Introduction
Irritable bowel syndrome (IBS) is a highly prevalent
functional gastrointestinal disorder characterized by abdominal
pain and alterations in bowel habits (1). IBS caused by viral, bacterial and
parasitic infection, observed in 3.7–36% of patients, has been
commonly referred to as PI-IBS (2). The pathophysiology of PI-IBS remains
to be elucidated, however, low-grade inflammation and chronic
alteration of the immune system at the molecular level have been
shown to target the mucosal secretory function, smooth muscle and
enteric nervous fibers (3–5). At present motility disorder is an
established typical character in PI-IBS patients, however,
alterations in the motility patterns in PI-IBS and the underlying
mechanisms remain to be elucidated.
The interstitial cells of Cajal (ICC) are present
throughout the gastrointestinal tract, from the esophagus to the
anus, with three main functions, including acting as
mechanoreceptors, transmitting enteric neuronal signals to the
smooth muscle cells and placing slow waves and regulating their
propagation (6). According to
their location, the ICC are divided into the myenteric plexus (MP)
ICC, intramuscular (IM) ICC, deep muscular plexus (DMP) ICC and the
submucosa (SMP) ICC (6). All the
subtypes are important components of gastrointestinal motility.
Although certain studies have reported that ICC changed in PI-IBS
patients and animal models (7,8), the
correlation between the patterns of gastrointestinal motor
dysfunction and changes of the ICC remain to be elucidated.
Therefore, the present study measured the feces and the c-kit
protein in the different intestinal segments in PI-IBS model mice
to elucidate the types of motility disorders and the association
with changes in the ICC.
Materials and methods
Animals
The experimental procedure was approved by the
Animal Welfare committee of Chongqing Medical University
(Chongqing, China). Specific pathogen-free female C56BL/6 mice (4–6
weeks old) were purchased from the Animal Center of The Third
Military Medical University (Chongqing, China). A total of 34 mice
were randomly assigned to a control group (n=17) and a model group
(n=17) and maintained in normal 12 h light/dark cycles in a
regulated environment (20–25°C). The study was approved by the
ethics committee of the First Affiliated Hospital of Chongqing
Medical University.
Trichinella spiralis infection
The infective muscle larvae were obtained from mice
infected with Trichinella spiralis at 30 days. The infected
mouse was sacrificed, skinned and eviscerated. The muscles
containing the encysted larvae were minced and digested in 0.5%
pepsin A (Biosharp, Hefei, China) and 0.5% hydrochloric acid for 16
h at 37°C. The isolated infective larvae were washed several times
with 0.85% sodium chloride and suspended in a balanced saline
solution. The mice in the model group were infected by oral
administration of 350–400 larvae in 0.2 ml saline solution, whereas
the control mice received only saline solution (9).
Sample collection and processing
In each group, three mice were humanely sacrificed
(ether inhalation and cervical dislocation) at days 14, 28 and 56
post-infection (PI). The terminal ileum and proximal colon were
sampled and flushed with physiological saline to remove the gut
contents. A single sample of 1 cm in length from each intestinal
tissue was fixed overnight in 4% paraformaldehyde and embedded in
paraffin for immunochemical and hematoxylin and eosin (H&E)
analysis. The intestine tissues of the remaining mice were removed
and preserved in liquid nitrogen immediately for subsequent mRNA
and protein extraction.
Abdominal withdrawal reflex (AWR)
The behavioral responses to colorectal distention
(CRD) were assessed at day 56 PI by measuring the AWR (10). Briefly, the distention was applied
using a balloon (2 mm external diameter, 10 cm length), which was
inserted rectally into the descending colon of the mildly sedated
mice, inserted with ether inhalation and fixed at the base of the
tail. The mice were then housed in glass cubicles (20×8×8 cm) and
were left to wake and acclimate for 1 h. The balloon was distended
with 0.25 ml, 0.35 ml, 0.5 ml and 0.65 ml water for 20 sec. Each
distention was repeated three times with 30 sec intervals and the
balloon was then deflated and withdrawn following assessment of the
AWR. The AWR score was assigned as follows: 0, no behavioral
response to CRD; 1, brief head movement followed by immobility; 2,
contraction of abdominal muscles; 3, lifting of abdomen and 4, body
arching and lifting of pelvic structures (11).
Measurement of intestinal motility
All the mice were orally administrated with 0.3 ml
activated carbon suspension and the time until production of the
first black fecal pellets was recorded to evaluate ITT. The fecal
pellets were collected every 15 min for 8 h until the color of
feces returned to normal. The collected stool was weighed and
desiccated every 2 h. This was performed by measuring the stool
weight prior to and following oven drying (24 h at 70°C in the
presence of desiccant) and the water content percentage was
calculated. The grain numbers, wet weights, percentage liquid
content, dry weights and Bristol scores were then compared with
each other. The criteria for stool scoring were established as
follows (8): 1, normal stool; 2,
soft and poorly formed stool 3, watery stool.
Immunohistochemical staining
The paraffin-embedded tissues were cut into 5
μm-thick sections. To deparaffinize the sections, they were
immersed in xylene at 56°C twice for 20 min and hydrated with
ethanol (twice with 100%, once with 95% and once with 75% ethanol)
for 5 min. The sections were then pretreated with 3% hydrogen
peroxide for 20 min at 37°C and antigen retrieval was performed by
boiling the sections in citrate buffer (0.01 M; pH 6.0) for 20 min.
Following cooling at room temperature for 1 h, the specimens were
treated with 5% bovine serum albumin for 30 min at 37°C followed by
overnight incubation with 1:200 diluted rabbit anti-mouse c-kit
polyclonal antibody (Boster Biotechnology, Ltd., Wuhan, China) at
4°C. Following washing in phosphate-buffered saline, the slides
were incubated for 60 min at 37°C with the corresponding secondary
biotinylated goat anti-rabbit antibody (Boster Biotechnology, Ltd.)
at a 1:300 dilution. Following washing, the slides were incubated
with Diaminobenzidine chromogen (Zsbio, Beijing China) for 5 min. A
nuclei counterstain was performed using Mayer’s hematoxylin (Boster
Biotechnology, Ltd., Wuhan, China). The slides were then washed
with water and immersed in 75, 85, 90 and 100% ethanol and 100%
xylene. The sealed slides were recorded digitally using a Leica
Microsystem (DM6000B, Leica, Mannheim, Germany). The
immunohistochemical analysis was scored as staining intensity and
the proportion of positive cells (12). The scale of staining intensity was
as follows: 0, not detected; 1, minimal; 2, mild; 3, moderate and
4, marked. The proportion of positive cells scale was: 0, no
staining, 1, c-kit-positive cells in <1% population; 2,
c-kit-positive cells in 1–10% population; 3, c-kit-positive cells
in 10–50% and 4, c-kit-positive cells in >50% population.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) mRNA assay
The total RNA in the intestinal mucosa was extracted
using TRIzol solution (Takara Bio, Inc., Shiga, Japan). The gene
expression of c-kit was assayed by RT-qPCR. The β-actin mRNA level
was determined as an internal reference and their levels of
expression were quantitated by optical densitometry following
electrophoresis on an agarose gel. The primers were designed based
on the following complementary DNA sequencs: β-actin (470 bp),
forward 5′-AGGCTGTGCTGTCCCTGTATG-3′ and reverse
5′-GAGGTCTTTACGGATGTCAACG-3′ and c-kit (214 bp), forward
5′-CGACGCAACTTCCTTATGAT-3′ and reverse 5′-AGGACCTTCAGTTCCGACAT-3′.
The RT was performed at 37°C for 15 min and 85°C for 5 sec. The PCR
cycling condition was 36 cycles at 94°C for 30 sec, 59°C (β-actin)
or 56°C (c-kit) for 30 sec and 72°C for 35 sec. The PCR end
products were electrophoresed on 4% agarose gel and stained with
ethidium bromide. The gray values of the bands were calculated
using Quantity One software (Bio-Rad). The relative mRNA expression
levels of target genes were normalized to the corresponding
internal standard.
Western blotting for protein assay
The frozen sample was fractured using an ultrasonic
disrupter (HD3100, Bandelin Electronic, Berlin, Germany), suspended
in radioimmunoprecipitation buffer (Takara Bio Inc.) for 20 min and
the protein levels in the supernatants were obtained following
centrifugation at 12,000 × g for 20 min and quantified using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Aliquots (60 μg) of the protein
were subjected to electrophorese on a polyacrylamide gel for the
c-kit protein. The primary antibodies were goat anti-mouse c-kit
polyclonal antibody (Boster Biotechnology, Ltd.) at a dilution of
1:500 and goat anti-mouse β-actin polyclonal antibody (Boster
Biotechnology, Ltd.) at a dilution of 1:1,000 The β-actin protein
was used as an internal standard. The signal was detected using an
enhanced chemiluminescence detection system (Bio-Rad). The
densities of the bands were assessed using Quantity One software
(Bio-Rad). The relative protein levels of the target protein were
obtained by correction with the corresponding internal
standard.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). Data are expressed as the
mean ± standard deviation. The independent sample t-test was used
to compare the results between the two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pathological examination
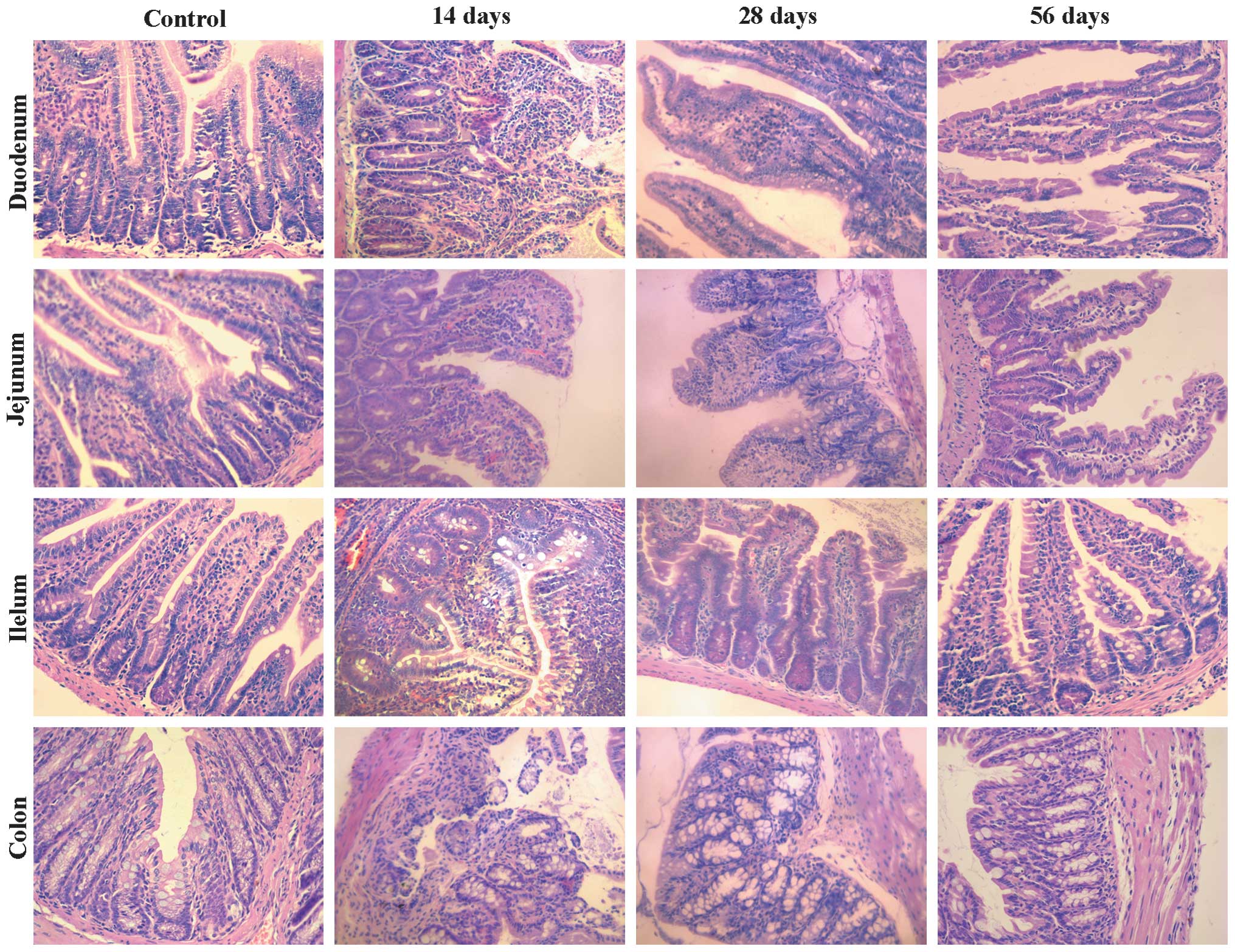
H&E staining (Fig.
1) revealed a marked infiltration by neutrophils in the lamina
propria and interstitial edema on day 14 PI. Infiltration and edema
gradually reduced between days 14 and 28 PI. At 56 days PI, no
obvious inflammatory infiltrate was observed and from this day, the
mice were deemed suitable as PI-IBS model mice.
AWR
At distention volumes of 0.35 and 0.5 ml, the AWR
scores in the model mice were higher compared with those in the
controls, which indicated that the visceral sensitivity to
colorectal distension was increased in the model mice. No
significant differences were observed at volumes of 0.25 or 0.65 ml
(Table I).
 | Table IAbdominal withdrawal reflex scores for
colon distention of mice in the model and control groups. |
Table I
Abdominal withdrawal reflex scores for
colon distention of mice in the model and control groups.
| Group | n | 0.25 ml | 0.35 ml | 0.5 ml | 0.65 ml |
|---|
| Model | 8 | 0.50±0.25 | 2.42±0.24a | 3.54±0.31a | 3.88±0.35 |
| Control | 8 | 0.33±0.31 | 1.83±0.44 | 2.92±0.43 | 3.75±0.46 |
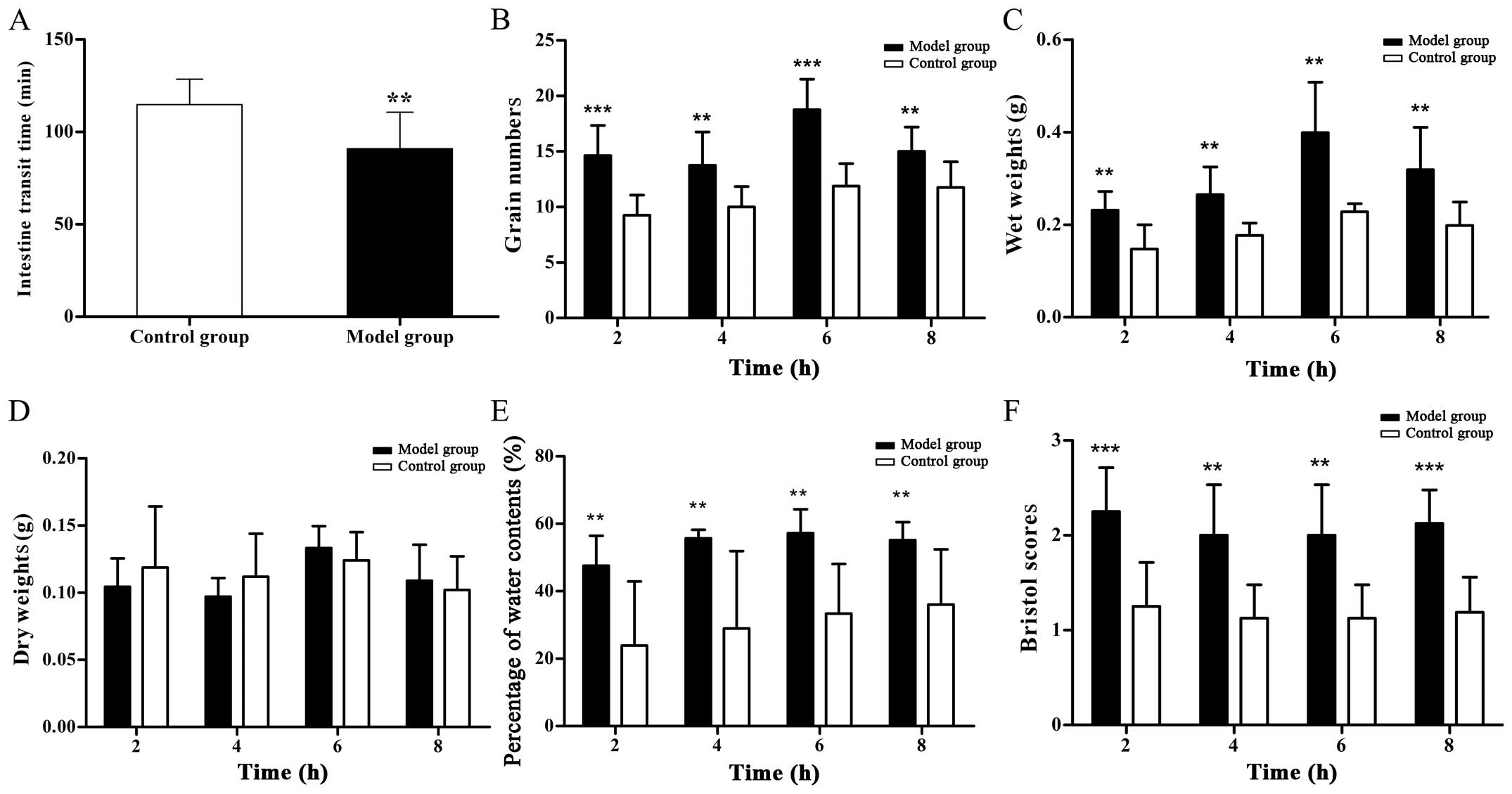
Measurement of intestinal motility
The ITT of the PI-IBS group was markedly shorter
compared with the control group. Compared with those of the control
group, the grain numbers, wet weights, Bristol scores and
percentage liquid contents were significantly higher, however, the
dry weights were not different between the two groups (Fig. 2).
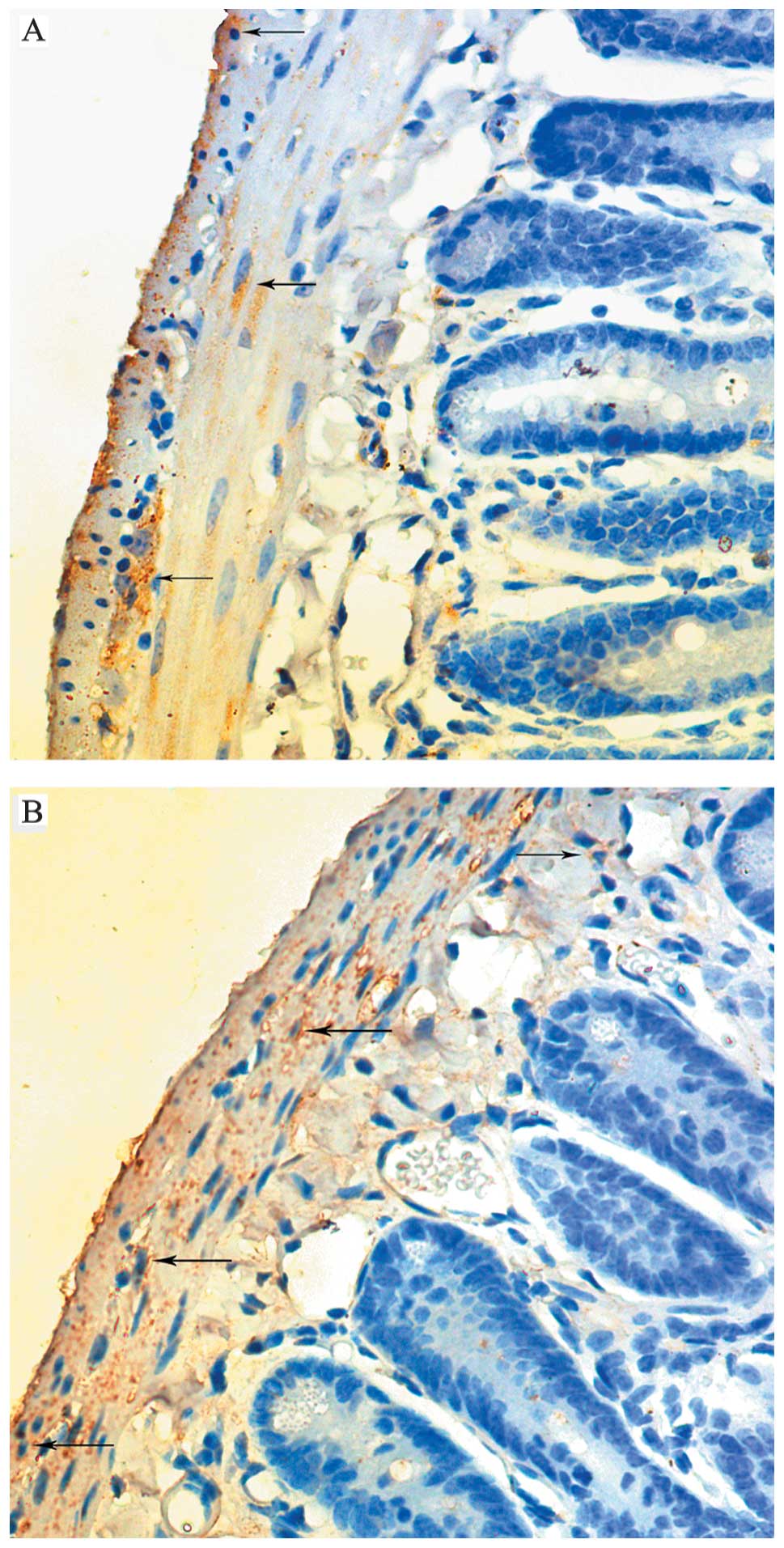
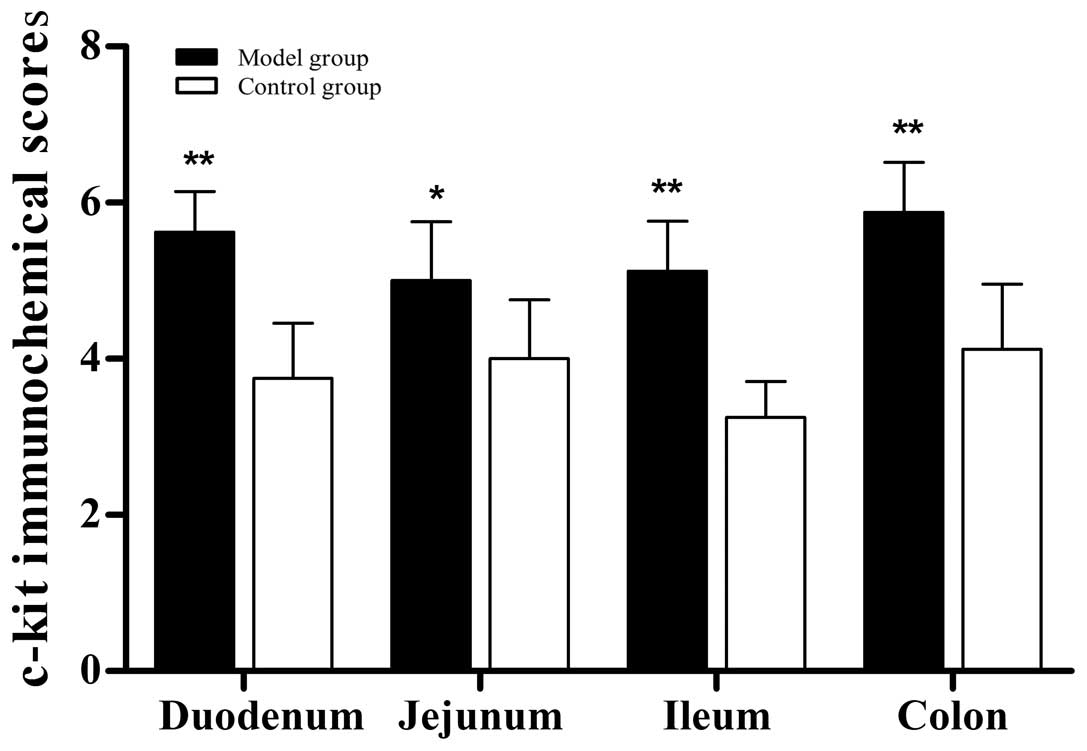
Immunohistochemical staining
The immunolabeling revealed that the c-kit signals
were detected predominantly in the submucosa and myenteron
(Fig. 3). The staining scores of
the entire intestines in the model group were notably higher
compared with those of the control group (Fig. 4).
RT-PCR and western blotting assays
The results of the RT-PCR and western blotting
assays demonstrated that the mRNA and protein levels of c-kit were
significantly higher in the duodenum, jejunum, ileum and colon of
the PI-IBS mice compared with the control mice (Figs. 5 and 6).
 | Figure 5Relative mRNA levels of c-kit in the
different intestinal segments. Reverse transcription polymerase
chain reaction was performed. Lanes 1, 3, 5 and 7 represent the
duodenum, jejunum, ileum and colon of the model mice, respectively;
2, 4, 6 and 8 represent the duodenum, jejunum, ileum and colon of
the control mice, respectively. Error bars represent the mean ±
standard deviation. n=8. *P<0.05, and
**P<0.01 compared with the control group. |
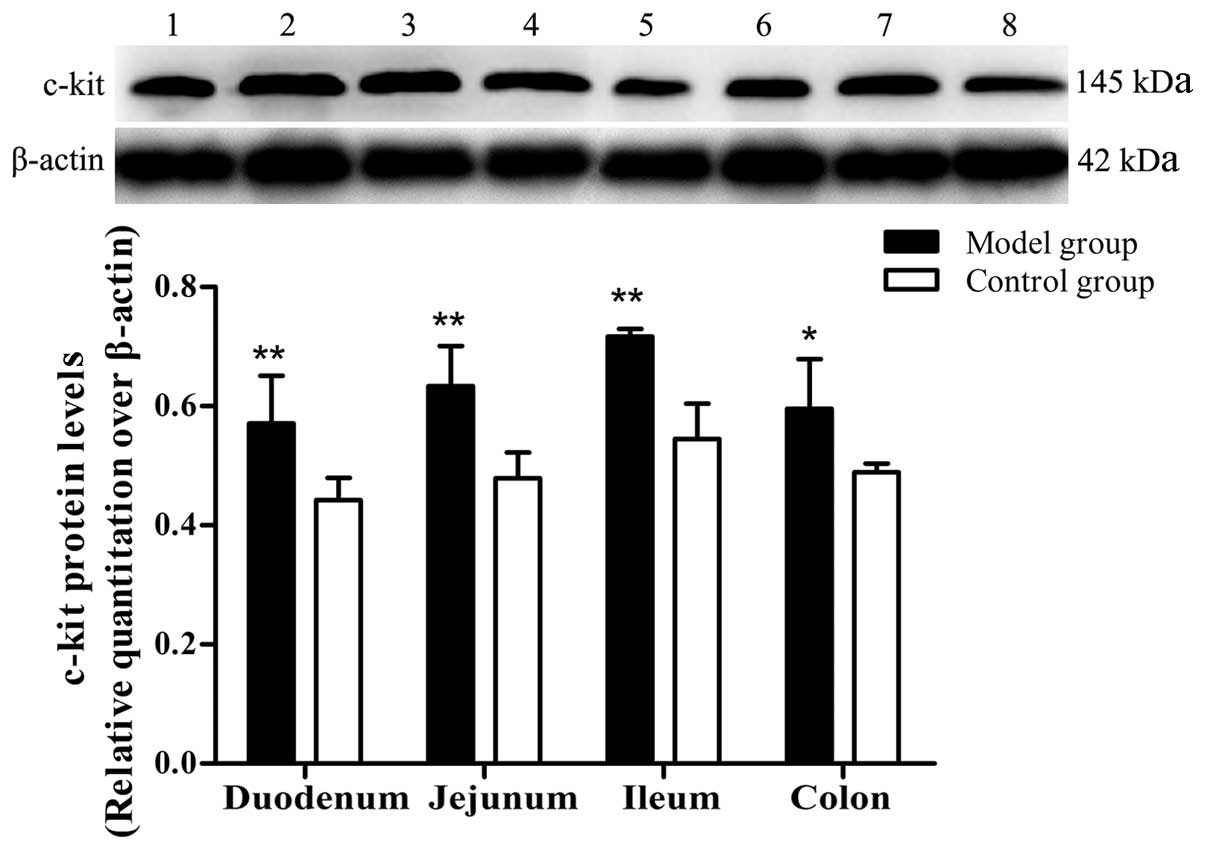 | Figure 6Relative protein levels of c-kit in
the different intestinal segments. Western blot analysis was
performed. Lanes 1, 2, 3 and 4 represent the duodenum, jejunum,
ileum and colon of the model mice, respectively; 5, 6, 7 and 8
represent the duodenum, jejunum, ileum and colon of the control
mice, respectively. Error bars represent the mean ± standard
deviation. n=8. *P<0.05 and **P<0.01
compared with the control group. |
Discussion
PI-IBS is defined as the acute onset of IBS symptoms
(meeting Rome III criteria) that develop when an individual, who
has not previously met the Rome criteria, experiences a
gastrointestinal infection with two or more of the following
characteristics: fever, vomiting, diarrhea or a stool culture
positive for an infectious agent (13). The Rome III criteria are as
follows: Recurrent abdominal pain or discomfort should be present
for at least three days per month over the past three months and be
associated with ≥2 of: involvement with defecation, onset
associated with a change in frequency of stool, onset associated
with a change in form of stool. These criteria must be fulfilled
over the past three months with symptom onset occurring at least
six months previously. Although the mechanism remains to be
elucidated, visceral hypersensitivity and intestinal motor
dysfunction are reported to be involved (14). The present study used a previously
described method to establish a PI-IBS mouse model (15). Through the dynamic observation of
intestinal histology and AWR scores, the infected mice were used as
PI-IBS mice when the intestine returned to normal. According to the
results of intestinal motility, the present study suggested that
the frequency and rhythm of intestinal peristalsis altered in the
model mice. The frequency and rhythm of bowel movement is completed
by ICC, smooth muscle cells and the enteric nervous system in
combination and ICC provides a pacemaker signal for coordinated
motility patterns. As ICC is important in bowel movement (16), the present study hypothesized that
ICC were changed in the PI-IBS model mice infected by
Trichinella spiralis. To confirm this hypothesis, the c-kit
protein was used to determine the number and function of the
intestinal ICC, which is an important biological symbol and is
expressed in the ICC of the intestinal muscular layer and lamina
propria (6). The mucosal
inflammation of the gut induced by Trichinella spiralis
infection resulted in loss of ICC function due to structural injury
and loss of c-kit positivity (17). In inflammation subsidence, the
rough endoplasmic reticulum and Golgi apparatus of the injured ICC
are required to synthesize proteins, including c-kit, to form a new
plasma membrane and to recover structure and function (17). The present study found that the
protein and mRNA expression of c-kit were significantly higher in
the PI-IBS mice compared with the control mice. Overexpressed c-kit
is required for development and maintenance of ICC, was also
necessary for development of coordinated motility patterns and for
the survival of ICC (18,19). In several diseases, an association
between macrophages and ICC injury has been identified (20,21).
However, in vivo, 5-hydroxytryptamine (5-HT) released from
macrophages and an increase in PI-IBS can combine with 5-HT2B
receptors, which are expressed on the plasma membrane of ICC and
this promotes proliferation of the ICC (22,23).
The present study found that the c-kit-positive cells increased in
the intestine of the PI-IBS mice.
On resolution of submucosal and myenteric
inflammation, the network of ICC, particularly that involved in
connecting ICC to each other and to smooth muscle cells, return to
a normal physiological state, whereas the increased ICC in PI-IBS
mice may induce intestinal motor disturbances similar to that
induced by inflammation (24,25).
The present study observed that the signals of c-kit were
predominantly detected in the submucosa and myenteron, where the
ICC mainly pace slow waves (26).
Increased ICC has the potential to place more slow waves and this
alteration can accelerate the intestinal muscular contraction and
peristaltic propagation (27). In
addition, increased cholecystokinin in PI-IBS can bind to
cholecystokinin A receptors expressed on ICC and promote
gastrointestinal emptying (28,29).
Furthermore, an increase in the number of IM-ICC can shorten the
intervals between the migratory motor complex (MMC) cycles and
decrease the bowel transmitting time (30). These mechanisms mean that the
reduced time of the whole gastrointestinal transmitting was closely
associated with the ICC changes.
The small intestine is the primary site of nutrient
absorption. As the frequency and rhythm of bowel movement increase,
the higher osmotic pressure of food residue entering the colon is
not conducive to water absorption. However, the reduced contact
time between food waste and intestinal wall results in a markedly
higher water content and higher Bristol scores of the feces
(31).
In the present study, the observed increase in feces
grains and similar dry weights between the two groups indicated
that the MMC frequency increased and the underlying mechanisms may
be associated with an increase in the number of slow waves and
short time intervals in the MMC cycles (30). However, an increase in the number
of IM-ICC leads to increased nitric oxide transmission and
subsequently decreased MMC frequency (15). Whether the association between ICC
and nitric oxide transmission was abnormal in PI-IBS requires
further investigation.
In conclusion, a PI-IBS model was successfully
established in the present study using C57BL\6 mice induced by
Trichinella spiralis. Alterations in the ICC numbers and
c-kit protein were closely associated with changes in the
intestinal motor patterns in PI-IBS. However, further studies are
required to confirm the association between the ICC and intestinal
motility disorders in PI-IBS.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81160057). The authors would
like to thank Professor Bao-Quan Fu (Chinese Academy of
Agricultural Sciences, Beijing, China) for offering Trichinella
spiralis and Professor Yu Li and Mr. Bing Liu (Department of
Anatomic Pathology, Chongqing Medical University) for their
technical assistance.
References
|
1
|
Choung RS and Locke GR III: Epidemiology
of IBS. Gastroenterol Clin North Am. 40:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spiller R and Garsed K: Postinfectious
irritable bowel syndrome. Gastroenterology. 136:1979–1988. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elsenbruch S, Holtmann G, Oezcan D, Lysson
A, Janssen O, Goebel MU and Schedlowski M: Are there alterations of
neuroendocrine and cellular immune responses to nutrients in women
with irritable bowel syndrome[quest]. Am J Gastroenterol.
99:703–710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liebregts T, Adam B, Bredack C, Röth A,
Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ and
Holtmann G: Immune activation in patients with irritable bowel
syndrome. Gastroenterology. 132:913–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarna SK: Are interstitial cells of Cajal
plurifunction cells in the gut? Am J Physiol Gastrointest Liver
Physiol. 294:112008.
|
|
7
|
Jee SR, Morales W, Low K, Chang C, Zhu A,
Pokkunuri V, Chatterjee S, Soffer E, Conklin JL and Pimentel M: ICC
density predicts bacterial overgrowth in a rat model of
post-infectious IBS. World J Gastroenterol. 16:36802010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pokkunuri V, Pimentel M, Morales W, Jee
SR, Alpern J, Weitsman S, Marsh Z, Low K, Hwang L, Khoshini R, et
al: Role of Cytolethal Distending Toxin in Altered Stool Form and
Bowel Phenotypes in a Rat Model of Post-infectious Irritable Bowel
Syndrome. J Neurogastroenterol Motil. 18:434–442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wheatcroft J, Wakelin D, Smith A, Mahoney
CR, Mawe G and Spiller R: Enterochromaffin cell hyperplasia and
decreased serotonin transporter in a mouse model of postinfectious
bowel dysfunction. Neurogastroenterol Motil. 17:863–870. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Distrutti E, Sediari L, Mencarelli A,
Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G,
Wallace JL and Fiorucci S: Evidence that hydrogen sulfide exerts
antinociceptive effects in the gastrointestinal tract by activating
KATP channels. J Pharmacol Exp Ther. 316:325–335. 2006. View Article : Google Scholar
|
|
11
|
Al-Chaer ED, Kawasaki M and Pasricha PJ: A
new model of chronic visceral hypersensitivity in adult rats
induced by colon irritation during postnatal development.
Gastroenterology. 119:1276–1285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1α in brain tumors: association with angiogenesis, invasion and
progression. Cancer. 88:2606–2618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghoshal UC and Ranjan P: Post-infectious
irritable bowel syndrome: the past, the present and the future. J
Gastroenterol Hepatol. 26:94–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spiller R and Garsed K: Postinfectious
irritable bowel syndrome. Gastroenterology. 136:1979–1988. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bercik P, Wang L, Verdu EF, Mao YK,
Blennerhassett P, Khan WI, Kean I, Tougas G and Collins SM:
Visceral hyperalgesia and intestinal dysmotility in a mouse model
of postinfective gut dysfunction. Gastroenterology. 127:179–187.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eshraghian A and Eshraghian H:
Interstitial cells of Cajal: a novel hypothesis for the
pathophysiology of irritable bowel syndrome. Can J Gastroenterol.
25:277–279. 2011.PubMed/NCBI
|
|
17
|
Wang XY, Vannucchi MG, Nieuwmeyer F, Ye J,
Faussone-Pellegrini MS and Huizinga JD: Changes in interstitial
cells of Cajal at the deep muscular plexus are associated with loss
of Distention-Induced Burst-Type Muscle Activity in Mice Infected
by Trichinella spiralis. Am J Pathol. 167:437–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rich A, Gordon S, Brown C, Gibbons SJ,
Schaefer K, Hennig G and Farrugia G: Kit signaling is required for
development of coordinated motility patterns in zebrafish
gastrointestinal tract. Zebrafish. 10:154–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghaith O, El-Halabi M, Hashash JG and
Sharara AI: Investigational agents for the irritable bowel
syndrome. Expert Opin Investig Drugs. 19:1161–1178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rumessen JJ: Ultrastructure of
interstitial cells of Cajal at the colonic submuscular border in
patients with ulcerative colitis. Gastroenterology. 111:1447–1455.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki T, Won K-J, Horiguchi K, Kinoshita
K, Hori M, Torihashi S, Momotani E, Itoh K, Hirayama K, Ward SM,
Sanders KM and Ozaki H: Muscularis inflammation and the loss of
interstitial cells of Cajal in the endothelin ETB receptor null
rat. Am J Physiol Gastrointest Liver Physiol. 287:638–646. 2004.
View Article : Google Scholar
|
|
22
|
Chun L and Bai Y: Experimental research on
modifying liver and spleen decoction for diarrhea-predominant IBD.
J Nanjing Univ Trad Med. 2:0192011.
|
|
23
|
Wouters MM, Roeder JL, Tharayil VS,
Stanich JE, Strege PR, Lei S, Bardsley MR, Ordog T, Gibbons SJ and
Farrugia G: Protein kinase Cγ mediates regulation of proliferation
by the serotonin 5-hydroxytryptamine receptor 2B. J Biol Chem.
284:21177–21184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Der T, Bercik P, Donnelly G, Jackson T,
Berezin I, Collins SM and Huizinga JD: Interstitial cells of cajal
and inflammation-induced motor dysfunction in the mouse small
intestine. Gastroenterology. 119:1590–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bettolli M, De Carli C, Cornejo PD, Jolin
DK, Wang XY, Huizinga J, Krantis A, Rubin S and Staines WA:
Interstitial cell of Cajal loss correlates with the degree of
inflammation in the human appendix and reverses after inflammation.
J Pediatr Surg. 47:1891–1899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ward SM, Harney SC, Bayguinov JR, McLaren
GJ and Sanders KM: Development of electrical rhythmicity in the
murine gastrointestinal tract is specifically encoded in the tunica
muscularis. J Physiol. 505:241–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eshraghian A and Eshraghian H:
Interstitial cells of Cajal: a novel hypothesis for the
pathophysiology of irritable bowel syndrome. Can J Gastroenterol.
25:277–279. 2011.PubMed/NCBI
|
|
28
|
Patterson L, Zheng H, Ward S and Berthoud
HR: Immunohistochemical identification of cholecystokinin A
receptors on interstitial cells of Cajal, smooth muscle, and
enteric neurons in rat pylorus. Cell Tissue Res. 305:11–23. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dizdar V, Spiller R, Singh G, Hanevik K,
Gilja OH, El-Salhy M and Hausken T: Relative importance of
abnormalities of CCK and 5-HT (serotonin) in Giardia-induced
post-infectious irritable bowel syndrome and functional dyspepsia.
Aliment Pharmacol Ther. 31:883–891. 2010.PubMed/NCBI
|
|
30
|
Powell AK and Bywater RA: Murine
intestinal migrating motor complexes: longitudinal components.
Neurogastroenterol Motil. 15:245–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Surawicz C: Mechanisms of diarrhea. Curr
Gastroenterol Rep. 12:236–241. 2010. View Article : Google Scholar : PubMed/NCBI
|