapy. Cancer Immunol Immunother. 53:153–159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chanphen R, Thebtaranonth Y,
Wanauppathamkul S and Yuthavong Y: Antimalarial principles from
Artemisia indica. J Nat Prod. 61:1146–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rashid S, Rather MA, Shah WA and Bhat BA:
Chemical composition, antimicrobial, cytotoxic and antioxidant
activities of the essential oil of Artemisia indica Willd. Food
Chem. 138:693–700. 2013. View Article : Google Scholar
|
|
18
|
Blunt JW and Stothers JB:
13C-NMR-spectra of steroids - A survey and commentary.
Org Magn Resonance. 9:439–464. 1977. View Article : Google Scholar
|
|
19
|
Abraham RJ and Monasterios JR:
13C nuclear magnetic resonance spectra of some
ergosta-dienes and -trienes. J Chem Soc Perkin Trans. 2:662–665.
1974. View Article : Google Scholar
|
|
20
|
Geissman TA and Griffin TS: Sesquiterpene
lactones of Artemisia carruthii. Phytochemistry. 11:833–835. 1972.
View Article : Google Scholar
|
|
21
|
Sosa VE, Oberti JC, Gil RR, et al:
10-Epideoxycumambrin b and other constituents of Stevia yaconensis
var. subeglandulosa. Phytochemistry. 28:1925–1929. 1989. View Article : Google Scholar
|
|
22
|
Harborne J, Tomás-Barberán F, Williams C
and Gill M: A chemotaxonomic study of flavonoids from european
teucrium species. Phytochemistry. 25:2811–2816. 1986. View Article : Google Scholar
|
|
23
|
Bohlmann F and Rode KM: Polyacetylene,
CVIII. About the ingredients of Artemisia pedemontana Balb. Chem
Ber. 99:2416–2418. 1966.(In German). View Article : Google Scholar
|
|
24
|
Bohlmann F, Burkhardt T and Zdero C:
Naturally Occurring Polyacetylenes. Academic Press; London: pp.
5471973
|
|
25
|
Kobayashi A, Morimoto S, Shibata Y,
Yamashita K and Numata M: C-10 polyacetylenes as allelopathic
substances in dominants in early stages of secondary succession. J
Chem Ecol. 6:119–131. 1980. View Article : Google Scholar
|
|
26
|
Bohlmann F, Kleine KM, Arndt C and Köhn S:
Polyacetylene LXXVIII: New ingredients in the genus Anthemis L.
Chem Ber. 98:1616–1622. 1965.(In German). View Article : Google Scholar
|
|
27
|
Miyakoshi N, Aburano D and Mukai C: Total
synthesis of naturally occurring diacetylenic spiroacetal enol
ethers. J Org Chem. 70:6045–6052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herz W, Bhat SV and Santhanam PS:
Coumarins of Artemisia dracunculoides and 3′,6
dimethoxy-4′,5,7-trihydroxyflavone in A. arctica. Phytochemistry.
9:891–894. 1970. View Article : Google Scholar
|
|
29
|
Jalmakhanbetova RI and Adekenov SM:
Chemical study of Artemisia filatovae. Chem Nat Compd. 43:347–348.
2007. View Article : Google Scholar
|
|
30
|
Esteban M, Gonzáles Collado L, Macías FA,
Massanet GM and Rodríguez Luis F: Flavonoids from Artemisia lanata.
Phytochemistry. 25:1502–1504. 1986. View Article : Google Scholar
|
|
31
|
Greger H: A new acetylenic ester from
Artemisia absinthium. Phytochemistry. 17:8061978. View Article : Google Scholar
|
|
32
|
Stavholt K and Sørensen NA: Studies
related to naturally-occurring acetylene compounds. V
Dehydromatricaria ester from the essential oil of Artemisia
vulgaris L. Acta Chem Scand. 4:1567–1574. 1950. View Article : Google Scholar
|
|
33
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar
|
|
34
|
Efferth T: Willmar Schwabe Award 2006:
antiplasmodial and antitumor activity of artemisinin - from bench
to beside. Planta Med. 73:299–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wons HF and Brown GD: Germacranolides from
Artemisia myriantha and their conformation. Phytochem. 59:529–536.
2002. View Article : Google Scholar
|
|
36
|
Kim JH, Kim HK, Jeon SB, et al: New
sesquiterpene-monoterpene lactone, artemisolide, isolated from
Artemisia argyi. Tetrahedron Lett. 43:6205–6208. 2002. View Article : Google Scholar
|