Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, due to its high incidence, malignant
characteristics and the lack of available effective treatment
strategies. Non-small cell lung cancer (NSCLC) accounts for ~85% of
lung cancer cases. Surgery can be performed to treat patients in
the early stages of the disease; however, the rates of recurrence
and metastasis remain high in the majority of lung cancer patients
(1). Thus, understanding the
molecular mechanisms underlying progression and metastasis is
essential and may aid the identification of novel therapeutic
targets by improving the treatment response of NSCLC.
Immunoglobulin (Ig)-like transcript 4 (ILT4) is a
member of the Ig-like inhibitory receptor family and contains four
Ig domains and three immunoreceptor tyrosine-based inhibitory
motifs (2). ILT4 is predominantly
expressed in lymphoid and myeloid cells (3,4),
binding to major histocompatibility complex-I molecules, including
human leukocyte antigen (HLA)-A2, HLA-G and HLA-F, or their viral
homologue, UL18 (5–8). Inflammatory stimuli or cytokines,
such as interleukin-10 or interferon-α, and growth factors
(9) have been demonstrated to
regulate the expression of ILT4. A previous study revealed that
ILT4 expression may be induced in NSCLC cells and was found to be
associated with lymph node metastasis (10). However, the precise role of ILT4 in
the progression and metastasis of NSCLC is poorly understood.
ILT4 and its mouse ortholog, paired Ig-like receptor
(PirB), were found to be expressed in human and mouse hematopoietic
stem cells (HSCs), respectively (11). ILT4 and PirB are receptors of
several angiopoietin-like proteins (ANGPTLs) (11). The binding of ANGPTLs to ILT4
maintains the stemness of normal adult stem cells and supports
acute myeloid leukemia (AML) development (11). Among the ANGPTLs, ANGPTL2 and 5
have been shown to possess the highest affinities for ILT4
(11).
ANGPTLs constitute a family of seven secreted
glycoproteins and play an important role in expansion, lipid
metabolism, angiogenesis and inflammation in HSCs (12–15).
ANGPTLs 1 to 7 possess an N-terminal coiled-coil domain and a
C-terminal fibrinogen-like domain, which are characteristics of
angiopoietins. ANGPTL2 is a causative mediator of chronic
inflammation in obesity and its related metabolic abnormalities
(12). In addition, ANGPTL2 has
been demonstrated to be involved in inflammatory carcinogenesis
(16). In patients with NSCLC,
increased ANGPTL2 mRNA expression in tumor tissues is correlated
with lymph node metastasis (17).
Furthermore, the protein expression of ANGPTL2 has also been
observed in a number of other tumor types, including ovarian cancer
(18) and sarcoma (19). The tumor cell-derived protein,
ANGPTL2, accelerates tumor metastasis by increasing tumor cell
migration in an autocrine/paracrine manner, in addition to
enhancing tumor angiogenesis (20). ANGPTL5, which is mainly expressed
in adult human heart tissues, regulates the lipoprotein metabolism
and supports the efficient expansion of HSCs without compromising
their repopulating potential (21–23).
However, the role of ANGPTL5 in cancer has not yet been
explored.
To the best of our knowledge, no previous studies
exist regarding the function and co-expression of ILT4 and ANGPTLs
in solid tumor cells. Since ANGPTL2 and 5 bind to ILT4-expressing
cells more efficiently compared with other ANGPTLs (11), the present study focused on
assessing the co-expression of ANGPTL2/ANGPTL5 and ILT4 in the
NSCLC cell lines and tissues. In addition, the correlation between
ANGPTL2/ANGPTL5 and ILT4 was analyzed in cases where ILT4
expression was up- or downregulated. Furthermore, the association
of ILT4/ANGPTL2 or ILT4/ANGPTL5 co-expression with
clinicopathological features and the survival time of patients were
determined using NSCLC specimens. The present study may aid future
studies on the interaction among ILT4 and ANGPTLs in human
NSCLC.
Materials and methods
Cell culture
The following NSCLC cell lines were used: H1650,
H226, H1299, H1975 and A549 (Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China). The cell lines were
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (GE Healthcare Life Sciences, Logan, UT, USA), 100 U/l
penicillin (Beyotime Institute of Biotechnology, Shanghai, China)
and 100 mg/l streptomycin (Beyotime Institute of
Biotechnology).
Cell transfection
The following plasmids were obtained from Genechem
Co., Ltd. (Shanghai, China): Pez-lv105-ILT4 (ILT4 vector),
pGPU6/GFP/Neo-shILT4-1 [short hairpin (sh)ILT4-1] and
pGPU6/GFP/Neo-shILT4-2 (shILT4-2). The plasmids were transfected
into H1650 cells using the X-tremeGENE HP DNA Transfection Reagent
(Roche Diagnostics, Basel, Switzerland), according to the
manufacturer’s instructions. A nontargeting plasmid was used as a
negative control (NC). The shRNA sequences were as follows:
shILT4-1, 5′-GAAGAAGAACACCCACAATGC-3′; shILT4-2,
5′-GCTATGGTTATGACTTGAACT-3′; and NC, 5′-GTTCTCCGAACGTGTCACGT-3′.
The transfected cells were collected at 72 h and the gene
expression was assessed prior to further experiments.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s protocol. cDNAs were synthesized from the total RNA
(2 μg) using random primers with a First Strand cDNA Synthesis kit
(Fermentas, Ontario, Canada). RT-PCR was performed using the
following primers: ILT4 forward, 5′-GCATCTTGGATTACACGGATACG-3′, and
reverse, 5′-CTGACAGCCATATCGCCCTG-3′; ANGPTL2 forward,
5′-CGCATCTCATCTCCAAACTACA-3′, and reverse,
5′-CCAAACATCCAACATCTCACAC-3′; ANGPTL5 forward,
5′-CTGTATGTGGCTTTGGAATCTG-3′, and reverse,
5′-CGGTCTTGTTATGGAGGTGACT-3′); GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′, and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′. The reaction was incubated for 35
cycles at 94°C for 20 sec, 55.2°C for 20 sec and 72°C for 45 sec.
GAPDH was amplified simultaneously as an internal control.
Western blot analysis
Total protein (30 μg) was separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Boston,
MA, USA). Unspecific binding was blocked with 5% skimmed milk in
Tris-buffered saline containing 0.1% Tween-20 for 1 h at room
temperature. Next, the blotted membranes were incubated overnight
at 4°C with specific antibodies. The following primary antibodies
were used: Mouse monoclonal (mAb) anti-human ILT4 (1:400; Abgent,
Inc., San Diego, CA, USA), rabbit polyclonal anti-human ANGPTL2
(1:1,000 Proteintech Group, Inc., Wuhan, China), rabbit polyclonal
anti-human ANGPTL5 (1:1,000; Proteintech Group, Inc.) and rabbit
mAb anti-human GAPDH (1:10,000; Proteintech Group, Inc.). Detection
was performed using horseradish peroxidase-conjugated goat
anti-mouse/rabbit secondary antibodies (1:5,000; Proteintech Group,
Inc.). Finally, western blots were developed using an enhanced
chemiluminescence reagent (Beyotime Institute of Biotechnology,
Nantong, China) and exposed to a Kodak X-ray film (XAR-5; Kodak,
Rochester, NY, USA). The intensities of the bands were calculated
by densitometric analysis using the Image lab 4.1 software
(Bio-Rad, Co., Hercules, CA, USA).
Patients and samples
Tumor specimens were obtained following surgical
resection for NSCLC at the Jinan Central Hospital Affiliated to
Shandong University (Jinan, China). The study was approved by the
Review Board and Ethics Committee of the Jinan Central Hospital
Affiliated to Shandong University, and written informed consent was
obtained from all patients. A total of 114 NSCLC patients (male,
86; female, 28; mean age at diagnosis, 61.6 years) who were not
subjected to any preoperative therapy were included in the current
study. Among these 114 tumors, 53 were adenocarcinomas, 48 were
squamous cell carcinomas and 13 were other tumor types, while 6
were well-differentiated, 74 were moderately-differentiated and 34
were poorly-differentiated. The patients were classified according
to the TNM classification of the International Union Against Cancer
(24). Following classification,
which indicated the prognosis of patient, with stage IV being the
worse prognosis, 34 patients were determined to be at stage I, 44
at stage II, 25 at stage III and 11 at stage IV.
Immunohistochemical analysis
The resected tissue specimens were fixed in formalin
(ComWin Biotech Co., Beijing, China) overnight and embedded in
paraffin (ComWin Biotech Co.). A series of 4-μm sections were
prepared for immunohistological staining. The sections were
deparaffinized and rehydrated, and antigens were retrieved in Tris
buffer by boiling in a microwave oven (600 W) for 10 min. The
endogenous peroxidase activity was blocked with 0.3% hydrogen
peroxide for 10 min. Subsequently, the sections were incubated
overnight at 4°C with the primary antibodies [anti-ILT4 mAb
(1:200), anti-ANGPTL2 mAb (1:50) and anti-ANGPTL5 mAb (1:100)]. In
order to detect primary antibody binding, the sections were
incubated with Elivision™ plus Polyer HRP (mouse/rabbit) IHC kit
(Maixin, Fuzhou, China) for 30 min at room temperature and with
streptavidin-conjugated peroxidase (ComWin Biotech Co.) for further
30 min. The sections were visualized using a 3,3′-diaminobenzidine
solution (MaiXin) and counterstained with hematoxylin. Negative
controls were prepared using normal mouse and rabbit IgG
(Proteintech Group, Inc.) instead of the primary antibody.
Immunohistochemical assays were performed simultaneously by two
independent investigators. The percentage of stained cells was
recorded at a magnification of ×400 using biological microscopes
(bx43; Olympus Co., Tokyo, Japan), in at least five fields, in
randomly selected tumor areas. Brown staining of >10% of cells
was considered as positive.
Statistical analysis
SPSS software version 18.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. The association among the
expression levels of ILT4/ANGPTL2/ANGPTL5 and clinicopathological
variables were analyzed using χ2 test. Spearman’s
correlation analysis was used to analyze the correlation between
the expression levels of ILT4 and ANGPTL2/ANGPTL5. The overall
survival time was measured from the date of initial diagnosis to
mortality or the last day of the follow-up evaluation. Survival
curves were constructed using the Kaplan-Meier method and compared
using the log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Co-expression of ILT4 and ANGPTL2/ANGPTL5
in NSCLC cell lines
The mRNA co-expression levels of ILT4 with ANGPTL2
or ANGPTL5 in the five NSCLC cell lines were determined using
RT-PCR (Fig. 1A). In addition, the
protein co-expression levels of ILT4 and ANGPTL2/ANGPTL5 were
determined using western blot analysis in all cell lines (Fig. 1B). The results showed that ILT4 and
ANGPTL2/ANGPTL5 were found to be co-expressed in all five NSCLC
cell lines at the mRNA as well as the protein level.
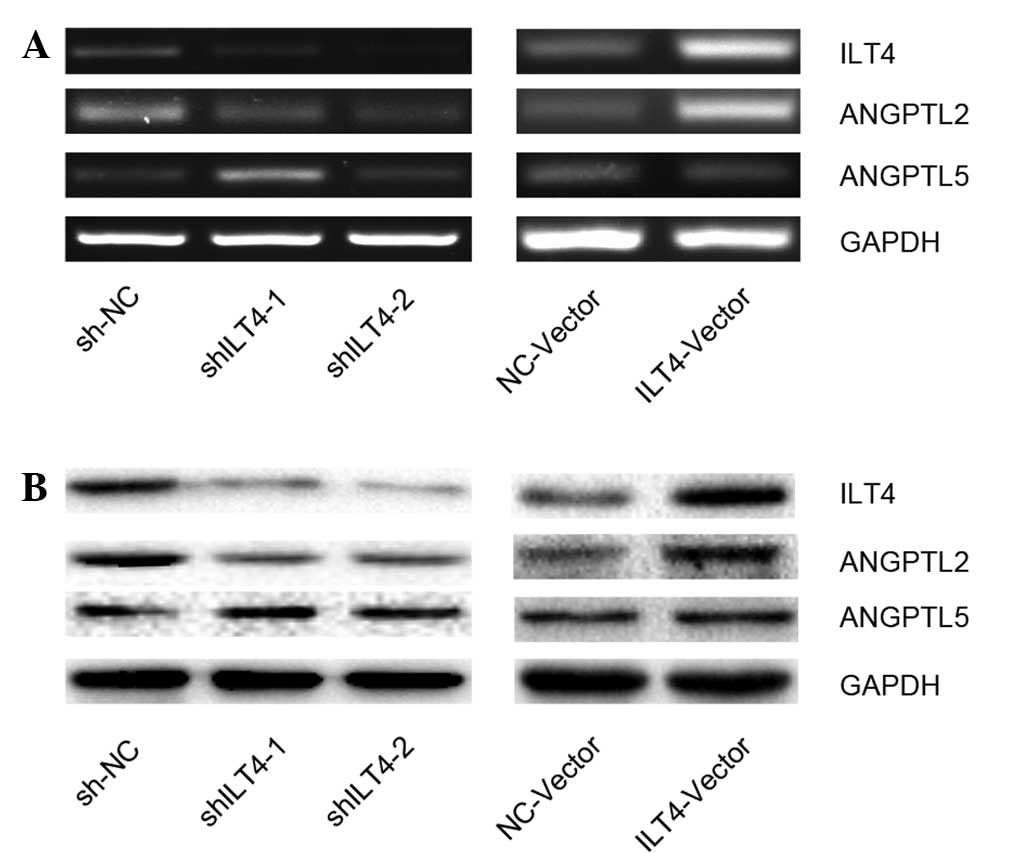
Effect of ILT4 expression on the
regulation of ANGPTL2 and ANGPTL5 in NSCLC cell lines
To determine the effect of ILT4 expression on
ANGPTLs, ILT4 expression was down- and upregulated using ILT4 shRNA
(shILT4-1 and shILT4-2) and ILT4 plasmids (ILT4-vector),
respectively. The cell line H1650 was selected to be used for all
subsequent experiments as the expression levels of ILT4 were
neither the highest or the lowest out of the cell lines examined.
Next, the expression levels of ANGPTL2 and ANGPTL5 were assayed at
the mRNA (Fig. 2A) and protein
levels (Fig. 2B). Downregulation
of ILT4 resulted in a reduced expression of ANGPTL2, whereas
upregulation of ILT4 was associated with an increased ANGPTL2
expression. However, the results demonstrated that silencing or
promoting ILT4 did not have a marked effect on the expression of
ANGPTL5 in H1650 cells compared with the NC group (Fig. 2).
Co-expression of ILT4 and ANGPTL2/ANGPTL5
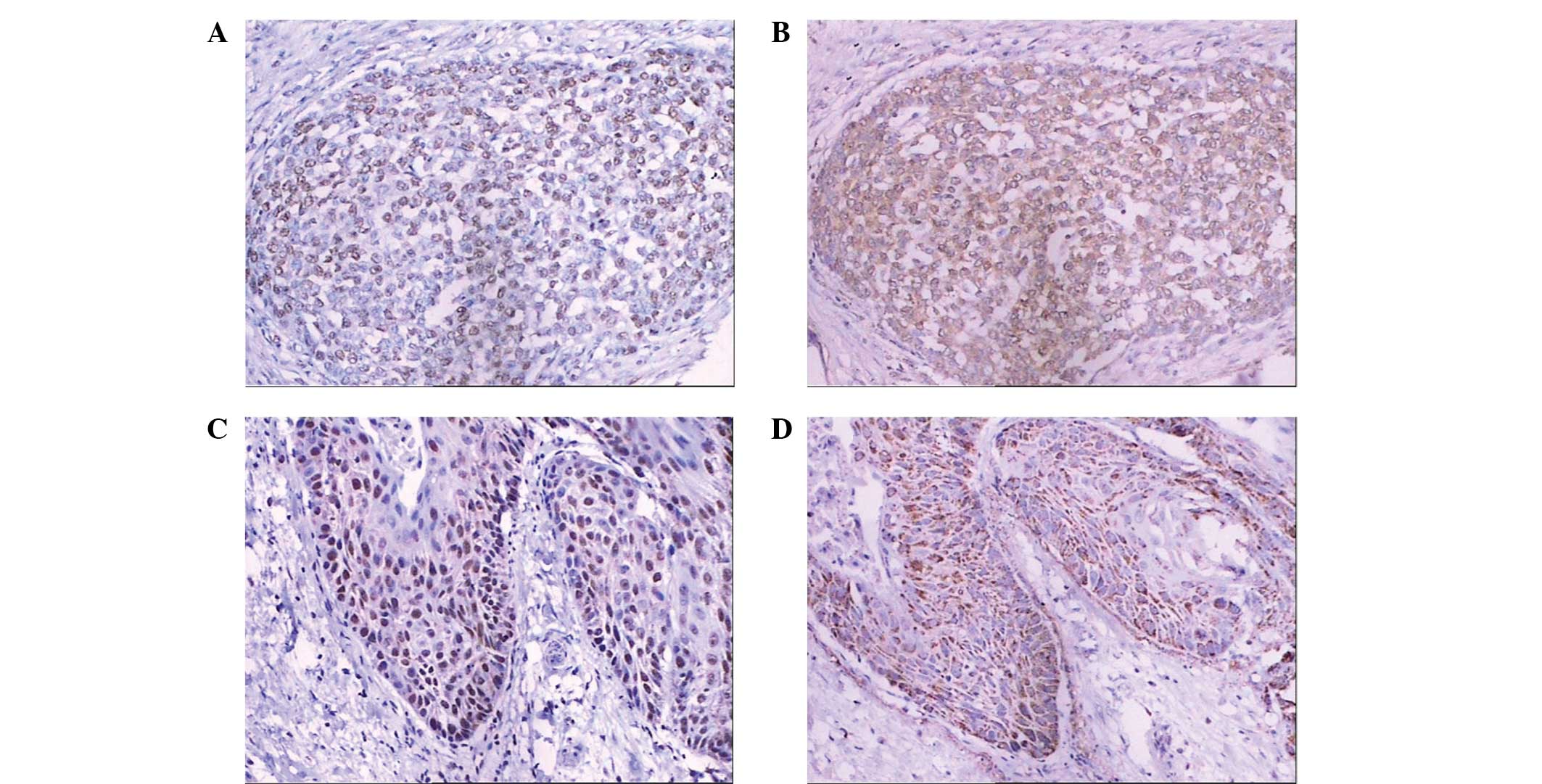
in primary human NSCLC tissues
Positive expression of ILT4 was observed in the cell
nucleus, membrane or cytoplasm using immunohistochemical staining
(Fig. 3), while positive ANGPTL2
or ANGPTL5 expression was identified in the cytoplasm of primary
NSCLC cells using brown staining. No staining of these molecules
was observed in the normal bronchial epithelium. For the NSCLC
tissue samples, the positive rates of ILT4, ANGPTL2 and ANGPTL5
expression were found to be 58.8 (67/114), 45.6 (52/114) and 55.3%
(63/114), respectively.
ILT4 and ANGPTL2/ANGPTL5 were found to be
co-expressed in primary NSCLC tissues. A significant association
was observed between the expression levels of ILT4 and ANGPTL2
(R=0.466, P=0.004; Table I).
However, no correlation was identified between the expression
levels of ILT4 and ANGPTL5 (R=0.142, P=0.131).
 | Table ICorrelation between the expression
levels of ILT4 and ANGPTL2/ANGPTL5 in primary human NSCLC
tissues. |
Table I
Correlation between the expression
levels of ILT4 and ANGPTL2/ANGPTL5 in primary human NSCLC
tissues.
| ANGPTL2a (No. of cases) | ANGPTL5b (No. of cases) |
|---|
|
|
|
|---|
| ILT4 | + | − | + | − |
|---|
| + | 38 | 29 | 41 | 26 |
| − | 14 | 33 | 22 | 25 |
Correlation between the co-expression of
ILT4-ANGPTL2 or ILT4-ANGPTL5 with clinicopathological factors in
primary human NSCLC tissues
As presented in Tables
II and III, the tumors were
classified as ILT4-positive or -negative. In the ILT4-positive
group, ILT4 and ANGPTL2 co-expression (ILT4-positive and
ANGPTL2-positive) was associated with lymph node metastasis
patients (P=0.011). A positive correlation was observed between
ANGPTL2 expression and TNM staging in the ILT4-negative group
(P=0.002). In addition, ILT4 and ANGPTL5 co-expression
(ILT4-positive and ANGPTL5-positive) presented a significant
correlation with cell differentiation (P<0.001). In the
ILT4-negative group, a positive correlation was observed between
ANGPTL5 expression and TNM staging (P=0.040). No significant
associations were identified among the ILT4-ANGPTL2 or ILT4-ANGPTL5
co-expression and the age, gender, smoking history, histological
types and tumor sizes.
 | Table IICorrelation between ILT4/ANGPTL2
co-expression and clinicopathological factors in primary human
NSCLC tissues. |
Table II
Correlation between ILT4/ANGPTL2
co-expression and clinicopathological factors in primary human
NSCLC tissues.
| ILT4 (+) (no. of
cases) | | ILT4 (−) (no. of
cases) | |
|---|
|
| |
| |
|---|
| Variable | ANGPTL2 (+) | ANGPTL2 (−) | P-value | ANGPTL2 (+) | ANGPTL2 (−) | P-value |
|---|
| Age (years) |
| ≤60 | 14 | 8 | 0.185 | 11 | 15 | 0.205 |
| >60 | 24 | 21 | | 3 | 18 | |
| Gender |
| Male | 32 | 19 | 0.075 | 10 | 25 | 0.731 |
| Female | 6 | 10 | | 4 | 8 | |
| Smoking history
(years) |
| <30 | 8 | 8 | 0.534 | 3 | 15 | 0.191 |
| ≥30 | 30 | 21 | | 11 | 18 | |
| Histology |
| ADC | 14 | 15 | 0.119 | 7 | 17 | 0.836 |
| SQCC | 21 | 9 | | 6 | 12 | |
| Others | 3 | 5 | | 1 | 4 | |
|
Differentiation |
| W/M | 25 | 23 | 0.224 | 9 | 23 | 0.742 |
| P | 13 | 6 | | 5 | 10 | |
| Primary tumor size
(cm) |
| ≤5 | 28 | 26 | 0.127 | 12 | 20 | 0.17 |
| >5 | 10 | 3 | | 2 | 13 | |
| Lymph node
metastasis |
| Yes | 35 | 19 | 0.011a | 7 | 14 | 0.633 |
| No | 3 | 10 | | 7 | 19 | |
| TNM stage
groupings |
| I, II | 23 | 15 | 0.471 | 8 | 32 | 0.002a |
| III, IV | 15 | 14 | | 6 | 1 | |
 | Table IIICorrelation between ILT4/ANGPTL5
co-expression and clinicopathological factors in primary human
NSCLC tissues. |
Table III
Correlation between ILT4/ANGPTL5
co-expression and clinicopathological factors in primary human
NSCLC tissues.
| ILT4 (+) (No. of
cases) | | ILT4 (−) (No. of
cases) | |
|---|
|
| |
| |
|---|
| Variable | ANGPTL5 (+) | ANGPTL5 (−) | P-value | ANGPTL5 (+) | ANGPTL5 (−) | P-value |
|---|
| Age (years) |
| ≤60 | 15 | 7 | 0.412 | 14 | 12 | 0.282 |
| >60 | 26 | 19 | | 8 | 13 | |
| Gender |
| Male | 30 | 21 | 0.565 | 18 | 17 | 0.331 |
| Female | 11 | 5 | | 4 | 8 | |
| Smoking history
(years) |
| <30 | 10 | 6 | 0.902 | 7 | 11 | 0.391 |
| ≥30 | 31 | 20 | | 15 | 14 | |
| Histology |
| ADC | 19 | 10 | 0.781 | 10 | 14 | 0.269 |
| SQCC | 17 | 13 | | 8 | 10 | |
| Others | 5 | 3 | | 4 | 1 | |
|
Differentiation |
| W/M | 23 | 25 | <0.001a | 14 | 18 | 0.539 |
| P | 18 | 1 | | 8 | 7 | |
| Primary tumor size
(cm) |
| ≤5 | 32 | 22 | 0.752 | 14 | 18 | 0.539 |
| >5 | 9 | 4 | | 8 | 7 | |
| Lymph node
metastasis |
| Yes | 32 | 22 | 0.752 | 12 | 9 | 0.202 |
| No | 9 | 4 | | 10 | 16 | |
| TNM stage
groupings |
| I, II | 20 | 18 | 0.100 | 16 | 24 | 0.040a |
| III, IV | 21 | 8 | | 6 | 1 | |
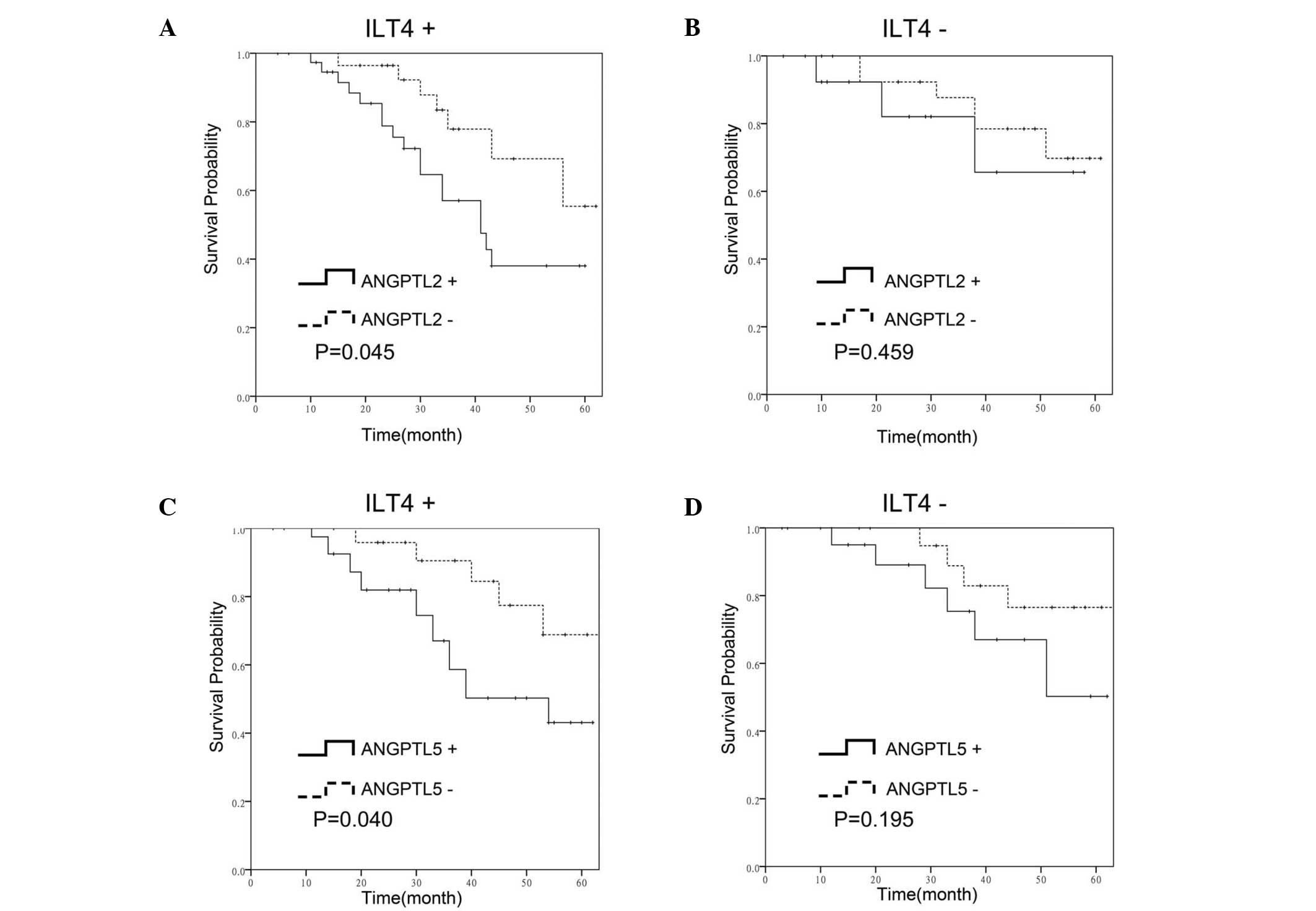
Association between ILT4-ANGPTL2 or
ILT4-ANGPTL5 co-expression and patient survival
In order to investigate whether the co-expression of
ILT4 and ANGPTL2/ANGPTL5 may be used to predict the prognosis of
patients with NSCLC, overall survival curves were constructed using
the Kaplan-Meier method and investigated using the log-rank test.
In the ILT4-positive group, the overall patient survival rate was
lower in the ANGPTL2-positive cases compared with the
ANGPTL2-negative cases (P=0.045; Fig.
4A). However, the difference in the overall survival between
ANGPTL2-positive and ANGPTL2-negative cases was not found to be
statistically significant in the ILT4-negative group (P=0.459;
Fig. 4B). In addition, survival
rate analysis revealed that ANGPTL5 expression was associated with
the overall survival rates of patients in the ILT4-positive group
(P=0.040; Fig. 4C), whereas the
overall survival rate difference was not found to be statistically
significant in the ILT4-negative cases (P=0.195; Fig. 4D).
Discussion
ANGPTLs constitute a group of growth factors known
to induce the expansion of mouse and human HSCs (13). ANGPTLs are also crucial in lipid
metabolism, inflammation and angiogenesis. A number of studies have
indicated that the aberrant expression of ANGPTLs is involved in
tumor progression, metastasis and tumor cell
epithelial-to-mesenchymal transitions (16,25,26).
These molecules were previously considered to be ‘orphan ligands’
since no receptor had been identified (27). However, a recent study has
demonstrated that ILT4 and its mouse ortholog, Pirb, were receptors
for ANGPTLs (11). ILT4 has also
been shown to be overexpressed in NSCLC cells (10). Furthermore, lymph node metastasis
was also more common in NSCLC cases with high ILT4 expression.
However, the co-expression of ILT4 and ANGPTLs was rarely observed
in cancer cells and their potential roles remain poorly
understood.
The existence of the growth factor and its receptor
in the same cancer cells is regarded as autocrine secretion. The
term ‘autocrine secretion’ was proposed by Sporn and Roberts
(28) and denotes the
self-stimulation by which a cell secretes a hormone-like substance
for which the cell itself has functional external receptors
(28). In the current study, the
presence of ILT4 and ANGPTL2/ANGPTL5 expression was determined in
five NSCLC cell lines. The results indicated that ILT4 and
ANGPTL2/ANGPTL5 were co-expressed in all the cell lines and primary
human NSCLC tissues; thus, the interactions between them were
investigated. The overexpression of ILT4 was found to enhance the
expression of ANGPTL2 in vitro. This effect was abolished by
the transfection of cells with shRNA targeted against ILT4. In
addition, the expression of ILT4 was positively correlated with the
expression of ANGPTL2 in clinical NSCLC samples (R=0.266, P=0.004).
However, ILT4 was not found to induce the expression of ANGPTL5 in
the NSCLC cell lines, and no relationship was observed between ILT4
and ANGPTL5 in the primary human NSCLC tissues. Therefore, these
results imply that the ILT4-ANGPTL2 interaction in NSCLC cells may
be self-stimulated whereby the NSCLC cells secrete ANGPTL2 for
which the cells themselves have the functional external ILT4
receptors, while ANGPTL5 may be regulated through other pathways.
Further studies are required to elucidate the detailed mechanisms
underlying the interaction between ILT4 and ANGPTL2 in NSCLC cells.
To the best of our knowledge, the present study is the first to
describe the co-expression and correlation between ILT4 and
ANGPTL2/ANGPTL5 in solid tumor cells.
Growth factors have been previously shown to be
closely associated with oncogenes that directly code for growth
factors or their receptors and amplify the mitogenic pathway
signals produced by the growth factor through its receptor
(28). In addition, the current
study assessed the effect of the correlation between the expression
of the growth factor, ANGPTL2, and its receptor, ILT4, on the
prognosis of NSCLC. Among the ILT4-positive cases, high expression
of ANGPTL2 was more common in the lymph node metastasis patients.
Notably, patients with a high expression of ANGPTL2 had a
significantly poorer prognosis. However, in the ILT4-negative
cases, ANGPTL2 did not serve as a prognosis factor. Recently, Zheng
et al (11) demonstrated
that the binding of ANGPTLs to PirB promoted the development of
leukemia by inhibiting the differentiation of AML cells, indicating
a potential role of the autocrine mechanism of ILT4 and ANGPTL2 in
the promotion of tumor growth and metastasis in NSCLC. Further
studies on the role of the ILT4-ANGPTL2 interaction in the
development of NSCLC are required to validate the findings of the
present study.
Although no direct influence of ILTE on ANGPTL5
expression was identified, ILT4 and ANGPTL5 co-expression was found
to be associated with low NSCLC differentiation and poor prognosis.
Therefore, more comprehensive investigations should be performed to
assess the underlying mechanistic interactions between ILT4 and
ANGPTL5 in order to improve the understanding on the role of these
interactions in the development of NSCLC.
In conclusion, the current study investigated the
co-expression of ILT4 and ANGPTL2 and their potential autocrine
mechanism in NSCLC cells. Co-expression of ILT4 and ANGPTL2/ANGPTL5
was found to be correlated with lower overall survival rates.
Therefore, co-expression of ILT4 and ANGPTL2/ANGPTL5 may be crucial
in the progression and development of NSCLC, and the identification
of an ILT4 and ANGPTL pathway may be required for the prevention
and treatment of NSCLC.
Acknowledgements
The present study was supported by a grant from the
Project of the National Natural Science Foundation of China (no.
81372334).
References
|
1
|
Postmus PE: Chemotherapy for non-small
cell lung cancer: the experience of the Lung Cancer Cooperative
Group of the European Organization for Research and Treatment of
Cancer. Chest. 113(Suppl): 28S–31S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fanger NA, Cosman D, Peterson L, Braddy
SC, Maliszewski CR and Borges L: The MHC class I binding proteins
LIR-1 and LIR-2 inhibit Fc receptor-mediated signaling in
monocytes. Eur J Immunol. 28:3423–3434. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borges L and Cosman D: LIRs/ILTs/MIRs,
inhibitory and stimulatory Ig-superfamily receptors expressed in
myeloid and lymphoid cells. Cytokine Growth Factor Rev. 11:209–217.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colonna M, Samaridis J, Cella M, et al:
Human myelomonocytic cells express an inhibitory receptor for
classical and nonclassical MHC class I molecules. J Immunol.
160:3096–3100. 1998.PubMed/NCBI
|
|
5
|
Colonna M, Navarro F, Bellón T, et al: A
common inhibitory receptor for major histocompatibility complex
class I molecules on human lymphoid and myelomonocytic cells. J Exp
Med. 186:1809–1818. 1997. View Article : Google Scholar
|
|
6
|
de Waal Malefyt R, Haanen J, Spits H, et
al: Interleukin 10 (IL-10) and viral IL-10 strongly reduce
antigen-specific human T cell proliferation by diminishing the
antigen-presenting capacity of monocytes via downregulation of
class II major histocompatibility complex expression. J Exp Med.
174:915–924. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lepin EJ, Bastin JM, Allan DS, et al:
Functional characterization of HLA-F and binding of HLA-F tetramers
to ILT2 and ILT4 receptors. Eur J Immunol. 30:3552–3561. 2000.
View Article : Google Scholar
|
|
8
|
Riteau B, Rouas-Freiss N, Menier C, Paul
P, Dausset J and Carosella ED: HLA-G2, -G3, and -G4 isoforms
expressed as nonmature cell surface glycoproteins inhibit NK and
antigen-specific CTL cytolysis. J Immunol. 166:5018–5026. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manavalan JS, Rossi PC, Vlad G, et al:
High expression of ILT3 and ILT4 is a general feature of
tolerogenic dendritic cells. Transpl Immunol. 11:245–258. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Liu J, Gao P, Wang Y and Liu C:
Expression of Ig-like transcript 4 inhibitory receptor in human
non-small cell lung cancer. Chest. 134:783–788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng J, Umikawa M, Cui C, et al:
Inhibitory receptors bind ANGPTLs and support blood stem cells and
leukaemia development. Nature. 485:656–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabata M, Kadomatsu T, Fukuhara S, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang CC, Kaba M, Ge G, et al:
Angiopoietin-like proteins stimulate ex vivo expansion of
hematopoietic stem cells. Nat Med. 12:240–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hato T, Tabata M and Oike Y: The role of
angiopoietin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oike Y, Yasunaga K and Suda T:
Angiopoietin-related/angiopoietin-like proteins regulate
angiogenesis. Int J Hematol. 80:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoi J, Endo M, Kadomatsu T, et al:
Angiopoietin-like protein 2 is an important facilitator of
inflammatory carcinogenesis and metastasis. Cancer Res.
71:7502–7512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki H, Suzuki A, Shitara M, et al:
Angiopoietin-like protein ANGPTL2 gene expression is correlated
with lymph node metastasis in lung cancer. Oncol Lett. 4:1325–1328.
2012.PubMed/NCBI
|
|
18
|
Kikuchi R, Tsuda H, Kozaki K, et al:
Frequent inactivation of a putative tumor suppressor,
angiopoietin-like protein 2, in ovarian cancer. Cancer Res.
68:5067–5075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teicher BA: Searching for molecular
targets in sarcoma. Biochem Pharmacol. 84:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endo M, Nakano M, Kadomatsu T, et al:
Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical
driver of metastasis. Cancer Res. 72:1784–1794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drake AC, Khoury M, Leskov I, et al: Human
CD34+ CD133+ hematopoietic stem cells
cultured with growth factors including Angptl5 efficiently engraft
adult NOD-SCID Il2rγ−/− (NSG) mice. PLoS One. 6:e183822011.
View Article : Google Scholar
|
|
22
|
Khoury M, Drake A, Chen Q, Dong D, Leskov
I, et al: Mesenchymal stem cells secreting angiopoietin-like-5
support efficient expansion of human hematopoietic stem cells
without compromising their repopulating potential. Stem Cells Dev.
20:1371–1381. 2011. View Article : Google Scholar :
|
|
23
|
Miida T and Hirayama S: Impacts of
angiopoietin-like proteins on lipoprotein metabolism and
cardiovascular events. Curr Opin Lipidol. 21:70–75. 2010.
View Article : Google Scholar
|
|
24
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th edition. John Wiley
& Sons; Hoboken, NJ: 2009
|
|
25
|
Huang XF, Han J, Hu XT and He C:
Mechanisms involved in biological behavior changes associated with
Angptl4 expression in colon cancer cell lines. Oncol Rep.
27:1541–1547. 2012.PubMed/NCBI
|
|
26
|
Marchiò S, Soster M, Cardaci S, et al: A
complex of α6 integrin and E-cadherin drives liver metastasis of
colorectal cancer cells through hepatic angiopoietin-like 6. EMBO
Mol Med. 4:1156–1175. 2012. View Article : Google Scholar
|
|
27
|
Hato T, Tabata M and Oike Y: The role of
angiopoitin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sporn MB and Roberts AB: Autocrine growth
factors and cancer. Nature. 313:745–747. 1985. View Article : Google Scholar : PubMed/NCBI
|