Introduction
Tachyplesin I is a disulfide-stabilized β-hairpin
antimicrobial peptide with 17 residues that can be isolated from
hemocytes of the horseshoe crab (Tachypleus tridentatus).
This peptide inhibits the growth of Gram-negative and Gram-positive
bacteria at particularly low concentrations (1,2). A
synthetic peptide from tachyplesin I has been demonstrated to
decrease the growth of tumor cells in vitro and in
vivo following linkage to the integrin homing
arginine-glycine-aspartic acid (RGD) domain (3). Several studies had demonstrated that
tachylesin inhibited the proliferation of tumor cells, including
gastric adenocarcinoma, human hepatocarcinoma, prostate cancer and
melanoma (3–5). In addition, tachyplesin I affects the
differentiation of tumor cells (6). These findings suggest that
tachyplesin I may be used to as an anti-tumor agent.
Malignant gliomas are aggressive brain tumors with
limited therapeutic options. According to the type of glial cell in
cancerous tissues, gliomas can be divided into four types,
astrocytoma, oligodendroglioma or ependymoma (7). Glioma are composed of heterogeneous
types of tumor cells that differ in their expression of markers and
growth capacities (8). At present,
glioma stem cells (GSCs) have been identified as the major reason
for chemotherapy resistance in high-grade glioma (9). In addition, stem-like cells
(CD133+) have been found in malignant brain tumors and
included cells expressing specific neural progenitor proteins,
including nestin, SOX2 and OCT4 (10,11).
These could initiate tumor formation following xenotransplantation
(12).
The cancer stem cell (CSC) hypothesis provides new
insight into the heterogeneity of types of malignant tumor
(8). CSCs account for several
important processes in carcinogensis, including tumor invasion,
angiogenesis and recurrence, and tumor heterogeneity is determined,
in part, by the presence of these CSCs (13,14).
Therefore, the strategy in tumor therapy is aimed at destroying the
tumorigenic CSCs (14,15). The present study aimed to examine
the effects of tachplesin I on GSCs cells obtained from glioma U251
cell lines. It was hypothesized that tachplesin I is able to
inhibit the proliferation of GSCs from glioma and is a potential
antitumor drug.
Materials and methods
Tachyplesin I synthesis and cell
culture
A peptide of tachyplesin containing the sequence
NH2-K-W-C-F-R-V-C-Y-R-G-I-C-Y-I-R-R-C-R-CONH2
was synthesized by Hanyu Bioengineering Company (Shenzhen, China)
with a purity of >95%. There were two disulfide bonds present in
two positions, located between the 3rd and 16th cysteine and
between the 7th and 12th cysteine. The NH2-terminal was
acetylated and the COOH-terminal was amidated to prevent its
degradation. Prior to use, the peptides were dissolved in PBS at a
concentration of 5 μg/μl.
U251 human glioma cells were purchased from the
Chinese Academy of Sciences Cell Band (Shanghai, China), cultured
in RPMI-1640 (Gibco-BRL Life Technologies, Grand Island, NY, USA)
and supplemented with 10% fetal bovine serum (Gibco-BRL), 1%
penicillin and streptomycin (Hyclone, Logan, UT, USA). The U251
cells were collected in the logarithmic growth phase. The U251
cells were thoroughly dissociated using 0.25% trypsin to prepare
single-cell suspensions and then plated onto 10 cm dishes for
culture in serum-free medium at a density of 30–50
cells/cm2. Following incubation for 7 days at in 5%
CO2 and 100% humidity, clones of different morphological
types were observed and adherent cells at the bottom were removed.
Subsequently, the single-cell suspensions were cultured in 24-well
plates at a clonal density and passaged at 1:2 or 1:3.
Self-renewal assay and induction of
differentiation
The suspended cells (1×104 cells/ml) were
cultured in neurobasal-A medium, which consisted of 50 ng/ml basic
fibroblast growth factor (bFGF; Gibco-BRL), 50 ng/ml epidermal
growth factor (EGF, Gibco-BRL) and 10 μl/ml B27 (Gibco-BRL) or
RPMI-1640 supplemented with 10% fetal bovine serum (Gibco-BRL), 1%
penicillin and streptomycin, and primary clone spheres were formed.
Subsequently, primary clone spheres were digested using stem cell
accutase (Gibco-BRL), centrifuged at 1,000 × g for 5 min and single
cells were obtained for further culture to form secondary clone
spheres.
Immunofluorescence microscopy and
dyes
The second or third generation U251 tumor spheres
were centrifuged and suspended in complete media. The cells were
then dropped onto a polylysine embedding slice and fixed using 4%
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 30 min.
Following this, the cells were permeabilized with 0.3% Triton X-100
in phosphate-buffered saline (PBS) for 15 min and inhibited with 3%
bovine serum albumin for 30 min before adding the following primary
antibodies: Mouse anti-human Nestin (Abcam, Cambridge, MA, USA) and
mouse anti-human CD133 (Abcam). The secondary goat anti-mouse
immunoglobulin (Ig)G labeled with fluorescein isothiocyanate or
cyanine-3 (Abcam) were added. Images were captured using an Olympus
fluorescence microscope (Olympus, Toykyo, Japan). The contrast and
brightness of the micrographs were then adjusted using Adobe
Photoshop CS software (Adobe Inc., San Jose, CA, USA) for data
presentation.
Administering of GSC with tachyplesin I
and 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetraolium bromide (MTT)
assay
The second or third generation stem cell spheres
were administered with tachyplesin I. Following seeding for 24 h,
the experimental groups were treated with reagent containing
tachyplesin I in a concentration gradient (0, 10, 20, 40, 80 and
160 μg/ml). After 24 h, images were captured using an Olympus
fluorescence microscope (Olympus).
Cell viability was determined using an MTT assay.
Initially, U251 tumor spheres were digested to single cells using
0.25 % trypsin and seeded in a 96 well-plate. Tachyplesin I was
used to treat cells when the concentration reached to
103–104 cells in each well. Following
treatment with different concentrations of tachyplesin I for 24 or
48 h, the cultures were washed with PBS. Subsequently, MTT (0.5
mg/ml) was added to each well and the mixture was incubated at 37°C
for 4 h. Dimethyl sulfoxide (DMSO) of an equal volume was used
instead of the culture medium to dissolve the formazan crysals. The
mixture was then agitated at room temperature for 10 min and the
absorbance of each well was determined at 490 nm using a microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). All
experiments were repeated six times and the data are expressed as
the mean ± standard deviation (SD). The inhibitory rate of
tyachyplesin was assessed using the following formula: inhibitory
rate = (OD490 value of controls − OD490 value of tachyplesin
I-treated U251 cells)/(OD490 value of controls − OD490 value of
blanks) × 100%.
Transmission electron microscopy
The cells were fixed in 0.1 M phosphate buffer (pH
7.2) containing 2.5% glutaraldehyde (Sigma-Aldrich) for 1 h. This
was followed by fixation in 0.1 M phosphate buffer (pH 7.2)
containing 1% OsO4 (Sigma-Aldrich) for 1 h. The
specimens were dehydrated in graded ethanol (Zhongshan Jinqiao,
Beijing, China), embedded in epoxy resin (Electron Microscopy
Sciences, Hatfield, PA, USA), cut into ultrathin sections and
stained using uranyl acetate and lead citrate (Zhongshan Jinqiao).
The stained ultrathin sections were observed by transmission
electron microscopy (H-9500; Hitachi, Tokyo, Japan).
Scanning electron microscopy
For standard scanning electron microscopy, the cells
were fixed in 0.1 M phosphate buffer (pH 7.2) containing 2.5%
glutaraldehyde for 1 h and subsequently fixed in 0.1 M phosphate
buffer (pH 7.2) containing 1% OsO4 for 1 h. The cells
were then dehydrated in graded ethanol and critical-point air
drying was performed, following treatment with isomyl acetate. The
samples were sputter coated with OsO4 and observed using
a scanning electron microscope (S-4800; Hitachi, Tokyo, Japan)
(17).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between the mean values for individual groups were
assessed using a Wilcoxon signed-rank test. Statistical analyses
were performed using GraphPad Prism 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum deprivation in U251 cells induces
tumor stem cell sphere formation
Initially, the U251 cells were inoculated with
serum-free medium. After 24 h, the U251 cells were adhesively grown
with irregular protrusions. No proliferation of the U251 cells was
observed (Fig. 1A). However,
following culture in serum-free medium supplemented with B27, bFGF
and EGF, the U251 tumor cells became non-adhesive and formed tumor
spheres after 3 days (Fig. 1B).
Subsequently, individual clonal spheres grew suspended in medium in
the following 2–3 days. In addition, the majority of the clonal
spheres demonstrated spherical or ovoid shapes and the cells
exhibited a high proliferative activity. By contrast, the adhesive
cells were programmed towards cell death. CD133 and nestin, markers
of brain CSCs, were detected using indirect immunofluorescence. As
shown in Fig. 1C and D, CD133 and
nestin were positively expressed in the suspended clone spheres.
Based on these results, the suspended spheres derived from U251
cells formed glioma stem cells.
U251 stem cell growth patterns in
different media
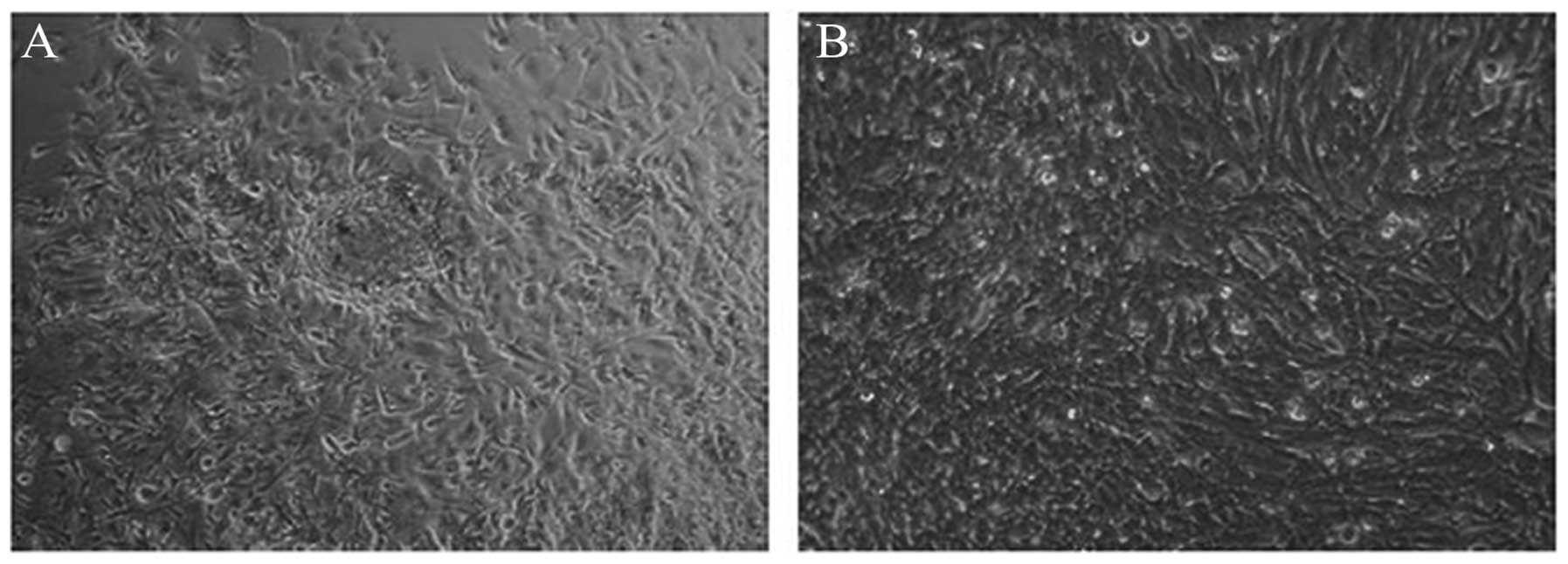
The U251 stem cells demonstrated different growth
patterns in serum and serum-free medium. When isolated GSCs were
cultured in medium with serum, after 4 h, the cell spheres grew
against the wall of the flask. Following 12 h culture, the single
cells had grown in the periphery of the stem cell spheres. As shown
in Fig. 2A, the cells were rounded
with some areas of cell flattening 24 h after culture. Adherent
cells formed a monolayer at day 3 (Fig. 2B). No differences were observed in
the morphological characteristics of GSCs following culture in
serum medium compared with routine methods of U251 cell culture.
However, when GSCs were cultured in serum-free medium, GSCs were
observed to grow in a suspended manner with tumor stem-like
morphology. In the following consecutive passages, conservation of
GSC sphere morphology, characteristics and proliferative capacity
was observed. Alterations in the growth pattern of the GSCs
coincided with the medium used during culture. In the medium
containing serum, the GSCs grew in an adherent manner, while the
cells were suspended following culture in serum-free medium.
Notably, the GSCs were plastic in response to their
environment.
Inhibition of GSC proliferation by
tachyplesin I is dose dependent
As GSCs are important in determining tumor
progression, the present study hypothesized that the inhibition of
GSCs contributed to limiting tumor proliferation. Therefore, the
present study examined the effects of tachyplesin I on the growth
of GSCs. An MTT assay was used to evaluate the proliferation of GSC
and U251 cells following treatment with various concentrations of
tachyplesin I for 24 or 48 h. As shown in Fig. 3A, GSC proliferation was inhibited
by tachyplesin I and the OD490 values were lower in the group
treated for 48 h compared with those treated for 24 h (P=0.0313).
Therefore, in addition to the decrease in OD490 with elevation in
tachyplesin I concentration, the OD490 values declined between 24
and 48 h (Fig. 3A). These results
indicated that the effects of tachyplesin I on GSC proliferation
occurred in a dose and time-dependent manner. The growth inhibitory
rates observed at the concentrations of 10, 20, 40, 80 and 160
μg/ml were 15.70, 25.82, 43.24, 91.70 and 96.45%, respectively, 24
h after treatment. After 48 h, the growth inhibitory rates were
33.47, 54.12, 79.44, 96.20 and 97.16%, respectively. These results
demonstrated that tachyplesin I had marked anti-proliferative
effects on the GSCs (Fig. 3B). In
addition, it was observed that the higher the concentration of
tachyplesin I, the stronger the anticancer-effects.
Tachyplesin I damages the structure of
GSCs
Following treatment of the GSC spheres with a series
of concentrations of tachyplesin I, observation using an inverted
microscope after 24 h demonstrated destruction of the morphological
characteristics.(Fig. 4).
Furthermore, a notable feature of the brain tumor-initiating cells
was the maintenance of tumor cells in an undifferentiated state.
For this reason, new therapeutic approaches that force initiating
cells to undergo differentiation to cease proliferation is being
sought to improve cancer treatment (18). When tachyplesin I was administered
at a concentration of 10–40 μg/ml, which induced GSC
differentiation, certain cells extended outward from the spheroids
in an adhesive manner (Fig 4B–D).
As the concentration of tachyplesin I increased to 80 μg/ml
(Fig 4E), there were several
vesicles within the spheres and the cell contents leaked out. A 160
μg/ml concentration of tachyplesin I induced cell death (Fig. 4F).
Imaging of intracellular deposition of
GSCs using transmission and scanning electron microscopy
To investigate the effects of tachyplesin I on GSCs,
transmission and scanning electron microscopy were used to examine
morphological changes. The primary cells were easily identified by
their lack of a nucleolus and small quantities of cytoplasm and
organelles (Fig. 5), consistent
with stem cells (19). Initially,
the GSCs had a clear cell structure (Figs. 5A and 6A) in the absence of tachyplesin I
treatment. The nuclei were darkly stained and located in the
central area with irregular morphologies. The cytoplasm contained
abundant mitochondria, endosomes, Golgi apparati and endoplasmic
reticula. The transmission electronmicrograph revealed the inner
GSC structures, with the cells containing dendritic processes and
folds. However, the cells treated with a low dose of tachyplesin I
had a disrupted plasma membrane, which led to the loss of
cytoplasmic organelles and a reduction in volume (Figs. 5B–D and 6B–D). Furthermore, a high dose of
tachyplesin I promoted cell death (Figs. 5E–F and 6E–F).
Discussion
Several studies have revealed that the progress of
carcinogenesis is driven and possibly accelerated by a subgroup of
cancer stem-like cells with higher self-renewal capacity and the
ability to differentiate (20).
Despite the development of neurosurgery, chemotherapy and
radiotherapy in previous decades, the mean survival rate of
patients with malignant glioma is limited to 2 years. GSCs may be a
potential target to improve the clinical efficacy of chemotherapy
(20). In the present study, it
was demonstrated that tachyplesin I inhibited tumor growth by
inducing GSC differentiation and death.
The separation of stem-like cells from cancer cell
lines offers a useful model for reflecting the biological
activities present in the human body during carcinogenesis
(22). valuable and accurate model
of disease is possible by the establishment of tumor cell lines,
which retain their stem cell properties to initiate cancer
(22). In the present study, a
large quantity of GSCs were obtained, which expressed CD133 and
nestin, the typical stem cell biomarkers (23). These cells may represent the most
malignant subset of tumor-initiating cells (9). The use of MTT assays revealed that
tachyplesin I inhibited the proliferation of GSCs in a dose and
time-dependent manner. However, due to a lack of sufficient
evidence, the detailed mechanism of how tachplesin disrupts the
progress of tumor stem-like cells remains to be elucidated. The
cationic amino acids of tachyplesin I have been detected in
negatively charged membranes, which interact with anionic head
groups of phospholipids (24). In
addition, tachyplesin I can destroy the integrity of the membrane
by disrupting the lipid bilayer with their amphipathic helices
(24). Above all, tachyplesin I is
more likely to disrupt prokaryotic and mictochondrial membranes, as
compared with the plasma membranes of eukaryotic cells due to the
presence of zwitterion phospholipids in eukaryotic cells. The
mechanism of the mitochondrial pathway, which induces tumor cell
apoptosis is important in inhibiting tumor progression. In previous
studies and in the present study, tachyplesin I was found to be
important in the disruption of mitochondrial membranes and,
therefore, it is proposed that tachyplesin I induces GSC apoptosis.
Chen et al demonstrated that RGD-tachyplesin I led to
upregulation in apoptosis associated with the mitochondrial and
death receptor pathways. RGD-tachyplesin I activated the expression
of either caspase 9, 8 and 3 or increased expression of the Fas
ligand, and induced cell apoptosis. Furthermore, RGD-tachyplesin I
also prevented tumor growth on the chorioallantoic membranes of
chicken embryos and in syngeneic mice (3). These results highlight the potential
use for tachyplesin I as an anti-tumor agent in clinical treatment
in the future.
Tachyplesin I may have other roles in regulating GSC
development. In the present study, tachyplesin I was found to
induce GSC differentiation. Li et al demonstrated that
thachyplesin downregulated the levels of mutant p52, cyclin D1 and
CDK4 proteins and c-myc mRNA. It also induced the differentiation
of human hepatocarcinoma cells (4)
and induced the differentiation of the human hepatocarcinoma cell
line, SMMC7721 (25). Furthermore,
tachyplesin I not only inhibits the proliferation of tumor cells,
but also regulates the cell cycle (4). In a previous study, tachyplesin I
arrested the cell cycle at the G0/G1 stage (4) and induced an immune reaction. Chen
et al (24) found that
classic complement cascade reactions were induced following the
linkage of tachyplesin I to hyaluronan in tumor cells or to C1q in
the serum. This resulted in tachyplesin I disrupting the integrity
of the tumor cell membrane and inducing cell death (24). Therefore, tachyplesin I has
multiple effects in regulating tumor cell growth, including the
induction of tumor cell apoptosis, differentiation and cell cycle
arrest at the G0/G1 stage.
In conclusion, the present study demonstrated that
tachyplesin I inhibited GSCs by disrupting the plasma membranes and
inducing GSC differentiation. Further investigation of tachyplesin
I, focussing on the detailed mechanisms of its role in inhibiting
tumor stem cells is required. In addition, it is necessary to
examine the role of tachyplesin I on glioma carcinogensis in
vivo.
Acknowledgements
This study was supported by the Shenzhen Basic
Research Key Projects (grant nos. JCYJ20130331151022276,
2111K3070010, JC201005280534A and JC201105201191A), the Guangdong
Province Natural Science Foundation (grant nos. 10151805501000007
and S2011020005160), GDPRSFS (2012), GDUHTP (2011 and 2013) and the
National Natural Science Funds of China (grant nos. 30800793 and
31272474).
References
|
1
|
Saravanan R, Mohanram H, Joshi M, et al:
Structure, activity and interactions of the cysteine deleted analog
of tachyplesin-1 with lipopolysaccharide micelle: Mechanistic
insights into outer-membrane permeabilization and endotoxin
neutralization. Biochim Biophys Acta. 1818:1613–1624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doherty T, Waring AJ and Hong M: Dynamic
structure of disulfide-removed linear analogs of tachyplesin-I in
the lipid bilayer from solid-state NMR. Biochemistry. 47:1105–1116.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Xu X, Hong S, et al:
RGD-Tachyplesin inhibits tumor growth. Cancer Res. 61:2434–2438.
2001.PubMed/NCBI
|
|
4
|
Li QF, Ou-Yang GL, Peng XX and Hong SG:
Effects of tachyplesin on the regulation of cell cycle in human
hepatocarcinoma SMMC-7721 cells. World J Gastroenterol. 9:454–458.
2003.PubMed/NCBI
|
|
5
|
Shi SL, Wang YY, Liang Y and Li QF:
Effects of tachyplesin and n-sodium butyrate on proliferation and
gene expression of human gastric adenocarcinoma cell line BGC-823.
World J Gastroenterol. 12:1694–1698. 2006.PubMed/NCBI
|
|
6
|
Wu XZ and Xie GR: Induced differentiation
of hepatocellular carcinoma by natural products. Afr J Tradit
Complement Altern Med. 5:325–331. 2008.PubMed/NCBI
|
|
7
|
Eyler CE, Wu Q, Yan K, et al: Glioma stem
cell proliferation and tumor growth are promoted by nitric oxide
synthase-2. Cell. 146:53–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
9
|
Gu C, Banasavadi-Siddegowda YK, Joshi K,
et al: Tumor-specific activation of the C-JUN/MELK pathway
regulates glioma stem cell growth in a p53-dependent manner. Stem
Cells. 31:870–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bleau AM, Howard BM, Taylor LA, et al: New
strategy for the analysis of phenotypic marker antigens in brain
tumor-derived neurospheres in mice and humans. Neurosurg Focus.
24:E282008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mo LJ, Ye HX, Mao Y, et al: B7-H4
expression is high in human U251 glioma stem-like cells and is
induced in monocytes cultured in U251 stem-like cell conditioned
medium. Chin J Cancer. 32:653–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rahman M, Deleyrolle L, Vedam-Mai V, et
al: The cancer stem cell hypothesis: failures and pitfalls.
Neurosurgery. 68:531–545. 2011. View Article : Google Scholar
|
|
13
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
14
|
Vermeulen L, Sprick MR, Kemper K, Stassi G
and Medema JP: Cancer stem cells - old concepts, new insights. Cell
Death Differ. 15:947–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vermeulen L, de Sousa e Melo F, Richel DJ
and Medema JP: The developing cancer stem-cell model: clinical
challenges and opportunities. Lancet Onco. 13:e83–e89. 2012.
View Article : Google Scholar
|
|
17
|
Akita M, Tanaka K, Murai N, et al:
Detection of CD133 (prominin-1) in a human hepatoblastoma cell line
(HuH-6 clone 5). Microsc Res Tech. 76:844–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sellheyer K: Stem cell markers can help
identify adnexal tumor differentiation when evaluated in the
context of morphology: methodology matters. J Cutan Pathol.
38:460–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue Z, Yan H, Li J, et al: Identification
of cancer stem cells in vincristine preconditioned SGC7901 gastric
cancer cell line. J Cell Biochem. 113:302–312. 2012. View Article : Google Scholar
|
|
20
|
Folkins C, Man S, Xu P, Shaked Y, Hicklin
DJ and Kerbel RS: Anticancer therapies combining antiangiogenic and
tumor cell cytotoxic effects reduce the tumor stem-like cell
fraction in glioma xenograft tumors. Cancer Res. 67:3560–3564.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu SC, Xiao HL, Jiang XF, et al: Connexin
43 reverses malignant phenotypes of glioma stem cells by modulating
E-cadherin. Stem Cells. 30:108–120. 2012. View Article : Google Scholar
|
|
22
|
Pollard SM, Yoshikawa K, Clarke ID, et al:
Glioma stem cell lines expanded in adherent culture have
tumor-specific phenotypes and are suitable for chemical and genetic
screens. Cell Stem Cell. 4:568–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji B, Chen Q, Liu B, et al: Glioma stem
cell-targeted dendritic cells as a tumor vaccine against malignant
glioma. Yonsei Med J. 54:92–100. 2013. View Article : Google Scholar
|
|
24
|
Chen J, Xu XM, Underhill CB, et al:
Tachyplesin activates the classic complement pathway to kill tumor
cells. Cancer Res. 65:4614–4622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li QF, Ouyang GL, Liu QR and Hong SG:
Tachyplesin-induced differentiation of human hepatocarcinoma cell
line SMMC-7721. Ai Zheng. 21:480–483. 2002.(In Chinese). PubMed/NCBI
|