Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are
the two most common forms of inflammatory bowel disease (IBD). They
are chronic inflammatory disorders, which may be progressive or
relapsing, and are characterized by a range of symptoms, including
abdominal pain, severe diarrhoea, rectal bleeding and wasting. The
pathological findings in IBD are correlated with a number of
factors, including genetic predisposition, environmental factors,
gut dysbiosis and an inadequate immune response (1–3).
Retrospective studies have shown that the balance between pro- and
anti-inflammatory cytokines is critical in maintaining normal gut
homeostasis in the colonic mucosa. A disturbance of the cytokine
profile, in particular, pro-inflammatory cytokine overexpression,
has been reported in IBD (4,5). The
present treatment for IBD comprises a number of approaches,
including the rapid induction of clinical remission, steroid-free
maintenance of clinical remission, mucosal healing and improvements
in quality of life. Other anti-tumor necrosis factor (TNF) agents
and novel biological therapies have been developed and introduced
into clinical trials for IBD (6,7).
Despite recent advances in the clinical management of IBD, the
long-term efficacy of these agents remains to be determined.
Mesenchymal stem cells (MSCs) are easily isolated
from a number of tissue sources, including bone marrow (BM), fat,
the umbilical cord and other tissues with the ability to
differentiate into multiple cell lineages, including adipocytes,
chondrocytes and osteocytes. They are a promising tool for use in
cell therapies (8). MSCs are able
to directly differentiate into multiple cell lineages and also
indirectly exhibit a range of immunomodulatory functions through
the secretion of proteins and cytokines. As BM-derived MSCs
(BM-MSCs) are easily isolated from adult sources, may be cultured
in vitro, display low expression of HLA and costimulatory
molecules, and are relatively free from ethical controversy, they
have been used in a number of preclinical and clinical studies
(9,10). An early study into MSC
immunobiology showed that MSCs acquire immunosuppressive or
immunostimulating properties within a typical inflammatory
environment (11). More recently,
MSCs were shown to exhibit immunomodulatory functions in innate and
adaptive immune responses. Other findings have demonstrated that
MSC transplantation therapies may be applied to graft versus host
disease and CD (12,13).
Increasing evidence indicated that a number of the
common immunological responses present in IBD are mediated by
cytokines. MSCs are also known to regulate the immune function by
modulating the secretion of pro-inflammatory cytokines and
chemokines in inflamed tissues. However, the effect of exogenously
administered MSCs on IBD remains to be elucidated. In the present
study, the effect of exogenously administered BM-MSCs in a
2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced rat model of
colitis was investigated in addition to the possible interactions
between MSCs and the pro-inflammatory response in mediating this
process.
Materials and methods
Animals
Female Sprague Dawley rats (age, 6–8 weeks), which
were specific pathogen-free and weighed 200–250 g, were purchased
from the Experiment Animal Center of Centers for Disease Control
(Hubei, China). Rats were randomly assigned to each group (n=6 per
group). Then were given ad libitum access to water and a
standard diet; rats were kept in a temperature controlled
enviroment (20–22°C), with a humidity of ~52% and a 12-h light/dark
cycle. The present study was approved by the ethics committee of
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China) and the experimental protocol was
approved by the Experimental Animal Center of Tongji Medical
College, Huazhong University of Science and Technology.
Isolation, culture and characterization
of BM-MSCs
The isolation and culture of BM-MSCs was conducted
as previously described (14).
Four-week-old rats were sacrificed by cervical dislocation. Rats
were immersed in 75% ethanol for 5 min, following which the bone
marrow was isolated bilaterally from femurs and tibias. BM
mononuclear cells were isolated by density gradient centrifugation
for 5 min at 350 × g. Cells were plated in a plastic tissue culture
flask (Corning Inc., Corning, NY, USA) and cultured in a
low-glucose complete cell culture medium consisting of a minimum
essential medium (a-MEM; GIBCO, Life Technologies, Grand Island,
NY, USA) with 10% fetal bovine serum (FBS; GE Healthcare, Life
Sciences, Logan, UT, USA). Nonadherent cells were removed by
replacing the medium at 48 h and every 3–4 days thereafter. All
cultures were maintained at 37°C in a 5% CO2 atmosphere.
Over the course of 1–2 weeks, adherent cells were collected using
0.25% trypsin solution (GIBCO). Cells were passaged once they
reached ~80% confluency. Third-passage cells were used in
subsequent experiments. To evaluate the surface marker phenotype of
the cultured MSCs, cells were trypsinized and incubated with the
following fluorescent anti-rat monoclonal antibodies for 30 min at
room temperature: Anti-CD29-phycoerythrin (PE)-Cy7;
anti-CD90-AlexaFluor®488; anti-CD45-PE; and
anti-CD11b-AlexaFluor®647 (BioLegend Inc., San Diego,
CA, USA). Cells were washed twice with phosphate-buffered saline
(PBS; Biosciences Co., Ltd., Wuhan, China), and then resuspended in
PBS. Detection of PE-Cy7/AlexaFluor488/PE and AlexaFluor647
labeling was conducted using flow cytometry (FACSCalibur;
Becton-Dickinson, Franklin Lakes, NJ, USA).
Transduction of BM-MSCs with green
fluorescent protein (GFP)
Third-passage BM-MSCs at ~40% confluence were seeded
in fibronectin-coated six-well plates (Corning Inc.). At 24 h
following plating, the medium containing 10% FBS was removed.
Transduction was conducted at a multiplicity of infection (MOI) of
15 units, according to the manufacturer’s instructions. Cells were
added to the recombinant replication-defective lentivirus carrying
GFP (LV-GFP; Genechem Co., Ltd., Shanghai, China) supernatant,
containing 5 μg/ml polybrene (Genechem Co., Ltd.), to obtain a
final volume of 3 ml. Following incubation with LV-GFP for 2 h, the
transduction medium was replaced with a fresh culture medium
containing 10% FBS. An additional transduction was conducted at 48
h. The expression of the GFP transgene in the BM-MSCs was observed
using fluorescence microscopy (GpJ9-TS100-F; Nikon, Co., Tokyo,
Japan). BM-MSCs were then trypsinized for five minutes and used in
the subsequent experiments.
Induction of experimental colitis and
treatment
In the present study, TNBS (Sigma-Aldrich, St.
Louis, MO, USA) was used to induce an experimental colitis as
described previously (15). On
days 0, 3 and 7, GFP-transduced BM-MSCs at a dose of
1×106 cells in 0.3 ml PBS were injected into the tail
vein of the rats with TNBS-induced colitis. In the control
experiments, animals received 0.3 ml PBS without BM-MSCs and
followed an otherwise identical protocol. The disease activity
index (DAI) was recorded as a combination of weight loss, stool
consistency and bleeding as previously described (16). Scores were assigned according to
the following criteria: (1) Weight loss (0, <1%; 1, 1–5%; 2,
5–10%; 3, 10–15%; and 4, >15%); (2) stool consistency (0,
normal; 2, soft stools; and 4, liquid stools); and (3) rectal
bleeding (0, negative; 2, positive; and 4, serious bleeding). On
day 15, mice were sacrificed and blood was collected by ventral
aortic puncture for the analysis of serum inflammatory cytokine
levels. In addition, the entire colon was excised and colon tissue
samples were harvested for histological examination and evaluation
of the mRNA expression of inflammatory cytokines in the intestinal
mucosa.
Histological examination
Colon samples were fixed in 4% paraformaldehyde,
embedded in paraffin (Rutgers Co., Ltd.), and cut into sections (4
μm) prior to staining with hematoxylin and eosin (Rutgers Co.,
Ltd.). Histological evaluation was completed semi-quantitatively
according to the scale described previously (17). The parameters that were evaluated
included the extent of mucosal injury, leukocyte infiltration,
crypt abscesses and loss of goblet cells. Each of these parameters
was graded on a 0–3 scale according to the following criteria: 0,
none; 1, slight; 2, moderate; 3, severe. The final histological
score was defined as the sum of the scores of these parameters.
Tracing of BM-MSCs by GFP labeling
BM-MSCs were transduced with LV-GFP as described
previously (18) in order to trace
the infused BM-MSCs in vitro. Colonic tissue samples,
excised from the inflamed and the non-inflamed regions of the
colon, were embedded in an optimum cutting temperature compound
(Sakura Finetechnical Co., Ltd., Tokyo, Japan), and frozen in dry
ice. One of these sections was used to detect GFP-positive cells
via fluorescence confocal microscopy (E600; Nikon Co.) and the
other was stained with an antibody against GFP (1:600; EMD
Millipore, Billerica, MA, USA) and visualized using a fluorescein
isothiocyanate (FITC)-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). In addition, further samples
were prepared in order to analyze the expression of the GFP protein
by western blotting.
Detection of serum pro-inflammatory
cytokines
Blood samples were centrifuged at 1,000 × g for 15
min and the sera were stored at −80°C prior to the evaluation of
cytokine levels. TNF-α concentration in the sera was determined
using a rat TNF-α enzyme-linked immunosorbent assay (ELISA) kit
(BioSource, Inc., Camarillo, CA, USA) according to the
manufacturer’s instructions.
Western blot analysis
Total protein was isolated and quantified using a
bicinchoninic acid protein assay kit (Pierce-Perbio Science,
Tattenhall, UK). The isolation of nuclear proteins was performed
using NE-PER® nuclear and cytoplasmic extraction
reagents (Thermo Fisher Scientific, Waltham, MA, USA). Ice-cold
nuclear extraction buffer (25 μl) was added and the mixture was
incubated for 30 min with intermittent mixing. Extracts were
centrifuged at 1,500 × g for 15 mins and the supernatant
(consisting of the nuclear extracts) was stored at −80°C prior to
use. The primary antibody against NF-κB p65 was used at a dilution
of 1:1,000. For evaluation of the levels of the control proteins,
anti-ACTION and anti-HISTON antibodies were used at a dilution of
1:10,000. Protein extracts were resolved by 0.1% SDS-PAGE
electrophoresis (Beyotime Co., Ltd., Shanghai, China) and
transferred onto polyvinylidene difluoride membranes (Rutgers Co.,
Ltd.), which were blocked with 5% bovine serum albumin and
incubated with the relevant antibody.
mRNA expression of TNF-α and NF-κBp65 in
the colon
Colonic segments were frozen in liquid nitrogen at
−80°C prior to use. RNA was isolated from colonic tissues using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. cDNA was
synthesized from 0.5 μg total RNA using a reverse transcription
(RT) kit (Toyobo Co., Ltd., Osaka, Japan) according to the
manufacturer’s instructions. Following this step, a quantitative
polymerase chain reaction (qPCR) using the SYBR-Green I Realtime
PCR Master mix (Takara Bio, Inc., Otsu, Japan) with a final volume
of 20 μl, was conducted using the ABI PRISM 7900 sequence detector
system (Applied Biosystems, Life Technologies, Foster City, CA,
USA). Primers (Takara Bio, Inc., Dalian, China) were designed
according to data from Gen-Bank and the sequences of primers for
the qPCR experiments were as follows: Forward,
5′-CCGTCTCCTACCAGACCAAGG-3′ and reverse,
5′-CTGGAAGACCCCTCCCAGATAG-3′ for TNF-α; forward,
5′-CTTCTCGGAGTCCCTCACTG-3′ and reverse, 5′-CCAATAGCAGCTGGAAAAGC-3′
for NF-κB; and forward, 5′-GGGGCTCTCTGCTCCTCCCTG-3′ and reverse,
5′-CGGCCAAATCCGTTCACACCG-3′ for GAPDH. The relative gene expression
levels (the amount of target mRNA, normalized to that of the
endogenous and control genes) was calculated using the comparative
Ct method via the formula 2−ΔΔCt.
Statistical analysis
Values are presented as the mean ± standard
deviation. The un-paired t-test and the Mann-Whitney U test were
used for parametric and non-parametric analyses between two groups,
which was assessed using SPSS 18.0 software (IBM, Armonk, NY, USA)
and Microsoft EXCEL 2003 (Microsoft Corp., Redmond, WA, USA).
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Characterization of BM-MSCs
Flow cytometric analysis confirmed that the BM-MSCs
were positive for CD29 and CD90, but that they stained negative for
the hematopoietic surface markers CD45 and CD11b (Fig. 1).
General conditions
All TNBS-treated rats developed looser, bloody and
purulent stools, and exhibited a marked weight loss following
administration of this compound. By contrast, rats that were not
treated with TNBS did not display any of these symptoms and gained
weight over time. Following infusion of BM-MSCs, the DAI score of
the TNBS-treated rats gradually decreased and the symptoms were
significantly alleviated in comparison with those in the
PBS-treated control group (Fig.
2).
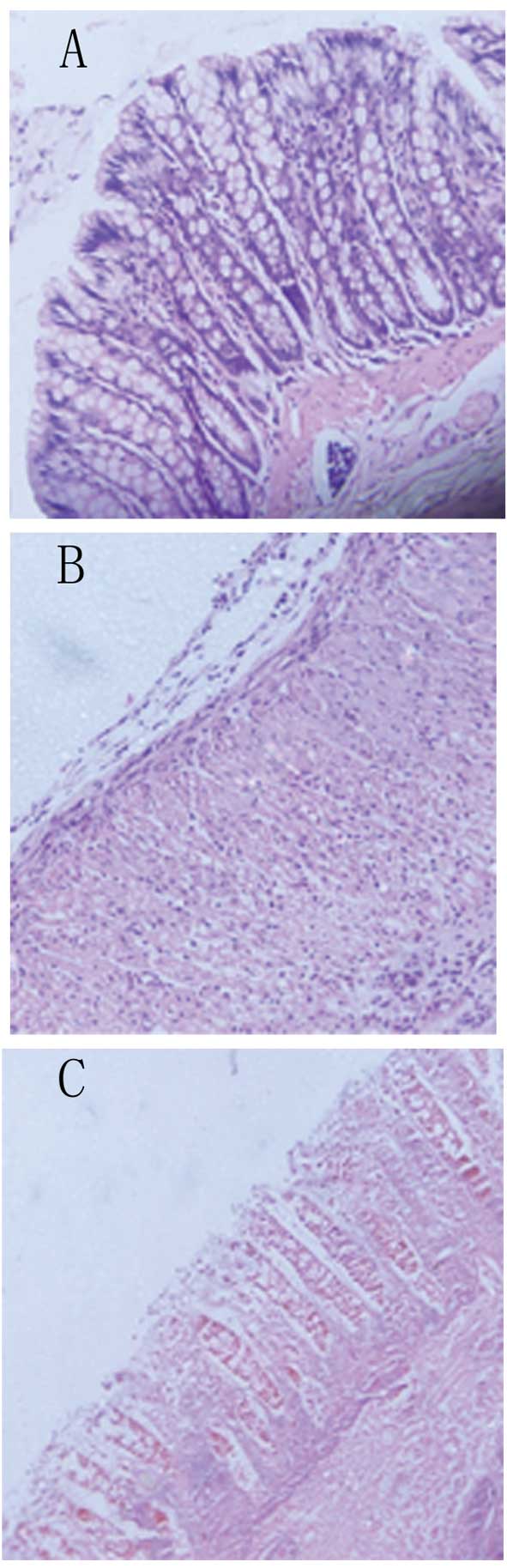
Histological findings
On microscopic examination, rats in the BM-MSC group
had a relatively intact structure of their colonic mucosa, with
more organized mucosal glands, more abundant goblet cells, a milder
degree of congestion and edema, and less infiltration of
inflammatory cells in the mucosa and sub-mucosa compared with the
PBS-treated control group (Fig.
3). The histological score, which was defined as the sum of
scores of the parameters outlined in the materials and methods
section, was significantly reduced in the BM-MSC-treated group
compared with that in the PBS-treated group (Fig. 4).
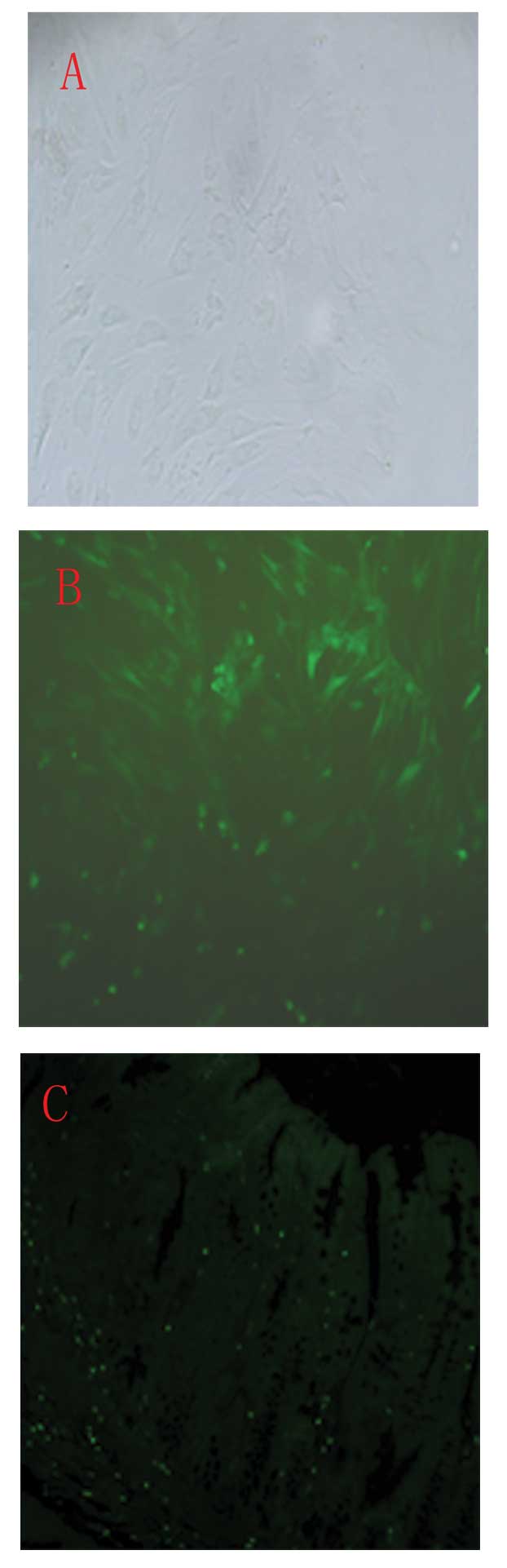
Localization of infused BM-MSCs in the
colon
In vitro, a large proportion of GFP-labeled
BM-MSCs were observed (Fig. 5B).
GFP fluorescence was not detected using a confocal microscope in
vivo. Distinct GFP-positive cells were observed when a GFP
antibody and FITC-conjugated secondary antibody were used as
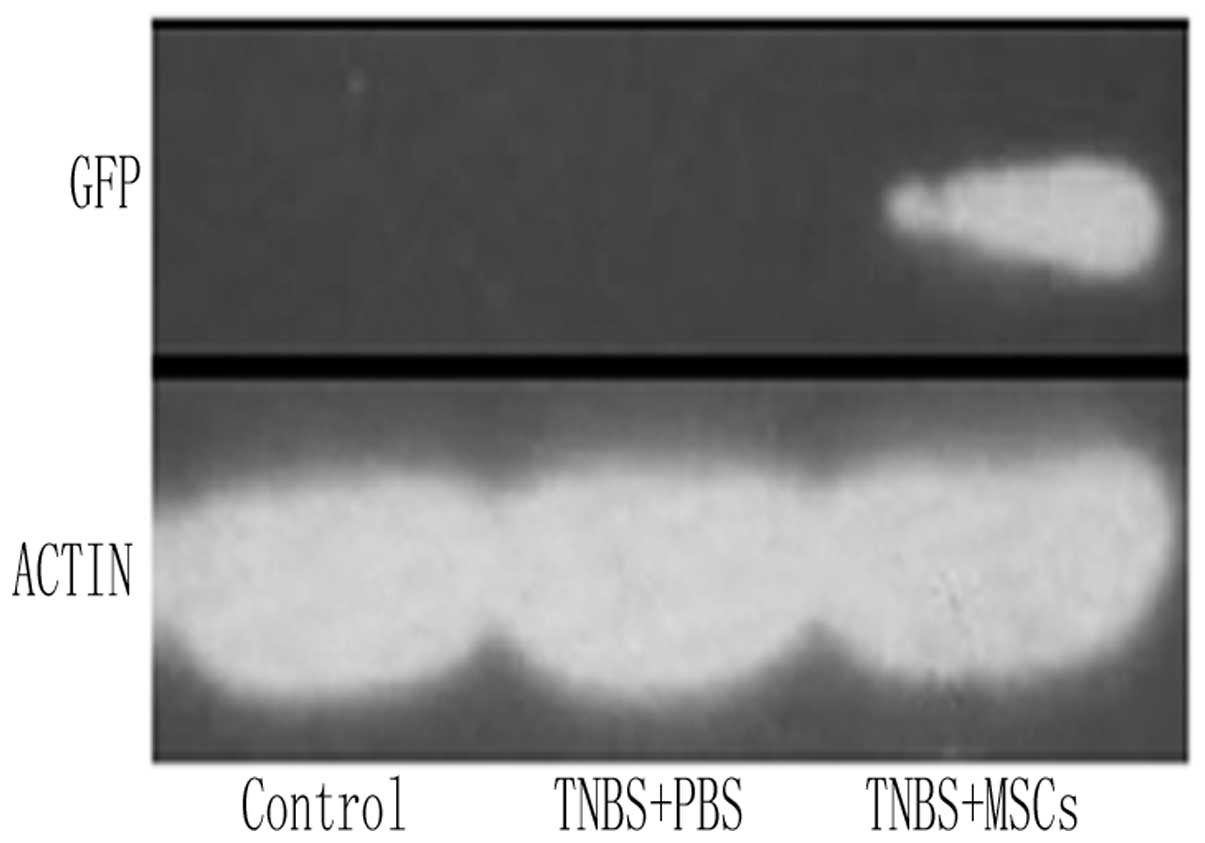
described in the materials and methods section (Fig. 5C). Furthermore, western blotting
revealed a high expression of GFP in the colonic tissue was
observed in the GFP-labeled BM-MSC group (Fig. 6). By contrast, no immunoreactivity
was detected in the PBS-treated control group or the normal control
group.
Concentration of serum TNF-α
The effect of administration of BM-MSCs on the serum
levels of TNF-α was evaluated using an ELISA. A significant
increase in TNF-α levels was observed in the PBS-treated group of
rats with TNBS-induced colitis. Following treatment with BM-MSCs,
the concentration of TNF-α was significantly reduced in the
treatment group compared with that in the PBS control group
(Fig. 7).
Protein expression of NF-κBp65 in colonic
mucosa
The total protein and nucleoprotein expression of
NF-κBp65 in the inflamed colon was investigated. Western blotting
showed that total protein and nucleoprotein were strongly expressed
in the PBS-treated colitis group. Systemic administration of
BM-MSCs markedly downregulated the expression of p65 in
nucleoprotein. However, no significant difference in the expression
of p65 in total protein was identified between the MSC-treated and
the PBS-treated groups (Fig.
8).
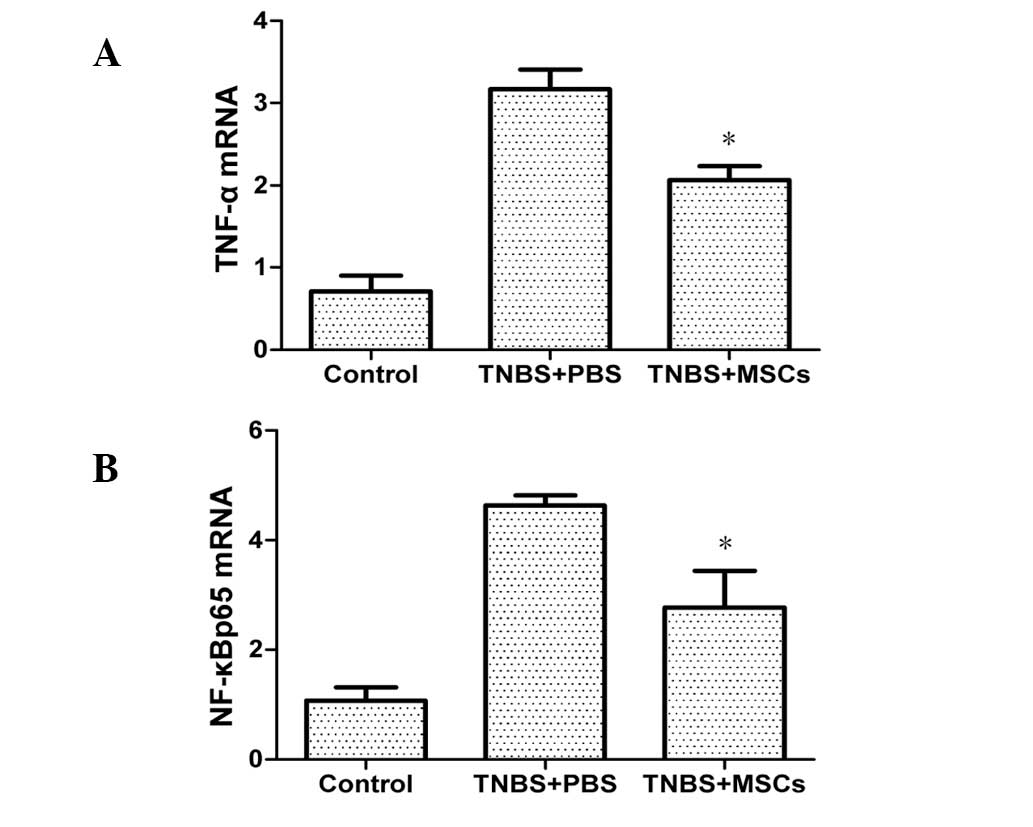
mRNA expression of TNF-α and NF-κBp65 in
the colonic mucosa
mRNA expression of TNF-α and NF-κBp65 in the colonic
mucosa was significantly increased in the PBS-treated group
compared with that in the control group. As shown in Fig. 9, treatment with BM-MSCs resulted in
a reduction of the expression of TNF-α and NF-κBp65.
Discussion
The mechanisms by which MSCs exert their reparative
benefits have remained elusive. However, numerous studies show that
BM-MSCs are present in areas of inflammation and promote tissue
repair by differentiation into a range of cell lineages. Recently,
MSCs have been demonstrated to possess immunomodulatory properties,
including the suppression of T-cell proliferation, influence on
dendritic cell maturation and function and the suppression of
B-cell proliferation and terminal differentiation, as well as the
immune modulation of other immune cells, including NK cells and
macrophages (19–21). The majority of in vitro
studies have demonstrated that MSCs limit T-cell expansion by
impairing interferon-γ and TNF-α production in addition to
increasing the production of IL-10 (22). Recently, Kazunari et al
(23) reported that in the
systemic injection of ex vivo-cultured BM-MSCs, these cells
accumulated exclusively in the region of the inflamed rectum and
localized in the lamina propria, in particular at the base of the
crypts. Another study demonstrated that MSCs may treat dextran
sulfate sodium-induced colitis via an interaction with immune
mediators, including TNF-α, interleukin-1β and cyclooxygenase-2
(24).
Members of the TNF protein superfamily exist in
either a membrane-bound or soluble form. The family contains 18
type 2 proteins, and the receptors for these ligands are type 1
transmembrane proteins (25,26).
Binding of TNF-like ligands to their receptors, including the
TNF/TNF-receptor protein superfamilies, triggers the activation of
intracellular pathways, which are involved in numerous components
of the immune response, including direct involvement in cell
proliferation, differentiation and survival (25–27).
TNF-α is one of the primary cytokines involved in the pathogenesis
of IBD (28). A number of possible
mechanisms underlying its protective effects in this disease have
been postulated. One factor may be that TNF-α effects the apoptotic
elimination of effector immunocytes in the lamina propria. A second
possible mechanism is the upregulation of endogenous
corticosteroids by TNF-α. A third possible factor is that TNF-α is
able to maintain the integrity of the epithelial barrier (29–31).
Subsequent research has shown that the serum levels of TNF-α are
negatively correlated with the clinical activity of UC and CD,
which may indicate the use of anti-TNF therapies for the treatment
of Crohn’s Disease (32,33). To the best of our knowledge, there
is currently no study reporting the administration of anti-TNF
agents as a first-line therapy in UC, but a recent study suggested
that biological therapies may have potential for use as first-line
treatments in the future. In the present study, serum TNF-α levels
were assayed by ELISA and mRNA expression of TNF-α in the colonic
mucosa was evaluated by RT-qPCR. The data showed that following the
administration of BM-MSCs, the concentration of TNF-α in the serum
and its mRNA expression in the colonic mucosa decreased
significantly compared with that in the PBS control group.
There are five members of the NF-κB family: RelA
(p65), RelB, C-Rel, p105 (NF-kB1; a precursor of p50) and p100
(NF-kB2; a precursor of p52) (34). The NF-κB family is a family of
transcription factors and has been hypothesized to be involved in
tumorigenesis, inflammation and cellular processes, including cell
proliferation and apoptosis (35).
Under unstimulated conditions, NF-κB is maintained in the cytoplasm
in an inactive form by interaction with a family of inhibitor
proteins, termed IκB proteins. NF-κB activation occurs in response
to various stimuli, when the rapid phosphorylation of IκB leads to
its degradation by the proteasome pathway, resulting in the
migration of NF-κB into the nucleus (36,37).
NF-κB is one of the primary factors involved in the formation of
the molecular network, which can lead to various changes in
cellular function that are associated with IBD. For example, IL-1,
TNF-α, IL-12 and IL-23 are NF-κB-dependent pro-inflammatory
mediators and are known to be upregulated in patients with IBD
(38,39). The role of NF-κB in the
transcriptional control of a number of inflammatory genes,
including cytokines, chemokines, growth factors and leukocyte
adhesion molecules, as well as the involvement of ROS, led to the
concept of NF-κB as a therapeutic target in numerous disorders. It
has been reported that antibodies targeting NF-κB and
pro-inflammatory cytokines, including TNF-α and IL-6 and their
signaling pathways, were effective in ameliorating the
inflammation-associated intestinal damage in patients with IBD
(40,41). In the present study, the protein
expression of NF-κBp65 in the inflamed colon was assessed by
western blot analysis. Systemic administration of BM-MSCs markedly
downregulated the expression of nucleoprotein but not that of the
total protein. However, data obtained from RT-qPCR suggested that
the BM-MSCs downregulated mRNA expression of NF-κBp65 in the
colonic mucosa. This information suggested that BM-MSCs may affect
TNBS-induced colitis via modulation of the NF-κB-mediated
pro-inflammatory response.
In conclusion, BM-MSCs may attenuate TNBS-induced
colitis in rats. Increased body weight and a marked histological
improvement were observed in the BM-MSC-treated group compared with
those in the PBS-treated control group. Therefore, the present
study demonstrated that modulation of the NF-κB-mediated
pro-inflammatory response was associated with exogenous
administration of BM-MSCs in the treatment of experimental colitis.
Future studies should focus on investigating the effect of MSCs on
the NF-κB-mediated pro-inflammatory signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81273906 and 81102690).
References
|
1
|
Schirbel A and Fiocchi C: Inflammatory
bowel disease: Established and evolving considerations on its
etiopathogenesis and therapy. J Dig Dis. 11:266–276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baumgart DC and Carding SR: Inflammatory
bowel disease: cause and immunobiology. Lancet. 369:1627–1640.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hisamatsu T, Kanai T, Mikami Y, et al:
Immune aspects of the pathogenesis of inflammatory bowel disease.
Pharmacol Ther. 137:283–297. 2013. View Article : Google Scholar
|
|
4
|
Műzes G, Molnár B, Tulassay Z and Sipos F:
Changes of the cytokine profile in inflammatory bowel diseases.
World J Gastroenterol. 18:5848–5861. 2012. View Article : Google Scholar :
|
|
5
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Zoeten E and Mamula P: What are the
guidelines for using biologics in pediatric patients? Inflamm Bowel
Dis. 14(Suppl 2): S259–S261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taba Taba Vakili S, Taher M and Ebrahimi
Daryani N: Update on the management of ulcerative colitis. Acta Med
Iran. 50:363–372. 2012.PubMed/NCBI
|
|
8
|
Parekkadan B and Milwid JM: Mesenchymal
stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyerrose T, Olson S, Pontow S, et al:
Mesenchymal stem cells for the sustained in vivo delivery of
bioactive factors. Adv Drug Deliv Rev. 62:1167–1174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le Blanc K, Tammik C, Rosendahl K,
Zetterberg E and Ringdén O: HLA expression and immunologic
properties of differentiated and undifferentiated mesenchymal stem
cells. Exp Hematol. 31:890–896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Blanc K, Tammik L, Sundberg B,
Haynesworth SE and Ringdén O: Mesenchymal stem cells inhibit and
stimulate mixed lymphocyte cultures and mitogenic responses
independently of the major histocompatibility complex. Scand J
Immunol. 57:11–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalal J, Gandy K and Domen J: Role of
mesenchymal stem cell therapy in Crohn’s disease. Pediatr Res.
71:445–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ringdén O, Uzunel M, Rasmusson I, et al:
Mesenchymal stem cells for treatment of therapy-resistant
graft-versus-host disease. Transplantation. 81:1390–1397. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun S, Guo Z, Xiao X, et al: Isolation of
mouse marrow mesenchymal progenitors by a novel and reliable
method. Stem Cells. 21:527–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morris GP, Beck PL, Herridge MS, et al:
Hapten-induced model of chronic inflammation and ulceration in the
rat colon. Gastroenterology. 96:795–803. 1989.PubMed/NCBI
|
|
16
|
Davaatseren M, Hwang JT, Park JH, et al:
Poly-γ-glutamic acid attenuates angiogenesis and inflammation in
experimental colitis. Mediators Inflamm. 2013:9823832013.
View Article : Google Scholar
|
|
17
|
González R, Rodríguez S, Romay C, et al:
Anti-inflammatory activity of phycocyanin extract in acetic
acid-induced colitis in rats. Parmacol Res. 39:55–59. 1999.
|
|
18
|
Guo Y, Su L, Wu J, et al: Assessment of
the green fluorescence protein labeling methods for tracking
implanted mesenchymal stem cells. Cytotechnology. 64:391–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi T and Song SU: Immunomodulatory
properties of mesenchymal stem cells and their therapeutic
applications. Arch Pharm Res. 35:213–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar
|
|
21
|
Tolar J, Le Blanc K, Keating A and Blazar
BR: Concise review: hitting the right spot with mesenchymal stromal
cells. Stem Cells. 28:1446–1455. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krampera M, Cosmi L, Angeli R, et al: Role
for interferon-gamma in the immunomodulatory activity of human bone
marrow mesenchymal stem cells. Stem Cells. 24:386–398. 2006.
View Article : Google Scholar
|
|
23
|
Tanaka F, Tominaga K, Ochi M, et al:
Exogenous administration of mesenchymal stem cells ameliorates
dextran sulfate sodium-induced colitis via anti-inflammatory action
in damaged tissue in rats. Life Sci. 83:771–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
25
|
Idriss HT and Naismith JH: TNF alpha and
the TNF receptor superfamily: structure-function relationship(s).
Microsc Res Tech. 50:184–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith CA, Farrah T and Goodwin RG: The TNF
receptor superfamily of cellular and viral proteins: activation,
costimulation, and death. Cell. 76:959–962. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogata H and Hibi T: Cytokine and
anti-cytokine therapies for inflammatory bowel disease. Curr Pharm
Des. 9:1107–1113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng L, Fisher G, Miller RE, et al:
Induction of apoptosis in mature T cells by tumour necrosis factor.
Nature. 377:348–351. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noti M, Corazza N, Mueller C, Berger B and
Brunner T: TNF suppresses acute intestinal inflammation by inducing
local glucocorticoid synthesis. J Exp Med. 207:1057–1066. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olson TS, Reuter BK, Scott KG, et al: The
primary defect in experimental ileitis originates from a
nonhematopoietic source. J Exp Med. 203:541–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reimund JM, Wittersheim C, Dumont S, et
al: Mucosal inflammatory cytokine production by intestinal biopsies
in patients with ulcerative colitis and Crohn’s disease. J Clin
Immunol. 16:144–150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armuzzi A, Lionetti P, Blandizzi C,
Caporali R, et al: Anti-TNF agents as therapeutic choice in
immune-mediated inflammatory diseases: focus on adalimumab. Int J
Immunopathol Pharmacol. 27(1 Suppl): 11–32. 2014.PubMed/NCBI
|
|
34
|
Chen FE and Ghosh G: Regulation of DNA
binding by Rel/NF-kappaB transcription factors: structural views.
Oncogene. 18:6845–6852. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: a pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
DiDonato JA, Hayakawa M, Rothwarf DM,
Zandi E and Karin M: A cytokine-responsive IkappaB kinase that
activates the transcription factor NF-kappaB. Nature. 388:548–554.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmitz ML, Bacher S and Kracht M: I kappa
B-independent control of NF-kappa B activity by modulatory
phosphorylations. Trends Biochem Sci. 26:186–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pizarro TT and Cominelli F: Cytokine
therapy for Crohn’s disease: advances in translational research.
Annu Rev Med. 58:433–444. 2007. View Article : Google Scholar
|
|
39
|
Elson CO, Cong Y and Weaver CT: Monoclonal
anti-interleukin 23 reverses active colitis in a T cell-mediated
model in mice. Gastroenterol. 132:2359–2370. 2007. View Article : Google Scholar
|
|
40
|
Atreya R, Mudter J, Finotto S, et al:
Blockade of interleukin 6 trans signaling suppresses T-cell
resistance against apoptosis in chronic intestinal inflammation:
evidence in crohn disease and experimental colitis in vivo. Nat
Med. 6:583–588. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suenaert P, Bulteel V, Lemmens L, et al:
Anti-tumor necrosis factor treatment restores the gut barrier in
Crohn’s disease. Am J Gastroenterol. 97:2000–2004. 2002. View Article : Google Scholar : PubMed/NCBI
|