Introduction
Hepatic fibrosis, a pathological condition
characterized by impaired hepatic function and nodule formation,
results from multiple types of liver injury, including drug
intoxication, viral hepatitis, alcohol abuse, autoimmunity and
non-alcoholic steatohepatitis (1–3).
According to 2004 statistics, chronic hepatitis B virus (HBV)
infection was the most common among all of the factors leading to
hepatic fibrosis in China, and in the majority of the European
countries and the USA, the main factors were hepatitis C virus
infection, alcohol abuse, and non-alcoholic steatohepatitis
(4–6). As hepatic fibrosis may contribute to
liver carcinoma, the mortality of patients with hepatic fibrosis is
gradually increasing (7). Certain
medicines, including corticosteroids, penicillamine, methotrexate,
silymarin and colchicines have been widely used in the treatment of
hepatic fibrosis; however, no definitive treatment has been
established (8–11). Consequently, novel and effective
therapeutic methods for treating hepatic fibrosis are urgently
required, as the fibrotic process is reversible and can be
controlled (12).
The Pacific oyster (Crassostrea gigas) is one
of the most economically important bivalves (13), and its global annual production
reached 4.2 million tons in 2007 (14). The protein extracts of the Pacific
oyster (PEPO) have been demonstrated to protect human epithelial
cells against oxidative stress and to increase glutathione and the
glutathione S-transferase activity levels in several organs of rats
(15,16). However, to the best of out
knowledge, no studies have examined the effects and underlying
mechanisms of the alleviation of hepatic fibrosis by PEPO.
Connective tissue growth factor (CTGF) is used as a
biomarker of hepatic fibrosis (17) as its levels have been associated
with the development of hepatic fibrosis (18–20),
and it can be used to evaluate the severity of cases of fibrosis
(21). Transforming growth factor
β (TGF-β), the key growth factor inducing the transcription of the
CTGF gene (22), is important in
the development hepatic fibrosis (23). Nuclear factor κB (NF-κB) can
accelerate recovery from hepatic fibrosis (24) as it functions to protect hepatic
stellate cells (HSCs) from apoptosis, and is key in the regulation
of TGF-β1 levels (25,26).
The present study was designed to explore whether
PEPO can alleviate the hepatic fibrosis induced by CCl4
in rats, and the main focus was the differential expression levels
of CTGF, TGF-β1 and NF-κB in liver tissues from rats exposed to
CCl4, with or without PEPO treatment.
Materials and methods
Materials
The Pacific oyster specimens were collected from the
waters around Zhoushan, China. Once the fresh whole bodies were
removed from the shells, they were stored at −20°C. The shelled
Pacific oysters (3.0 kg) were chopped and homogenized, which were
then processed with hot water (75°C) for 3.5 h. Once cooled to room
temperature, the homogenized mixture was filtered with Celite
powder (Linjiang Dahua Cellite Products Co., Ltd., Jilin, China)
and filter paper (Shijazhuang Golden Link Science Laboratory
Equiptment Co., Ltd., Shijiazhuang, China). The filtrate produced
was used as the crude PEPO in the present study. Colchicine and
CCl4 were provided by Sigma-Aldrich, St. Louis, MO,
USA.
Animals and groups
Sixty male Sprague-Dawley rats weighing 180–220 g
were provided by the Experimental Animal Centre, School of
Medicine, Zhejiang University (Hangzhou, China). All rats were
housed in a temperature-controlled room with a 12-h light/dark
cycle and maintained at a constant temperature of 25°C and a
humidity of 55%. They were fed with standard pellet food and tap
water ad libitum for 1 week. The current study was performed
according to The Care and Use of Laboratory Animals protocol of the
National Research Council, and was approved by the Ethics Committee
of The Third Clinical College of Zhejiang Chinese Medicine
University. The hepatic fibrosis model rats were established
through intragastric administration of CCl4 (mixed 1:1
with olive oil) at 2 ml/kg body weight twice a day for 12
consecutive weeks. The normal control rats were administered the
equivalent dosage of olive oil only (27–29).
The rats were maintained for 1 week prior to further experiments to
allow CCl4 penetration. A randomization chart
constructed in Microsoft Excel (Microsoft Corporation, Redmond, WA,
USA) was used to assign rats into six groups (n=10 in each group).
Each group received individual treatments that were orally
administered for 12 weeks: Group A (normal control group), olive
oil (2 ml/kg) twice a day; group B (model group), CCl4
(2 ml/kg) twice a day; group C (high-dose PEPO group),
CCl4 (2 ml/kg) twice a day and PEPO (8 mg/kg) once a
day; group D (medium-dose PEPO group), CCl4 (2 ml/kg)
twice a day and PEPO (4 mg/kg) once a day; group E (low-dose PEPO
group), CCl4 (2 ml/kg) twice a day and PEPO (2 mg/kg)
once a day; and group F (colchicine group), colchicine (2 mg/kg)
once a day. During the treatment period, the numbers of animal
mortalities were 0, 3, 0, 1, 2 and 2 in groups A, B, C, D, E and F,
respectively.
Sample collection and measurement
On the day following the 12-week treatments, after
fasting for 12 h, the body weight of each rat was measured. The
rats were then intraperitoneally anesthetized with urethane (1.2
g/kg). Blood samples were drawn from the abdominal aorta into
heparinized injectors (Huayi Biotech, Co., Ltd., Shanghai, China)
and then centrifuged at 550 xg at 4°C for 10 min. The supernatant
serum was then transferred to clean Eppendorf tubes and stored at
−80°C until required for the assay. Following the collection of
blood samples, the animals were sacrificed using cervical
dislocation, and the livers were immediately removed, washed with
physiological saline and weighed. The left lateral lobe of the
liver was sliced, and the tissue slices were fixed in 10%
neutral-buffered formalin (Beijing Reagen Biotechnology Co. Ltd.,
Beijing, China) for 24 h in preparation for the histological
examinations. The other parts of the livers were frozen and stored
at −80°C until required for the assay. Using commercial standard
assay kits (Wuhan Boster Bio-Engineering Limited Company, Wuhan,
China) the following enzymes were detected: Alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
γ-glutamyltransferase (GGT) and alkaline phosphatase (ALP). The
serum levels of hyaluronic acid (HA), laminin (LN), collagen type
IV (IV-C) and procollagen III (PC III) were detected by
radioimmunoassay kits (HaiYan Medical Biotechnology Center,
Shanghai, China). All measurements were performed in duplicate and
were conducted according to the manufacturer’s instructions. Intra-
and inter-assay coefficients of variation were <10%. The liver
index was calculated according to the following formula: (Liver
weight/rat weight) × 100.
Histological examinations and
immunohistochemistry
Once the fixed liver tissue slices were embedded in
paraffin, sectioned, deparaffinized and rehydrated, they were cut
into sections and mounted on slides. The slides were then stained
with hematoxylin and eosin (HE) for histopathological examination.
For immunohistochemical staining of CTGF, TGF-β1 and NF-κB, a
number of the sections were incubated with the monoclonal CTGF
antibody (rabbit-anti-rat; 1:200 dilution; Sigma, St Louis, MO,
USA), monoclonal TGF-β1 (rabbit-anti-rat; 1:400 dilution; Sigma)
and monoclonal NF-κB antibody (rabbit-anti-rat; 1:300 dilution;
Sigma) at 4°C overnight. Following washing of the slides with
Tris-buffered saline (TBS) twice, biotinylated secondary antibody
and horseradish peroxidase-conjugated streptavidin (Google
Biotechnology Co., Ltd., Wuhan, China) were applied to the liver
sections, and the expression was visualized by adding
3,3′-diaminobenzidine substrate (Sinopharm Chemical Reagent Co.,
Ltd, Shanghai, China) using an inversion fluorescence microscope
(Olympus IX71S1F-3; Olympus Corporation, Tokyo, Japan).
Detection of CTGF, NF-κB and TGF-β1 mRNA
expression in liver tissues with quantitative polymerase chain
reaction (qPCR)
Total RNA was isolated with RNAiso™ reagent (Takara
Biotechnology, Dalian, China) according to the instructions of the
manufacturer. The purity and concentration of the RNAs were
detected with a NanoDrop® ND-1000 spectrophotometer
(Thermo Fisher Scientific Inc., Waltham, MA, USA). The cDNA was
prepared from 500 ng total RNA by reverse transcription (RT) with
the PrimeScript™ RT Reagent kit (Perfect Real Time; Takara
Biotechnology). The cDNA samples were then diluted in DNase- and
RNase-free water at a proportion of 1:3 prior to further analysis.
PCR was performed using the iCycler iQ Real-Time PCR Detection
system (Bio-Rad, Hercules, CA, USA). The rat CTGF, TGF-β1 and NF-κB
gene-specific primers were provided by Sangon Biological
Engineering Technology (Shanghai, China). The sequences of the
primers were as follows: CTGF forward, 5′-GGCCCTGTGAAGCTGACCTA-3′
and reverse, 3′-CAGCCAGAAAGCTCAAACTTGAC-5′; NF-κB forward,
5′-AAAAACGCATCCCAAGGTGC-3′ and reverse, 3′-AAGCTCAAGCCACCATACCC-5′;
TGF-β1 forward, 5′-CACCGGAGAGCCCTGGATA-3′ and reverse,
3′-TCCAACCCAGGTCCTTCCTA-5′; GAPDH forward, 5′-GCAAGTTCAACGGCACAG-3′
and reverse, 3′-CGCCAGTAGACTCCACGAC-5′. PCR reactions were
performed using 2 μl cDNA, 10 μM each primer, and 2X
SYBR® Premix Ex Taq™ (Takara Biotechnology) in 20-μl
reactions. Thermal cycling conditions were as follows: 95°C for 10
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. A
final melting curve was used to verify single-product formation.
Gene starting quantity was based on the cycle threshold (Ct)
method. Each value was normalized to GAPDH, a housekeeping gene, to
control the amount of input cDNA. The Ct value for GAPDH mRNA was
subtracted from that of the target gene, and the mRNA levels of the
target gene were expressed as 2−ΔCt.
Detection of CTGF, NF-κB and TGF-β1
protein expression in liver tissues by western blotting
The protein lysates were prepared by homogenizing
the frozen liver tissue. A Bicinchoninic Acid Protein Assay kit
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used to
determine the protein concentration. Protein (40 μg) was incubated
in the loading buffer at 95°C for 5 min, cooled and then loaded
into lanes. Gel electrophoresis was performed on Mini-Protean III
gel apparatus (Bio-Rad) using 8% gel with 0.1% (w/v) SDS under a
constant current of 26 mA prior to being transferred to
nitrocellulose membranes (Dingguo Biotechnology, Beijing, China)
for 2 h. The membranes were then blocked for 1.5 h at room
temperature with 5% milk in TBS with Tween (TBST; 10 mM Tris pH
7.6, 150 mM NaCl and 0.05% Tween-20). Membranes were incubated with
the following primary antibody (rabbit-anti-rat) dilution: CTGF
antibody from Abcam, Cambridge, MA, USA, 1:2,000; NF-κB antibody
(rabbit-anti-rat) from Cell Signaling Technology, Inc., Danvers,
MA, USA, 1:1,500; TGF-β1 antibody (rabbit-anti-rat) from Abcam,
1:800; and β-actin antibody (rabbit-anti-rat) from Santa Cruz
Biotechnology, Inc., 1:5,000) overnight at 4°C. Once washed, the
membranes were incubated with their corresponding secondary
antibody (1:5,000) at room temperature for 2 h. The proteins were
detected with Enhanced Chemiluminescence reagent (Amersham
Biosciences, Piscataway, NJ, USA). Densitometric intensity was
measured with a GS-800 densitometer (Bio-Rad) and normalized
against the internal control β-actin.
Statistical analysis
All data were analyzed with Statistical Package for
Social Sciences (SPSS, version 13.0 for Windows; SPSS, Inc.,
Chicago, IL, USA). Analysis of variance was employed to analyze all
data. Two-tailed tests were used for all hypothesis tests and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Body and liver weights
No significant differences in the initial body
weights were identified among the different groups (P>0.05;
Table I). Following the 12-week
treatment, the average final body weight of the normal control rats
(group A) was significantly higher, while the liver weight and
liver index were significantly lower than those of all other groups
(P<0.05). Compared with the model group (group B), each of the
groups C–F displayed a significantly greater average final body
weight and significantly lower liver weight and liver index
(P<0.05). The weight of the rats in group B decreased markedly
over the treatment period and they displayed eminent irritability
and aggression. PEPO treatment inhibited the reduction of body
weight and the increase of liver weight and liver index in a
dose-dependent manner. High-dose PEPO treatment was demonstrated to
significantly inhibit the reduction of body weight and the increase
in liver weight and liver index compared with the colchicine group
(P<0.05). No significant differences in the final body weight
and liver index were identified between the medium-dose PEPO
treatment group and the colchicine treatment group (P>0.05);
however, the rats in the medium-dose PEPO treatment group exhibited
a significantly lower liver weight (P<0.05). Significant
differences in the final body weight and liver index were
identified between the low-dose PEPO treatment group and the
colchicine treatment group (P<0.05); however, no difference was
noted in their average liver weights (P>0.05).
 | Table IBody and liver weights. |
Table I
Body and liver weights.
| | Body weight
(g) | | |
|---|
| |
| | |
|---|
| Group | n | Initial | Final | Liver weight
(g) | Liver index
(%) |
|---|
| A | 10 | 200.2±13.3 | 475.6±41.7 | 7.1±0.8 | 1.5±0.2 |
| B | 7 | 201.2±8.7 | 295.2±23.5a | 13.2±1.6a | 4.5±0.5a |
| C | 10 | 197.8±10.9 | 423.4±30.7a–c | 9.2±0.5a–c | 2.2±0.2a–c |
| D | 9 | 201.1±11.2 | 367.6±39.1a,b | 10.4±0.9a-c | 2.9±0.5a,b |
| E | 8 | 203.0±9.9 | 322.6±22.5a–c | 11.4±1.1a,b | 3.6±0.5a–c |
| F | 8 | 198.1±13.7 | 360.4±30.3a,b | 11.2±0.5a,b | 3.1±0.3a,b |
Histopathological findings
Normal lobular architecture with central veins and
radiating hepatic cords were observed in the normal control rats,
whilst the hepatic fibrosis model rats displayed marked fat
degeneration, portal inflammation and necrosis, evident collagen
deposition, perihepatocyte fibrosis and hepatocyte loosening
(Fig. 1A). All PEPO treatment and
colchicine treatment groups demonstrated an amelioration in the
severity of hepatic fibrosis; however, high-dose and medium-dose
PEPO treatment were the most effective.
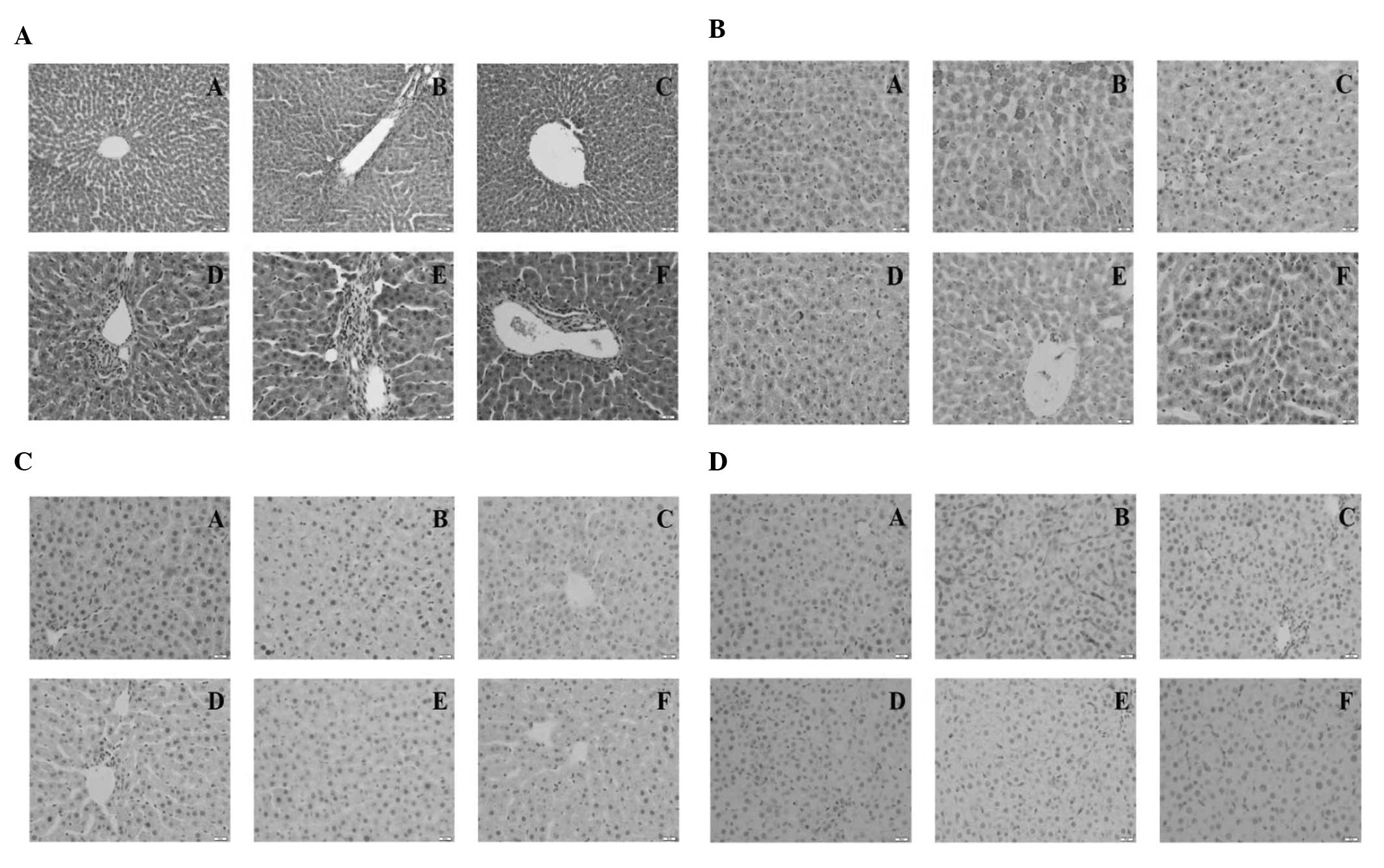 | Figure 1Representative photomicrographs of
histopathological findings. (A) Rat liver tissues with HE stain.
(B) CTGF immunohistochemistry. (C) NF-κB immunohistochemistry. (D)
TGF-β1 immunohistochemistry. Magnification, ×200. Group A, normal
control group (2 ml/kg olive oil); group B, model group (2 ml/kg
CCl4); group C, high-dose PEPO group (8 mg/kg PEPO+2
ml/kg CCl4); group D, medium-dose PEPO group (4 mg/kg
PEPO+2 ml/kg CCl4); group E, low-dose PEPO group (2
mg/kg PEPO+2 ml/kg CCl4); group F, colchicine group (2
mg/kg colchicine+2 ml/kg CCl4). HE, hematoxylin and
eosin; CTGF, connective tissue growth factor; NF-κB, nuclear factor
κB; TGF-β, transforming growth factor β; PEPO, protein extract of
Pacific oyster. |
Immunohistochemistry
The results of the immunohistochemical staining
demonstrated that the protein expression levels of CTGF, NF-κB and
TGF-β1 were lowest in the normal control group and highest in the
model group (Fig. 1B–D). All of
the PEPO and colchicine treatment groups exhibited decreased
expression levels of the three genes compared with those of the
model group.
Serum levels of ALT, AST, GGT and
ALP
The serum levels of ALT, AST, GGT and ALP in the
normal control rats (group A) were significantly lower than those
in all the other groups (P<0.05) (Fig 2A–D). Groups C–F exhibited
significantly lower levels of ALT, AST, GGT and ALP in the serum
compared with those of group B (P<0.05). The PEPO treatment
decreased the serum levels of ALT, AST, GGT and ALP in a
dose-dependent manner. High-dose PEPO treatment significantly
reduced the serum levels of ALT, AST, GGT and ALP compared with
those in the colchicine group (P<0.05). The medium-dose PEPO
treatment group displayed significantly lower levels of AST and ALP
than the colchicine treatment group (P<0.05); however, no
significant differences in their ALT and GGT levels were detected
(P>0.05). Significant differences in the serum levels of ALT,
AST and GGT were detected between the low-dose PEPO and colchicine
treatment groups (P<0.05); however, no difference was noted in
their ALP levels (P>0.05).
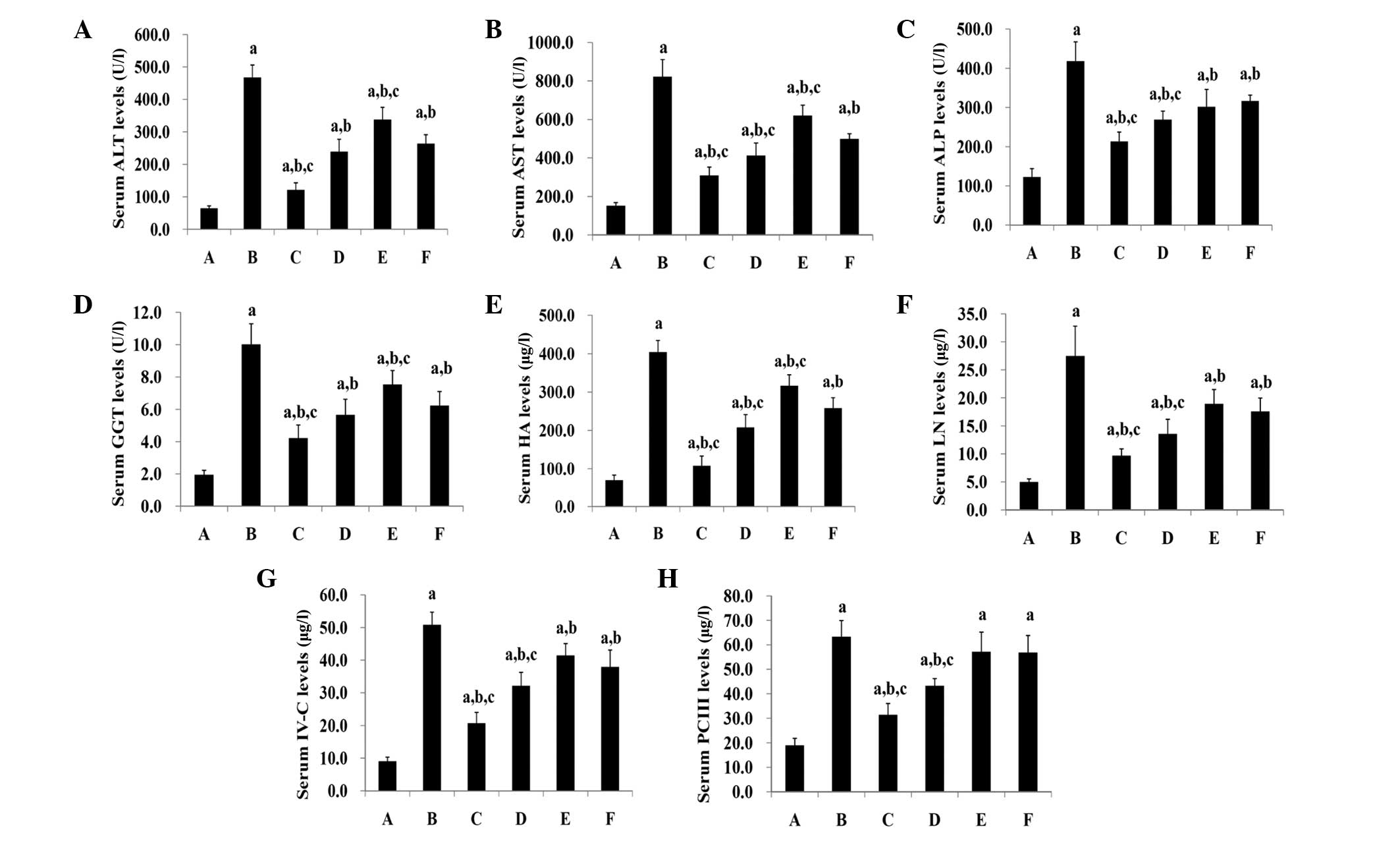 | Figure 2Serum levels of (A) ALT, (B) AST, (C)
ALP, (D) GGT, (E) HA, (F) LN, (G) IV-C and (H) PC III. Group A,
normal controls (2 ml/kg olive oil); group B, models (2 ml/kg
CCl4); group C, high-dose PEPO (8 mg/kg PEPO+2 ml/kg
CCl4); group D, medium-dose PEPO (4 mg/kg PEPO+2 ml/kg
CCl4); group E, low-dose PEPO (2 mg/kg PEPO+2 ml/kg
CCl4); group F, colchicine (2 mg/kg colchicine+2 ml/kg
CCl4). Data are presented as the mean ± standard
deviation. (n=10, 7, 10, 9, 8 and 8 in groups A, B, C, D, E and F,
respectively). P<0.05 was considered to indicate a statistically
significant difference. aP<0.05 vs. group A;
bP<0.05 vs. group B; cP<0.05 vs. group
F (analysis of variance). ALT, alanine aminotransferase; AST,
aspartate aminotransferase; ALP, alkaline phosphatase; GGT,
γ-glutamyltransferase; HA, hyaluronic acid; LN, laminin; IV-C,
collagen type IV; PC III, procollagen III; PEPO, protein extract of
Pacific oyster. |
Serum levels of HA, LN, IV-C and PC
III
The serum levels of HA, LN, IV-C and PC III in the
normal controls (group A) were significantly lower than those in
all other groups (P<0.05) (Fig.
2E–H). Groups C-F displayed significantly lower levels of HA,
LN, and IV-C in the serum compared with those of group B
(P<0.05). High-dose and medium-dose PEPO treatment significantly
decreased the serum PC III levels, compared with those of group B
(P<0.05); however, no significant differences in the serum PC
III levels were identified between group B and groups E and F
(P>0.05). The PEPO treatment reduced the serum levels of HA, LN,
IV-C and PC III in a dose-dependent manner. High- and medium-dose
PEPO treatment significantly decreased the serum levels of HA, LN,
IV-C and PC III compared with the colchicine group (P<0.05). A
significant difference between the low-dose PEPO and colchicine
treatment groups was identified in the serum levels of HA
(P<0.05), but not in those of LN, IV-C and PC III
(P>0.05).
CTGF, NF-κB and TGF-β1 protein expression
in liver tissues
The CTGF, NF-κB and TGF-β1 protein expression levels
in liver tissues of the normal control rats (group A) were
demonstrated to be significantly lower than those in all other
groups (P<0.05) and the levels in the model group (group B) were
significantly higher than those in groups C-F (P<0.05) (Fig. 3A and B). The PEPO treatment
decreased the protein expression of CTGF and NF-κB in liver tissues
in a dose-dependent manner. High-dose PEPO treatment significantly
reduced the expression levels of all three proteins in liver
tissues compared with the levels in the colchicine treatment group
(P<0.05). Medium-dose PEPO treatment significantly decreased the
protein expression levels of CTGF and NF-κB in the liver tissues
compared with those in the colchicine treatment group (P<0.05);
but it did not significantly affect the TGF-β1 levels (P>0.05).
A significant difference was noted in the CTGF protein expression
levels between the low-dose PEPO and colchicine treatment groups
(P<0.05); but not in those of NF-κB or TGF-β1 (P>0.05).
 | Figure 3(A) CTGF and NF-κB; and (B) TGF-β1
protein expression in liver tissues. Group A, normal controls (2
ml/kg olive oil); group B, models (2 ml/kg CCl4); group
C, high-dose PEPO (8 mg/kg PEPO+2 ml/kg CCl4); group D,
medium-dose PEPO (4 mg/kg PEPO+2 ml/kg CCl4); group E,
low-dose PEPO (2 mg/kg PEPO+2 ml/kg CCl4); group F,
colchicine group (2 mg/kg colchicine+2 ml/kg CCl4). Data
are presented as the mean ± standard deviation. (n=10, 7, 10, 9, 8
and 8 in groups A, B, C, D, E and F, respectively). P<0.05 was
considered to indicate a statistically significant difference.
aP<0.05 vs. group A; bP<0.05 vs. group
B; cP<0.05 vs. group F (analysis of variance). CTGF,
connective tissue growth factor; NF-κB, nuclear factor κB; TGF-β,
transforming growth factor β; PEPO, protein extract of Pacific
oyster. |
CTGF, NF-κB and TGF-β1 mRNA expression in
liver tissues
The CTGF and NF-κB mRNA expression levels in liver
tissues of the normal control group (group A) were significantly
lower than those of all other groups (P<0.05), whilst the levels
in the model group (group B) were significantly higher than those
in all other groups (P<0.05) (Fig.
4A–C). The PEPO treatment reduced the mRNA expression levels of
CTGF and NF-κB in the liver tissues in a dose-dependent manner.
High-dose and medium-dose PEPO treatment significantly decreased
the mRNA expression levels of these two genes in liver tissues
compared with those of the colchicine treatment group (P<0.05).
No significant differences in CTGF and NF-κB mRNA expression levels
were identified between the low-dose PEPO and colchicine treatment
groups (P<0.05). With regards to TGF-β1, the normal control rats
(group A) displayed significantly lower mRNA expression levels than
those in the other groups (P<0.05), with the exception of the
high-dose PEPO treatment group (group E) (P>0.05). TGF-β1 mRNA
expression levels in the model group (group B) were indicated to be
significantly higher than those in the high-dose and medium-dose
PEPO treatment groups (P<0.05). No significant differences in
TGF-β1 mRNA expression levels were identified between the three
PEPO treatment groups and the colchicine treatment group
(P>0.05).
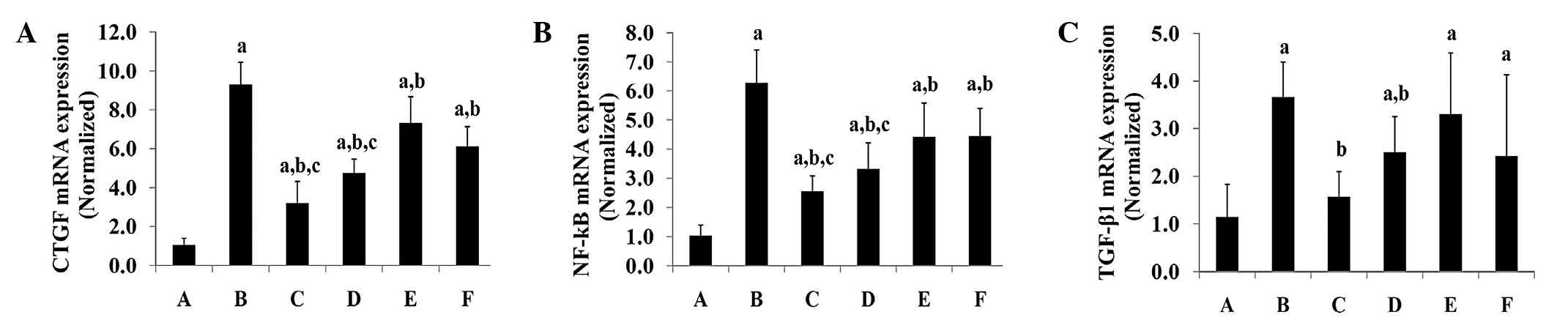 | Figure 4(A) CTGF; (B) NF-κB; and (C) TGF-β1
mRNA expression in liver tissues. Group A, normal controls (2 ml/kg
olive oil); group B, models (2 ml/kg CCl4); group C,
high-dose PEPO (8 mg/kg PEPO+2 ml/kg CCl4); group D,
medium-dose PEPO (4 mg/kg PEPO+2 ml/kg CCl4); group E,
low-dose PEPO (2 mg/kg PEPO+2 ml/kg CCl4); group F,
colchicine (2 mg/kg colchicine+2 ml/kg CCl4). Data are
presented as the mean ± standard deviation. (n=10, 7, 10, 9, 8 and
8 in groups A, B, C, D, E and F, respectively). P<0.05 was
considered to indicate a statistically significant difference.
aP<0.05 vs. group A; bP<0.05 vs. group
B; cP<0.05 vs. group F (analysis of variance). CTGF,
connective tissue growth factor; NF-κB, nuclear factor κB; TGF-β,
transforming growth factor β; PEPO, protein extract of Pacific
oyster. |
Discussion
Corticosteroids, penicillamine, methotrexate,
silymarin and colchicines have been widely applied to treat hepatic
fibrosis clinically, but none of them have been recognized as a
definitive intervention (8–11).
In the current study, it was demonstrated that PEPO successfully
alleviates the hepatic fibrosis induced by CCl4 and
reverses hepatotoxicity by regulating the expression of serum
enzymes and decreasing the expression levels of CTGF, TGF-β1 and
NF-κB in liver tissues. These findings may provide a novel
treatment option for patients with hepatic fibrosis in the
future.
In the present study, CCl4 was used to
establish model rats with hepatic fibrosis. This method has been
widely used in previous studies (30,31).
The hepatoprotective and anti-hepatofibrosis effects of PEPO were
evaluated in the current study by measuring body and liver weights,
examining histological changes, detecting serum levels of ALT, AST,
GGT, ALP, HA, LN, IV-C and PC III, and determining alterations in
the liver tissue protein and mRNA expression levels of CTGF, NF-κB
and TGF-β1, which are overall indicators of hepatic function and
hepatic fibrosis conditions. It was demonstrated that PEPO
treatment significantly reduced the serum levels of ALT, AST, GGT,
ALP, HA, LN, IV-C and PC III in hepatic fibrosis model rats
compared with those in untreated models. HE staining exhibited that
PEPO treatment markedly alleviated the fibrosis.
CTGF is synthesized in hepatocytes, and has been
demonstrated to alter cellular responses of growth factors
(17,32). The expression of CTGF has been
demonstrated to be eminently induced in fibrotic liver, and its
levels are significantly elevated in fibrotic lesions (33–35).
Serum levels of CTGF have been associated with the development of
hepatic fibrosis (18–20). For patients with HBV-induced
hepatic fibrosis, the serum CTGF levels can be used to distinguish
between mild and severe cases of fibrosis (21).
TGF-β is a well-known profibrotic cytokine, which
can stimulate the activation and proliferation of HSCs to induce
their transition to myofibroblast-like cells (17,36–38).
TGF-β1 may be the principal growth factor inducing the
transcription of the CTGF gene (22). The key roles of TGF-β1 in the
induction of fibrosis have been demonstrated by various
experimental models (17,39–41).
As the main HSC-transformation promoting cytokine in Kupffer cells
and infiltrating mononuclear cells, TGF-β1 is key in the
development of hepatic fibrosis (23). The secretion of CTGF depends on
levels of TGF-β1 (17,42–44),
and CTGF expression levels, which are linked to TGF-β pathways in
fibro-proliferative diseases, are increased in fibrotic human liver
(45). Tissue fibrosis has been
indicated to be associated with increased TGF-β and CTGF
production, and there exists a coordinate expression of TGF-β
before CTGF in regenerating tissues (46). As an enhancer of profibrogenic
TGF-β1, CTGF functions to mediate fibre-fibre, fibre-matrix and
matrix-matrix interactions (44).
NF-κB is important during the processes of hepatocyte
survival/damage, stellate and inflammatory cell activation and
inflammatory cytokine production (47,48).
As NF-κB has been demonstrated to protect HSCs from apoptosis, it
may be useful to accelerate recovery from hepatic fibrosis
(24). The activation of HSCs and
other liver-originated cells have been demonstrated to be closely
correlated with activation of the transcription factors TGF-β/Smad
and NF-κB (25,48,49).
As NF-κB is key in regulating TGF-β1 levels, a potential crosstalk
mechanism between NF-κB and TGF-β/Smad has been proposed previously
(47), and potential NF-κB binding
sites in the promoter of the CTGF gene have been located (25,26).
In the present study, it was demonstrated that the protein and mRNA
expression levels of CTGF, TGF-β1 and NF-κB in the liver tissues
were significantly reduced by PEPO treatment. It was therefore
concluded that PEPO successfully alleviated the hepatic fibrosis
induced by CCl4 and reversed hepatotoxicity through
regulating the levels of serum enzymes and decreasing the
expression levels of CTGF, TGF-β1 and NF-κB in the liver tissues.
However, further in vitro experiments using HSCs are
required to elucidate other mechanisms underlying PEPO alleviation
of hepatic fibrosis induced by CCl4.
In conclusion, in the present study, PEPO
successfully alleviated the hepatic fibrosis induced by
CCl4 and reversed hepatotoxicity by regulating serum
enzymes and reducing the expression of CTGF, TGF-β1 and NF-κB in
liver tissues.
Acknowledgements
The study was supported by the Open Research Fund of
Zhejiang First-foremost Key Subject-Acupuncture and Moxibustion
(ZTK 2010A 15) the International Science and Technology Cooperation
Program of Zhejiang Province (2012C24017) and a grant from the
Doctoral Program of Higher Education of China (no.
20133326120006).
Abbreviations:
|
CTGF
|
connective tissue growth factor
|
|
TGF-β1
|
transforming growth factor β1
|
|
NF-κB
|
nuclear factor κB
|
References
|
1
|
Friedman SL: Mechanisms of disease:
Mechanisms of hepatic fibrosis and therapeutic implications. Nat
Clin Pract Gastroenterol Hepatol. 1:98–105. 2004. View Article : Google Scholar
|
|
2
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poli G: Pathogenesis of liver fibrosis:
role of oxidative stress. Mol Aspects Med. 21:49–98. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Afdhal NH: The natural history of
hepatitis C. Semin Liver Dis. 24(Suppl 2): 3–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McMahon BJ: The natural history of chronic
hepatitis B virus infection. Semin Liver Dis. 24(Suppl 1): 17–21.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment, and current and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brenner DA: Molecular pathogenesis of
liver fibrosis. Trans Am Clin Climatol Assoc. 120:361–368.
2009.PubMed/NCBI
|
|
8
|
Rosenbloom J, Castro SV and Jimenez SA:
Narrative review: fibrotic diseases: cellular and molecular
mechanisms and novel therapies. Ann Intern Med. 152:159–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lissoos TW, Beno DW and Davis BH: Hepatic
fibrogenesis and its modulation by growth factors. J Pediatr
Gastroenterol Nutr. 15:225–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedman SL: Seminars in medicine of the
Beth Israel Hospital, Boston. The cellular basis of hepatic
fibrosis Mechanisms and treatment strategies. N Engl J Med.
328:1828–1835. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mavier P and Mallat A: Perspectives in the
treatment of liver fibrosis. J Hepatol. 22(Suppl): 111–115.
1995.PubMed/NCBI
|
|
12
|
Iredale J: Defining therapeutic targets
for liver fibrosis: exploiting the biology of inflammation and
repair. Pharmacol Res. 58:129–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaura K, Takahashi KG and Suzuki T:
Identification and tissue expression analysis of C-type lectin and
galectin in the Pacific oyster, Crassostrea gigas. Comp Biochem
Physiol B Biochem Mol Biol. 149:168–175. 2008. View Article : Google Scholar
|
|
14
|
Fishery and Aquaculture Statistics 2007.
Food and Agriculture Organization of the United Nations; Rome: pp.
542009
|
|
15
|
Gaté L, Schultz M, Walsh E, et al: Impact
of dietary supplement of Crassostrea gigas extract (JCOE) on
glutathione levels and glutathione S-transferase activity in rat
tissues. In Vivo. 12:299–303. 1998.PubMed/NCBI
|
|
16
|
Gaté L, Paul J, Ba GN, Tew KD and Tapiero
H: Oxidative stress induced in pathologies: the role of
antioxidants. Biomed Pharmacother. 53:169–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gressner OA and Gressner AM: Connective
tissue growth factor: a fibrogenic master switch in fibrotic liver
diseases. Liver Int. 28:1065–1079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamatani T, Kobayashi H, Tezuka K, et al:
Establishment of the enzyme-linked immunosorbent assay for
connective tissue growth factor (CTGF) and its detection in the
sera of biliary atresia. Biochem Biophys Res Commun. 251:748–752.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Wang NY, Yang CB, et al: The
clinical value of serum connective tissue growth factor in the
assessment of liver fibrosis. Dig Dis Sci. 55:767–774. 2010.
View Article : Google Scholar
|
|
20
|
Guo-Qiu W, Nai-Feng L, Xiao-Bo V, et al:
The level of connective tissue growth factor in sera of patients
with hepatitis B virus strongly correlates with stage of hepatic
fibrosis. Viral Immunol. 23:71–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piao RL, Brigstock DR, Zhu J, Zhang ML and
Gao RP: Clinical significance of connective tissue growth factor in
hepatitis B virus-induced hepatic fibrosis. World J Gastroenterol.
18:2280–2286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dessein A, Arnaud V, He H, et al: Genetic
analysis of human predisposition to hepatosplenic disease caused by
schistosomes reveals the crucial role of connective tissue growth
factor in rapid progression to severe hepatic fibrosis. Pathol Biol
(Paris). 61:3–10. 2013. View Article : Google Scholar
|
|
23
|
Torre F, Bellis L, Delfino A, et al:
Peripheral blood serum markers for apoptosis and liver fibrosis:
are they trustworthy indicators of liver realness? Dig Liver Dis.
40:441–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Papa S, Zazzeroni F, Bubici C, et al:
Gadd45 beta mediates the NF-kappa B suppression of JNK signalling
by targeting MKK7/JNKK2. Nat Cell Biol. 6:146–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu M, Zhang J, Zhu X, et al: Peroxisome
proliferator-activated receptor gamma inhibits transforming growth
factor beta-induced connective tissue growth factor expression in
human aortic smooth muscle cells by interfering with Smad3. J Biol
Chem. 276:45888–45894. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen A and Zheng S: Curcumin inhibits
connective tissue growth factor gene expression in activated
hepatic stellate cells in vitro by blocking NF-kappaB and ERK
signalling. Br J Pharmacol. 153:557–567. 2008. View Article : Google Scholar
|
|
27
|
Sun H, Che QM, Zhao X and Pu XP:
Antifibrotic effects of chronic baicalein administration in a
CCl4 liver fibrosis model in rats. Eur J Pharmacol.
631:53–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erman F, Balkan J, Cevikbaş U, Koçak-Toker
N and Uysal M: Betaine or taurine administration prevents fibrosis
and lipid peroxidation induced by rat liver by ethanol plus carbon
tetrachloride intoxication. Amino Acids. 27:199–205. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Zhang S, Huang Q, et al: Protective
effect of Fufang-Liu-Yue-Qing, a traditional Chinese herbal
formula, on CCl4 induced liver fibrosis in rats. J
Ethnopharmacol. 142:548–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Basu S: Carbon tetrachloride-induced lipid
peroxidation: eicosanoid formation and their regulation by
antioxidant nutrients. Toxicology. 189:113–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh WY, Pyo S, Lee KR, et al: Effect of
Holotrichia diomphalia larvae on liver fibrosis and hepatotoxicity
in rats. J Ethnopharmacol. 87:175–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dendooven A, Gerritsen KG, Nguyen TQ, Kok
RJ and Goldschmeding R: Connective tissue growth factor (CTGF/CCN2)
ELISA: a novel tool for monitoring fibrosis. Biomarkers.
16:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abou-Shady M, Friess H, Zimmermann A, et
al: Connective tissue growth factor in human liver cirrhosis.
Liver. 20:296–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hora C, Negro F, Leandro G, et al:
Connective tissue growth factor, steatosis and fibrosis in patients
with chronic hepatitis C. Liver Int. 28:370–376. 2008. View Article : Google Scholar
|
|
35
|
Williams EJ, Gaça MD, Brigstock DR, Arthur
MJ and Benyon RC: Increased expression of connective tissue growth
factor in fibrotic human liver and in activated hepatic stellate
cells. J Hepatol. 32:754–761. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Wen XM, Lui EL, et al: Therapeutic
targeting of the PDGF and TGF-beta-signaling pathways in hepatic
stellate cells by PTK787/ZK22258. Lab Invest. 89:1152–1160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gressner OA, Lahme B, Demirci I, Gressner
AM and Weiskirchen R: Differential effects of TGF-beta on
connective tissue growth factor (CTGF/CCN2) expression in hepatic
stellate cells and hepatocytes. J Hepatol. 47:699–710. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar
|
|
40
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakamura T, Sakata R, Ueno T, Sata M and
Ueno H: Inhibition of transforming growth factor beta prevents
progression of liver fibrosis and enhances hepatocyte regeneration
in dimethylnitrosamine-treated rats. Hepatology. 32:247–255. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Povero D, Busletta C, Novo E, et al: Liver
fibrosis: a dynamic and potentially reversible process. Histol
Histopathol. 25:1075–1091. 2010.PubMed/NCBI
|
|
43
|
Tache D, Bogdan F, Pisoschi C, et al:
Evidence for the involvement of TGF-β1-CTGF axis in liver
fibrogenesis secondary to hepatic viral infection. Rom J Morphol
Embryol. 52(Suppl): 409–412. 2011.
|
|
44
|
Weng HL, Ciuclan L, Liu Y, et al:
Profibrogenic transforming growth factor-beta/activin receptor-like
kinase 5 signaling via connective tissue growth factor expression
in hepatocytes. Hepatology. 46:1257–1270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kovalenko E, Tacke F, Gressner OA, et al:
Validation of connective tissue growth factor (CTGF/CCN2) and its
gene polymorphisms as noninvasive biomarkers for the assessment of
liver fibrosis. J Viral Hepat. 16:612–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Igarashi A, Okochi H, Bradham DM and
Grotendorst GR: Regulation of connective tissue growth factor gene
expression in human skin fibroblasts and during wound repair. Mol
Biol Cell. 4:637–645. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fox ES, Kim JC and Tracy TF: NF-kappaB
activation and modulation in hepatic macrophages during cholestatic
injury. J Surg Res. 72:129–134. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Demirbilek S, Akin M, Gürünlüoğlu K, et
al: The NF-kappaB inhibitors attenuate hepatic injury in bile duct
ligated rats. Pediatr Surg Int. 22:655–663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rippe RA, Schrum LW, Stefanovic B,
Solís-Herruzo JA and Brenner DA: NF-kappaB inhibits expression of
the alpha1(I) collagen gene. DNA Cell Biol. 18:751–761. 1999.
View Article : Google Scholar : PubMed/NCBI
|


















