Introduction
Gastric cancer (GC) is the fourth most frequent type
of cancer around the world (1). In
East Asia, it is the second leading cause of cancer-associated
mortalities, and ~934,000 new patients are diagnosed with gastric
cancer each year (2). In spite of
advanced chemotherapy and surgical resection, the majority of
patients are already at the advanced stage at diagnosis, negating
the use of a surgical operation. Patients with an identical tumor,
nodes and metastasis stage may even have different prognoses
(3). Therefore, the application of
novel chemotherapeutic agents and combinations of regimens may
provide an improved therapy for advanced GC and reduce the toxicity
of these drugs.
The compound 5-fluorouracil (5-FU) remains a first
line antineoplastic treatment in clinical practice (4). However, GC cells are becoming
resistant to existing chemotherapeutic compounds, including 5-FU.
Therefore, it is necessary to explore novel chemotherapeutic
regimens for the treatment of advanced GC including metastatic GC.
Puerarin
[8-(β-d-glucopyranosyl-7-hydroxy-3-(4-hydroxyphenyl)-4-H-1-benzopyran-4-one],
with the molecular formula
C21H20O9, was isolated from the
herbal medicine Radix puerariae (5,6).
Previous studies have shown that puerarin has beneficial effects on
cardiovascular, neurological and hyperglycemic disorders (7–9).
Researchers found that puerarin has an anticancer effect on HT29
cells and enhances the antiproliferative effects of other
antineoplastic agents (10,11).
However, to the best of our knowledge there have been no studies on
the effectiveness of puerarin on GC cells, and the mechanism of its
protective effects has remained elusive.
The aim of the present study was to evaluate the
combined effects of puerarin and 5-FU on BGC-823 gastric carcinoma
cells in vitro and in vivo.
Materials and methods
Cells and reagents
The BGC-823 human gastric cancer cell line was
purchased from the Shanghai Cell Collection (Shanghai, China). The
cells were maintained in DMEM (Gibco-BRL Gaithersburg, MD, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS) and
1% antibiotic solution (penicillin 100 U/ml and streptomycin 100
g/ml; Beyotime, Shanghai, China) at 37°C in a humidified atmosphere
of 95% air/5% CO2. Puerarin and 5-FU were obtained from
Sigma-Aldrich (P5555, F6627; St. Louis, MO, USA) and were 98% pure
by reverse-phase high-performance liquid chromatography.
Estimation of cell proliferation
Cell proliferation was quantified with a Cell
Counting kit-8 assay (CCK-8; Dojindo, Shanghai, China). Cells were
suspended in a complete DMEM and subsequently seeded in 96-well
microtiter plates at a density of 1×105 per well.
Following exposure to puerarin (400, 800, 1600, 3200 or 6400 μM),
5-FU (20, 40, 80, 160 or 320 μM) or puerarin+5-FU for 48 h, cells
were incubated at 37°C for another 4 h with CCK-8 (10 μl per well).
Absorbance was measured at a wavelength of 490 nm using an iMark
microplate reader (Bio-Rad, Hercules, CA, USA). Each sample was
subjected to three independent experiments.
Evaluation of the combined effects of
puerarin and 5-FU
As previously described (12,13),
the following equation was used to evaluate the nature of the
interaction between puerarin and 5-FU: D = Dm
[fa/(1-fa)]1/m, where D is the dose, Dm is
the median effect dose required to produce (analogous to the
IC50), fa is the fraction of the system affected by D,
and m is a Hill-type coefficient signifying the sigmoidicity of the
dose-effect curve. The combination index (CI) was obtained by using
the Biosoft CalcuSyn software (Biosoft Version 2.0, Cambridge, UK)
written in BASIC for automatic graphing of the CI with respect to
fa. When smaller than 1, equal to 1, or greater than 1, CI
indicates synergism, summation, antagonism, respectively.
Hoechst asssay
Detection of apoptotic morphological features was
performed using Hoechst 33258 staining (Beyotime). Cells were
cultured in a six-well plate and incubated with puerarin (1,600
μM), 5-FU (80 μM) or puerarin+5-FU for 48 h. Then, 20 μM Hoechst
reagent was added to each well with for 10 min at room temperature.
Changes in nuclear morphology were observed with a fluorescence
microscope (BX53F, Olympus, Tokyo Japan). In each group, ten
microscopic fields were selected randomly and counted.
Annexin V/propidium iodide (PI)
staining
An Annexin V/PI apoptosis kit was used to quantify
the percentage of cells undergoing apoptosis (Multisciences,
Hangzhou, China). Cells were incubated for 48 h with either
puerarin, 5-FU or puerarin and 5-Fu. Following incubation, the
cells were washed twice with cold phosphate-buffered saline and
resuspended in binding buffer (500 μl) at a concentration of
1×106 cells/μl. Then, 5 μl Annexin V-fluorescein
isothiocyanate and 10 μl PI were added and the cells were incubated
at room temperature for 5 min in the dark. At the end of the
incubation period, cell apoptosis was analyzed on a FACScan flow
cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Western blot analysis
A wet transfer system was used to detect the protein
levels. Proteins were resolved by SDS-PAGE (10%, Beyotime) and
transferred onto nitrocellulose membranes (Bio-Rad). Membranes were
blocked with 10% non-fat dry milk and incubated with primary
antibodies (Bcl-2, Bax, GAPDH mouse monoclonal antibodies; 1:1,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
The following day, horseradish peroxidase-conjugated polyclonal
goat anti-rat secondary antibodies (1:2,000; Santa Cruz
Biotechnology, Inc.) were added, and the cells were incubated for 2
h at room temperature. Then, the membranes were placed in an
enhanced chemiluminescence system (Millipore, Bedford, MA, USA) and
imaged in a dark room. The antibodies were used as follows: B-cell
lymphoma-2 (Bcl-2), Bcl-2-associated X (Bax) and GAPDH (1:1,000,
Santa Cruz Biotechnology, Austin, TX, USA).
Tumor xenograft experiment in nude
mice
Male BALB/c-nu/nu nude mice, [weighing 16–18 g, 4–6
weeks of age, purchased from the Center of Experimental Animals of
Wuhan University, (Wuhan, China)] were caged in groups of six and
fed with a standard diet and water ad libitum. The study was
approved by the ethics committee of Remnin Hospital of Wuhan
University. Cells (5×106 cells/mouse) were injected
subcutaneously into the right dorsal area. When tumors grew to a
mean volume of 160 mm3, the mice were treated were as
follows: i) Control, 0.9% saline; ii) puerarin group,
30 mg/kg/day puerarin, iii) 5-FU group, 12 mg/kg/day 5-FU;
iv) puerarin+5-FU group, combination of puerarin (30
mg/kg/day) and 5-FU (12 mg/kg/day), three times a week for three
weeks. Tumor volume (TV) and weight were recorded every three days.
TV was calculated by the following formula: TV (mm3) =
d2 × D/2, where d and D are the shortest and longest
diameters, respectively. Following three weeks of treatment, the
mice were sacrificed by orbital sinus bleeding and the tumors were
segregated and weighed. Liver and renal injury were measured
through detection of the levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST), blood urea nitrogen (BUN) and
serum creatinine (Cr). The tumor tissues were harvested for
pathological examination to confirm that they were the same type of
tumor. The levels of apoptosis in the cells of the tumor xenograft
were measured by terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL) assay according to the manufacturer’s
instructions (Roche Diagnostics, Branchburg, NJ, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). All data are
expressed as the mean ± the standard deviation. The mean values of
the different groups were compared using non-parametric analysis
(Mann-Whitney rank sum test), and a value of P<0.05 was
considered to be statistically significant.
Results
Cytotoxic effects of puerarin and 5-FU on
BGC-823 cells
After 48 h of drug exposure, growth of BGC-823 cells
was clearly inhibited in a dose-dependent manner in vitro.
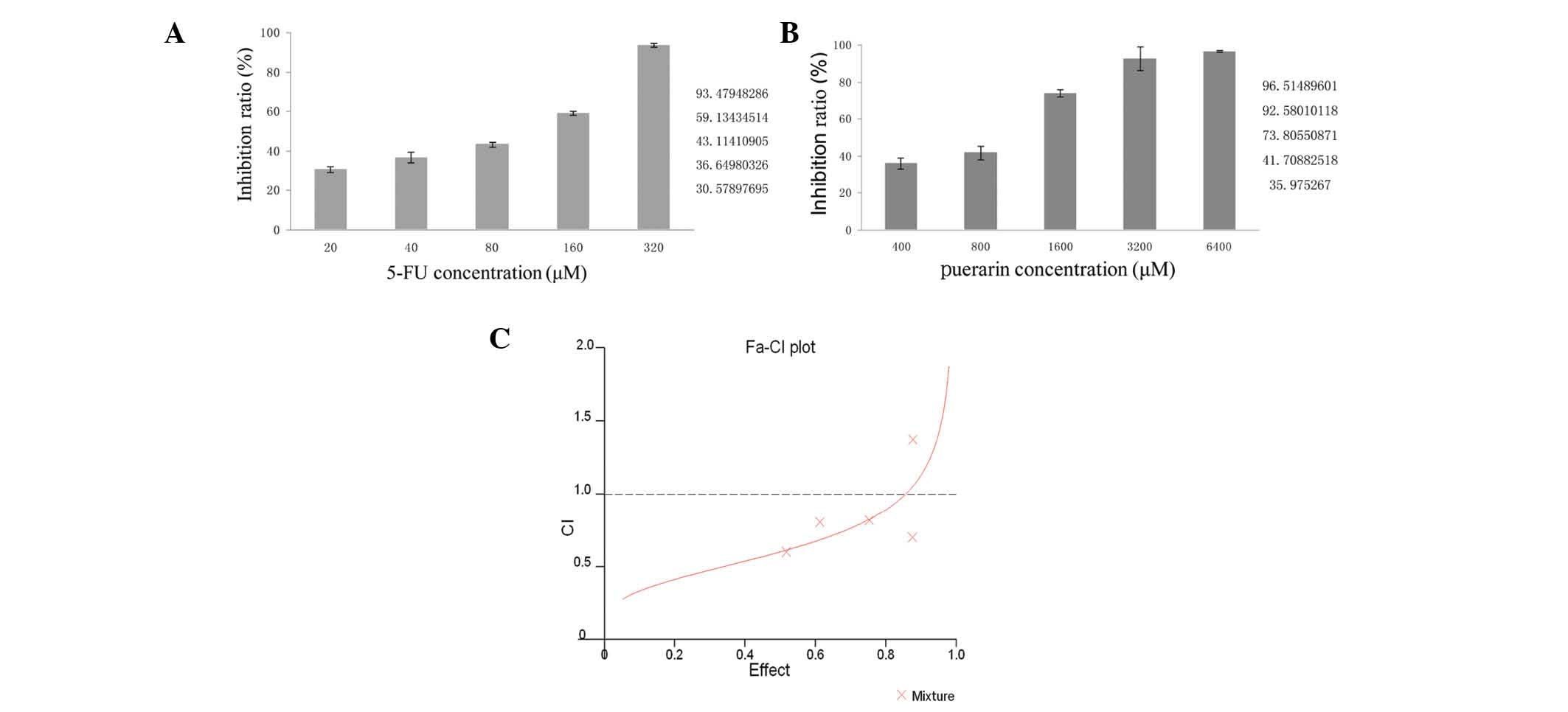
As shown in Fig. 1, 20 μM 5-FU had
an inhibition rate of 30.58%, which increased to 93.48% at 320 μM
(Fig. 1A). The inhibition rates
caused by puerarin were 35.90% (400 μM) and 96.51% (6,400 μM;
Fig. 1B). Combined treatment with
these two drugs significantly decreased the viability of BGC-823
cells compared with the effect of either drug used alone.
Synergistic effect of puerarin and 5-FU
on BGC-823 cell growth
Following exposure to the drugs for 48 h, the
inhibition rate was tested. The combination effects were studied by
the method of Chou and Talalay (12). Treatment with 2,400 μM puerarin or
240 μM 5-FU produced nearly 75% growth inhibition when used alone.
The same inhibition rate was achieved with a combination of 1,600
μM puerarin and 80 μM 5-FU (20:1). Therefore, puerarin was able to
decrease the efficient dose of 5-FU by two-fold. The experiments
were repeated independently in triplicate. The CI value was <1
when the fractions affected were <0.8775, as shown in Fig. 1C, which indicated that puerarin and
5-FU had a synergistic effect on inhibiting BGC-823 cells.
Apoptosis induced by puerarin and
5-FU
Apoptosis was one of the predominant types of
programmed cell death which involved a series of biochemical events
leading to specific cell morphology characteristics, including cell
shrinkage, nuclear fragmentation, chromatin condensation and
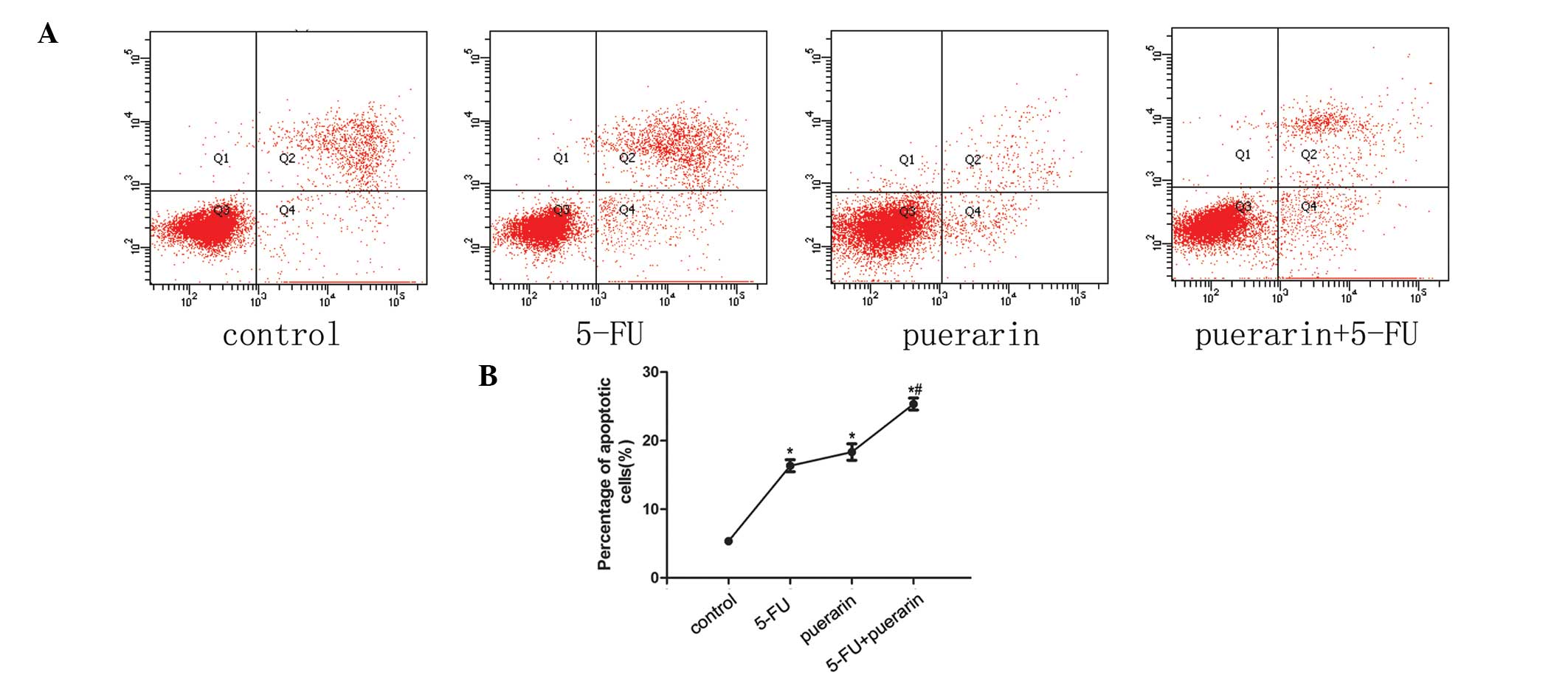
chromosomal DNA fragmentation. As shown in Fig. 2A and B, nuclear fragmentation and
chromatin condensation in the puerarin + 5-FU group were brighter
than in the groups treated with one of the drugs alone (P<0.05)
and the control group (P<0.05). BGC-823 cells were treated with
low concentrations of puerarin (1,600 μM) and 5-FU (80 μM) for 48 h
alone or in combination, respectively. The Annexin V/PI apoptosis
kit was used to examine the proportion of apoptotic cells. Data
indicated that the combined effect was greater than the effects of
puerarin and 5-FU individually (P<0.01, Fig. 3).
Mitochondrial pathway protein expression
by western blot
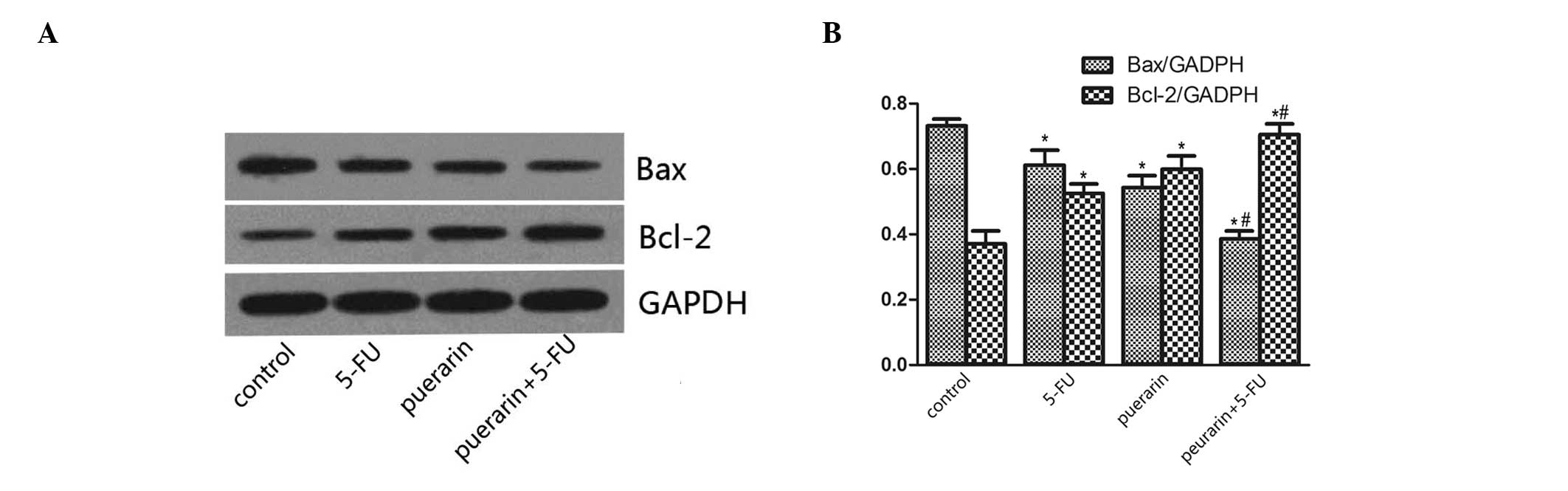
In order to verify the apoptotic mechanism, the
levels of the mitochondrial pathway proteins Bax and Bcl-2 were
detected. As shown in Fig. 4, the
Bax/GAPDH protein expression levels were increased in the puerarin
and 5-FU treatment groups. By contrast, the Bcl-2/GAPDH protein
expression levels were reduced. Results showed that the effect of
combined treatment was greater than that of individual
treatment.
Effect of puerarin and 5-FU on tumor
development in vivo
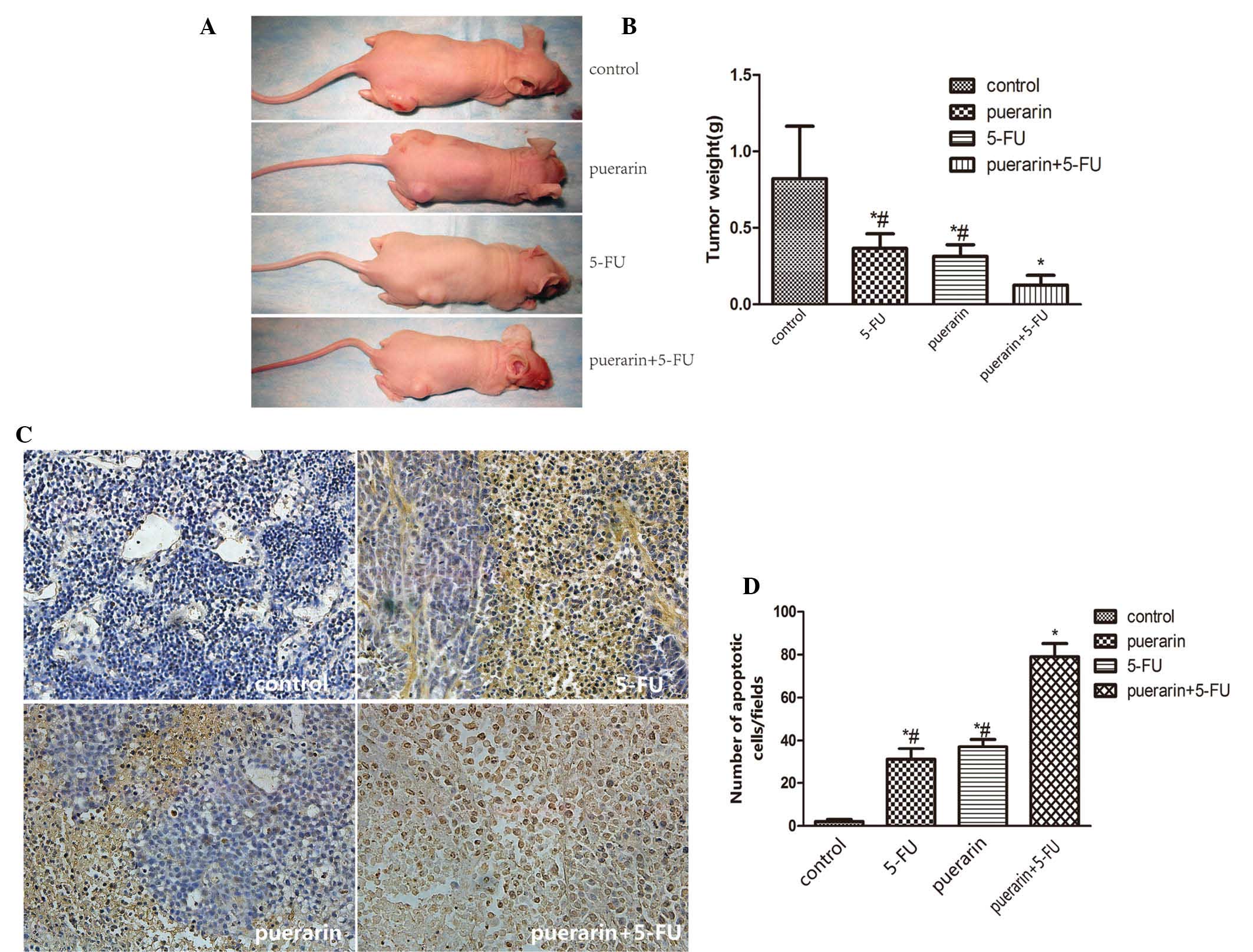
The effect of puerarin and 5-FU on the growth of
primary tumor xenografts in nude mice was examined. Tumor volume
was recorded every three days. The volumes of the tumors in the
treatment groups were clearly reduced compared with those in the
control group, while the inhibition rate in the combination group
was 90.65%, which was a more significant inhibition than that in
the other three groups (P<0.05; Table I). The data showed that the effect
of the combined treatment was superior to the effect of puerarin or
5-FU individually. The mean tumor weight in the combination group
was only 0.125 g at the end of the experiment compared with the
control group (0.822 g) (Fig. 5A and
B). TUNEL assays of the subcutaneous tumor tissue sections
demonstrated that puerarin combined with 5-FU produced clear cell
apoptosis in the tumor mass, while little apoptosis was observed in
the control group (P<0.05, Fig. 5C
and D).
 | Table IInhibitory effects of puerarin and
5-FU on BGC-823 tumor volume in nude mice. |
Table I
Inhibitory effects of puerarin and
5-FU on BGC-823 tumor volume in nude mice.
| Group | n | Volume
(mm3) | Inhibition rate
(%) |
|---|
| Puerarin | 6 | 236.02±40.50a,b | 68.37 |
| 5-FU | 6 | 194.70±35.33a,b | 73.90 |
| Puerarin+5-FU | 6 | 69.76±24.01b | 90.65 |
| Control | 6 | 746.10±114.56 | |
Evaluation of side effects
At the end of the experiment, 24 mice were
necropsied to assess the side effects. There were no injuries to
the liver or kidney observable with the naked eye. ALT, AST, BUN
and Cr levels in the serum were detected to evaluate liver or renal
injuries. The results were not indicative of any injury and there
were no significant variations among the four tested groups
(P>0.05, Table II).
 | Table IIEffect of puerarin combined with 5-FU
or alone on hepatic and renal function. |
Table II
Effect of puerarin combined with 5-FU
or alone on hepatic and renal function.
| Group | n | ALT (U/l) | AST (U/l) | Urea (μmol/l) | Cr (μmol/l) |
|---|
| Puerarin | 6 | 34.33±4.72 | 131.50±12.88 | 7.25±2.50 | 17.69±2.48 |
| 5-FU | 6 | 33.67±3.44 | 129.17±26.63 | 6.96±1.29 | 15.72±1.29 |
| Puerarin+5-FU | 6 | 35.50±10.37 | 136.33±17.50 | 7.34±0.53 | 19.21±5.59 |
| Tumor control | 6 | 31.67±10.56 | 124.50±22.49 | 7.01±0.44 | 15.81±2.91 |
| Normal control | 6 | 32.17±8.70 | 130.17±15.08 | 7.04±0.81 | 16.62±2.48 |
Discussion
GC remains the most commonly diagnosed type of
cancer worldwide, exhibiting a high annual mortality (2,3).
Early diagnosis and combined chemotherapies have increased the
survival rate. Numerous chemotherapeutic agents, including 5-FU,
cisplatin, doxorubicin and paclitaxel, have been used for clinical
treatment of patients for years. However, the overall outcome
remains poor due to drug resistance, radiotherapy or side effects.
Studies have shown that the combination of Chinese herbs with these
drugs enhanced the efficacy of therapy of human tumors, including
gastric cancer (5). Therefore,
combination chemotherapy has become one of the most important means
of improving survival of gastric cancer patients.
5-FU, which is widely used for the treatment of
several human malignancies, has been used in clinical treatments
for years (14). However,
administration of 5-FU alone often leads to undesirable side
effects and drug resistance. In addition, 5-FU has been used in
combination with other agents, which resulted in fewer side
effects, superior pharmacological properties and an improved
antitumor effect (15–18). Therefore, it is necessary to
identify novel agents enhancing the anticancer effect of 5-FU.
Recently, traditional medicines have been exploited to treat
tumors, particularly in China. Puerarin (isolated from a Chinese
Medicinal herb), has been used in China for years. However, little
is known about the effects of puerarin on GC cells. In the present
study, the inhibitory effect of puerarin combined with 5-FU on GC
was investigated in vitro and in vivo.
The results of the present study revealed that
puerarin or 5-FU alone significantly inhibited the proliferation of
BGC-823 cells in a dose-dependent manner (puerarin, 400–6,400 μM;
5-FU, 20–320 μM). These results showed that puerarin combined with
5-FU at appropriate concentrations had a synergistic effect on
BGC-823 cells, compared with that of puerarin or 5-FU alone. Wang
et al (11) examined the
effect of puerarin on HT-29 cells, and their results showed that
puerarin, with a higher tolerance in vitro, inhibits the
growth of HT-29 cells by the induction of early apoptosis. A study
by Wang et al (19) showed
that using puerarin reversed multidrug resistance (MDR) in a nude
mouse model of human GC and reduced the expression of
P-glycoprotein and multidrug resistance protein (19). Hien et al (20) found that puerarin downregulated
MDR1 expression via nuclear factor κ-B and CRE transcriptional
activity-dependent upregulation of 5′ adenosine
monophosphate-activated protein kinase in MCF-7/adr cells.
Furthermore, a study by Han et al (21) indicated that puerarin increased Bax
expression and reduced Bcl-2 expression, inhibiting the cell cycle
in G0/G1 phase. In the present study, the results of the flow
cytometric analysis showed that puerarin and 5-FU inhibited the
early apoptosis of BGC-823 cells, with a synergistic anticancer
effect observed when puerarin and 5-FU were used together. The
mechanism of this synergistic effect of puerarin (1,600 μM) and
5-FU (80 μM) on BGC-823 cells was investigated by western blot
analysis. The results demonstrated that the cytotoxic effects of
5-FU were potentiated by the addition of puerarin. The side effects
of puerarin and 5-FU were also observed in vivo. Serological
indicators were not significantly different between combined or
single treatment and control groups.
In conclusion, the present study indicated that
low-dose 5-FU combined with puerarin inhibited cell viability more
effectively, compared with 5-FU and puerarin alone. Puerarin may
potentiate the antiproliferative effect of 5-FU and reduce the
therapeutically required dose of 5-FU, without increasing the
toxicity, which provided a valuable novel treatment for patients
with GC.
Acknowledgements
This study was performed in the Key Laboratory of
Hubei Province for Digestive System Disease, with the assistance of
Mr. Hong Xia. The study was supported by a grant from the
Fundamental Research Funds for the Central Universities of China
(no. 2012302020208)
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008. GLOBOCAN. 2008. Int J Cancer. 127:2893–2917. 2008. View Article : Google Scholar
|
|
3
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geo-graphic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahn MS, Kang SY, Lee HW, Jeong SH, Park
JS, Lee KJ, et al: 5-fluorouracil, mitomycin-C, and
polysaccharide-K versus uracil-ftorafur and polysaccharide-K as
adjuvant chemoimmunotherapy for patients with locally advanced
gastric cancer with curative resection. Onkologie. 36:421–426.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanagihara K, Ito A, Toge T and Numoto M:
Antiproliferative efects ofisoflavones on human cancer cell lines
established from the gastrointestinal tract. Cancer Res.
53:5815–5821. 1993.PubMed/NCBI
|
|
6
|
Tian F, Xu LH, Zhao W, Tian LJ and Ji XL:
The optimal therapeutic timing and mechanism of puerarin treatment
of spinal cord ischemia-reperfusion injury in rats. J
Ethnopharmacol. 134:892–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, et al: Puerarin attenuates pressure overload-induced cardiac
hypertrophy. J Cardiol. 63:73–81. 2014. View Article : Google Scholar
|
|
8
|
Zhao LX, Liu AC, Yu SW, Wang ZX, Lin XQ,
Zhai GX and Zhang QZ: The permeability of puerarin loaded poly
(butylcyanoacrylate) nanoparticles coated with polysorbate 80 on
the blood-brain barrier and its protective effect against cerebral
ischemia/reperfusion injury. Biol Pharm Bull. 36:1263–1270. 2013.
View Article : Google Scholar
|
|
9
|
Kim KM, Jung DH, Jang DS, Kim YS, Kim JM,
Kim HN, Surh YJ and Kim JS: Puerarin suppresses AGEs-induced
inflammation in mouse mesangial cells: a possible pathway through
the induction of heme oxygenase-1 expression. Toxicol Appl
Pharmacol. 244:106–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Z and Li W: Induction of apoptosis by
puerarin in colon cancer HT-29 cells. Cancer Lett. 238:53–60. 2006.
View Article : Google Scholar
|
|
11
|
Wang Y, Ma Y, Zheng Y, Song J, Yang X, Bi
C, Zhang D and Zhang Q: In vitro and in vivo anticancer activity of
a novel puerarin nanosuspension against colon cancer, with high
efficacy and low toxicity. Int J Pharm. 441:728–735. 2013.
View Article : Google Scholar
|
|
12
|
Chou TC and Talalay P: Quantitative
analysis of dose effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar
|
|
13
|
Chou TC, Motzer RJ, Tong Y and Bosl GJ:
Computerized quantitation of synergism and antagonism of taxol,
topotecan and cisplatin against human teratocarcinoma cell growth:
a rational approach to clinical protocol design. J Natl Cancer
Inst. 86:1517–1524. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashraf N, Hoffe S and Kim R: Adjuvant
treatment for gastric cancer: chemotherapy versus radiation.
Oncologist. 18:1013–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imazawa M, Kojima T, Boku N, Onozawa Y,
Hironaka S, et al: Efficacy of sequential methotrexate and
5-fluorouracil (MTX/5FU) in improving oral intake in patients with
advanced gastric cancer with severe peritoneal dissemination.
Gastric Cancer. 12:153–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SL, Kim SH, Trang KT, Kim IH, Lee SO,
et al: Synergistic antitumor effect of 5-fluorouracil in
combination with parthenolide in human colorectal cancer. Cancer
Lett. 335:479–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Liu W, Zhao Q, Qi Q, Lu N, et al:
Synergistic effect of 5-fluorouracil with gambogic acid on BGC-823
human gastric carcinoma. Toxicology. 256:135–140. 2009. View Article : Google Scholar
|
|
18
|
Nishino T, Yamamoto Y, Ikeda M, Morimoto
M, Furukawa T, Goto M, Furukita Y, Takechi H, Seike J, Tangoku A
and Fujiwara H: Pathological complete response in a case of
advanced esopha-geal cancer invading aorta treated by preoperative
chemotherapy with docetaxel and cisplatin plus 5-FU. Gan To Kagaku
Ryoho. 40:643–646. 2013.(In Japanese). PubMed/NCBI
|
|
19
|
Wang L, Wei PK and Xu YP: Experimental
study on puerarin injection reverse multidrug resistance of nude
mice of human gastric carcinoma constructed using orthotopic
transplantation. J Chendu Uni TCM. 28:42–46. 2005.(In Chinese).
|
|
20
|
Hien TT, Kim HG, Han EH, Kang KW and Jeong
HG: Molecular mechanism of suppression of MDR1 by puerarin from
pueraria lobata via NF-kappaB pathway and cAMP-responsive element
transcriptional activity-dependent up-regulation of AMP-activated
protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res.
54:918–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han P, Pei LY, Li J, Zhang L, Zhang HQ and
Xu D: Effect and mechanism of pueraria crude extract puerarin on
lung cancer H446 cell proliferation. Shandong Med J. 48:7–9.
2008.
|