Introduction
Age-associated macular degeneration (AMD) is the
leading cause of legal blindness among individuals aged >60
years in developed countries (1).
AMD is divided into two subtypes: Wet and dry. The development of
choroidal neovascularization (CNV) is a key feature of wet AMD,
which induces bleeding and scar formation in the macula and
severely reduces the vision of patients (1). To date, no cure exists for AMD. Since
2003, numerous agents targeting vascular endothelial growth factors
(VEGFs) have been approved by the US Food and Drug Administration
for the treatment of CNV in AMD (2). Targeting VEGFs is an effective
treatment method; however, there are concerns regarding repeated
injections, CNV recurrence and the safety of long term application.
Although VEGF plays an important role in the development of CNV,
other mechanisms are also involved in this disorder (3). Alternative agents for the treatment
of CNV are currently investigated, in order to improve their
efficacy and safety and ensure that they are non-invasive. In 2012,
Jin et al(4) devised a
novel traditional Chinese medicine (TCM) composition for wet AMD
(patent no. WO2012079419). This pharmaceutical composition was
shown to enhance and stabilize the vision of patients with AMD,
promote absorption of hemorrhage and reduce CNV fluorescein leakage
and size (5). The pharmaceutical
composition consisted of eight TCM components, including
Astragalus membranaceus Bunge, Angelica sinensis,
Poria cocos Wolf, Fritillaria thunbergii, Panax
pseudoginseng, charred Radix et Rhizoma Rhei (CRRR),
pollen Typhae and Curcuma aromatica Salisb. In
addition, Jin et al (4)
(patent no., WO2012079419) tested another composition for the
treatment of CNV in AMD for four years; however, the clinical
outcomes of the study were unsatisfactory. The previously used
composition included Astragalus membranaceus, Angelica
sinensis, Panax pseudoginseng, pollen Typhae,
Citrus reticulata, Fritillaria thunbergii, Curcuma
aromatic Salisb, Fructus and Poria cocos Wolf.
The significant alteration between the previous and the present
(WO2012079419) composition was the replacement of Citrus
reticulata with CRRR. Thus, CRRR may play a vital role in the
treatment of wet AMD. To the best of our knowledge, currently no
publication exists regarding the mechanism of CRRR in this CNV
treatment method. The aim of the present study was to explore the
effect of CRRR treatment in a CVD murine model.
Materials and methods
Animals
All the animal experiments conformed to the
Association for Research in Vision and Ophthalmology guide
(www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research/)
for the Use of Animals in Ophthalmic and Vision Research and were
approved by the Institutional Animal Care and Use Committee of
Shanghai Jiao Tong University, Shanghai, China (permit no.
2014KY110). A total of 30 male C57BL/6 mice (eight-weeks-old;
Laboratory Animal Center of Shanghai Jiao Tong University) were
used in the present study. The mice were fed normal mouse chow and
supplied with water ad libitum. Prior to laser treatment and
during subsequent retinal imaging, the mice were anesthetized by
intraperitoneal (i.p.) injection of sodium pentobarbital (40 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) and the pupils were dilated
using topical 1% tropicamide (Beijing Double-Crane Pharmaceutical
Co., Ltd., Beijing, China).
Laser-induced CNV in mice
Laser treatment was performed on the retinas of the
mice. A coverslip was mounted onto the mouse cornea in order to
view the retina and perform laser burns. Six laser burns were
performed between the retinal vessels around the optical nerve head
of each eye (laser parameters: 150 mW; 100 msec; laser spot size,
0.05 mm) using a diode laser system (Coherent, Inc., Santa Clara,
CA, USA). Rupture of the Bruch’s membrane was confirmed by the
formation of a bubble immediately following laser application. The
inclusion criteria of the lesions induced by laser, included the
successful rupture of the Bruch’s membrane and the absence of
subretinal hemorrhage. The inclusion criteria of the present study
included the formation of a bubble immediately following laser
application and the absence of subretinal hemorrhage. Following
full recovery from anesthesia, the mice were returned to the animal
care facility.
Preparation of CRRR and treatment
The water extract (0.6 g/100 ml) of CRRR was
prepared and packed by the Department of Pharmaceutical Sciences of
the First People’s Hospital (Shanghai, China) using a standardized
procedure. Briefly, the lyophilized powder (Pingcheng Biotech Co.
Ltd., Xi’an, China) was dissolved in distilled water (0.6g/100ml)
prior to use. The mice were randomly divided into two groups (15
mice per group) and treated as follows: i) Control group, receiving
laser treatment on day 0 and administered 0.9% saline (1 ml/0.1 kg,
twice a day by oral gavage) between days 0 and 21; and (ii) CNV
with CRRR group, receiving laser treatment on day 0 and
administered CRRR (1 ml/0.1 kg, twice a day by oral gavage) between
days 0 and 21. The concentration and dosage of CRRR used in the
present study were calculated based on the concentration used in
the previously described pharmaceutical composition (patent no.,
WO2012079419).
Fundus fluorescein angiography (FFA)
FFA of the CNV lesions was conducted using a
Heidelberg Retina Angiograph 2 imaging system (Heidelberg
Engineering, Inc., Vista, CA, USA). Fluorescein treatment (0.02 ml
25% fluorescein; Alcon Laboratories, Irvine, CA, USA) was delivered
by i.p. injection, on days 7, 14, and 21 following laser treatment.
Timing began immediately after the fluorescein injection. Early-,
middle- and late-phase fundus angiograms were obtained at 45 sec, 2
min and 7 min after the fluorescein injection, respectively. The
fluorescein leakage of each CNV lesion was graded by two
independent retina specialists, unaware of the experimental design,
according to a method previously described by Marneros et al
(6). Briefly, the early-, middle-
and late-phase angiograms were compared in order to determine the
severity of the lesions: Grade 0 lesions showed no
hyperfluorescence; grade 1 lesions exhibited hyperfluorescence
without leakage; grade 2a lesions exhibited hyperfluorescence in
the early-phase or midtransit images and late-phase leakage; and
grade 2b lesions exhibited bright hyperfluorescence in the transit
images and late-phase leakage beyond the treated areas. Only
lesions with grade 2b leakage were considered as clinically
significant (Fig. 1).
Immunofluorescence and volumetric
analysis
At 22 days after laser treatment, the mice were
sacrificed by cervical dislocation and their eyes were enucleated.
The eyes were fixed for 1 h in 4% paraformaldehyde. Then the
anterior segments and the retinas were removed. The eye cups were
washed with phosphate-buffered saline (PBS), then immersed in 2%
normal donkey serum (Sigma-Aldrich) and 1% Triton X-100 (Invitrogen
Life Technologies, Carlsbad, CA, USA) in PBS for 3–10 min. After
incubation with isolectin B4 from Griffonia simplicifolia
(AlexaFluor® 568; 25 μg/ml; Invitrogen Life
Technologies), phalloidin (AlexaFluor® 488; 5 U/ml;
Invitrogen Life Technologies) and Hoechst 33258 (20 μg/ml;
Invitrogen Life Technologies), the eye cups were washed with PBS
and cut with four peripheral radial cuts. The flat mounts were
covered with a coverslip using ProLong® Gold antifade
reagent (Invitrogen Life Technologies). A Zeiss LSM-510 Meta Laser
Confocal microscope (Carl Zeiss, Inc., Thornwood, NY, USA) was used
for sample observation and image capture. An image stack was
collected for each lesion, which included the area from the retinal
pigment epithelium (RPE) surface to the bottom of the lesion.
Volumetric analysis of the images was performed using the Image J
software (National Institutes of Health, Bethesda, MA, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from the posterior part of
the eyeball (retina, RPE and choroid) at day 7 after the laser
treatment using Isogen (Nippon Gene Co, Ltd., Tokyo, Japan)
(7). Next, the total RNA was
reverse-transcribed into cDNA using a Superscript III First Strand
Synthesis system (Invitrogen Life Technologies) and random hexamer
primers, according to the manufacturer’s instructions. The gene
expression levels were analyzed by qPCR using TaqMan®
Gene Expression Assays (Applied Biosystems Life Technologies,
Foster City, CA, USA). A realtime PCR cycler (ABI Prism 7800
Sequence Detection System; Applied Biosystems Life Technologies)
was used for the experiment, according to the manufacturer’s
instructions. The assay identification numbers used in this study
were as follows: interleukin-10 (IL-10), Mm00439614_m1; and VEGF,
Mm00437304_m1. β-actin was used as the housekeeping gene, to
normalize for differences in the efficiency of sample extraction or
cDNA synthesis by reverse transcriptase. For relative
quantification, the 2−ΔΔCt method was used.
Western blot analysis
Extracts from the retina-RPE-choroid complexes of
five mice in each group were prepared for western blot analysis at
day seven following laser photocoagulation, according to the method
of Andrews and Faller (8).
Briefly, total protein was obtained by lysing in a buffer
containing 1 M Tris-HCl (pH 7.5), 1% Triton X-100, 1% nonidet p-40,
10% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 0.5 M EDTA,
10 μg/ml leupeptin, 10 μg/ml aprotinin and 1 mM
phenylmethylsulfonyl fluoride. Protein was separated by 10%
SDS-PAGE and transferred to polyvinylidine difluoride filter
membranes (EMD Millipore, Bedford, MA, USA). The membranes were
blocked in Tris-buffered saline (TBS) containing 5% milk and 0.1%
Tween-20 for 1 h at room temperature, then incubated at 4°C
overnight with the following primary antibodies: Rat monoclonal
anti-IL-10 (1:200; sc-73309; Santa Cruz Biotechnology Inc., Dallas,
TX, USA), rat polyclonal anti-VEGF (1:300; sc-507; Santa Cruz
Biotechnology Inc.) and rat polyclonal anti-β-actin antibody
(1:500; SAB2100037; Sigma-Aldrich). The membranes were then washed
three times for 10 min each in TBS containing 0.1% Tween-20.
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rat immunoglobulin G secondary
antibody (1:200; sc-45100; Santa Cruz Biotechnology, Inc.) for 2 h.
After three 10-min washes in TBS containing 0.1% Tween-20, the
protein bands were visualized using an enhanced chemiluminescence
system (Pierce Biotechnologies Inc., Rockford, IL, USA).
Densitometric analysis was performed using the Quantity One
analysis software version 4.2.1 (Bio-Rad Laboratories, Hercules,
CA, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS software
version 19.0 (IBM, Armonk, NY, USA). Statistical significance was
analyzed by one-way analysis of variance, followed by Student’s
t-test for CNV volume analysis, Mann-Whitney test for qPCR
experiments and χ2 test for CNV leakage grading
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Fluorescein leakage in laser treated
eyes
The percentage of lesions showing a clinically
significant leakage was determined by FFA (Fig. 1). In the control group, the
percentage of lesions with grade 2b fluorescein leakage was 79.65,
56.67 and 46.67% at days 7, 14 and 21 after laser treatment,
respectively. In the CRRR-treated group, the percentage of lesions
with grade 2b leakage was 74.23, 50.00 and 43.33% at the same time
points. No statistically significant differences were observed in
the leakage grade between the control and CRRR-treated groups at
days 7, 14 or 21 days after laser treatment (day 7, P=0.73; day 14,
P=0.69; day 21, P=0.79).
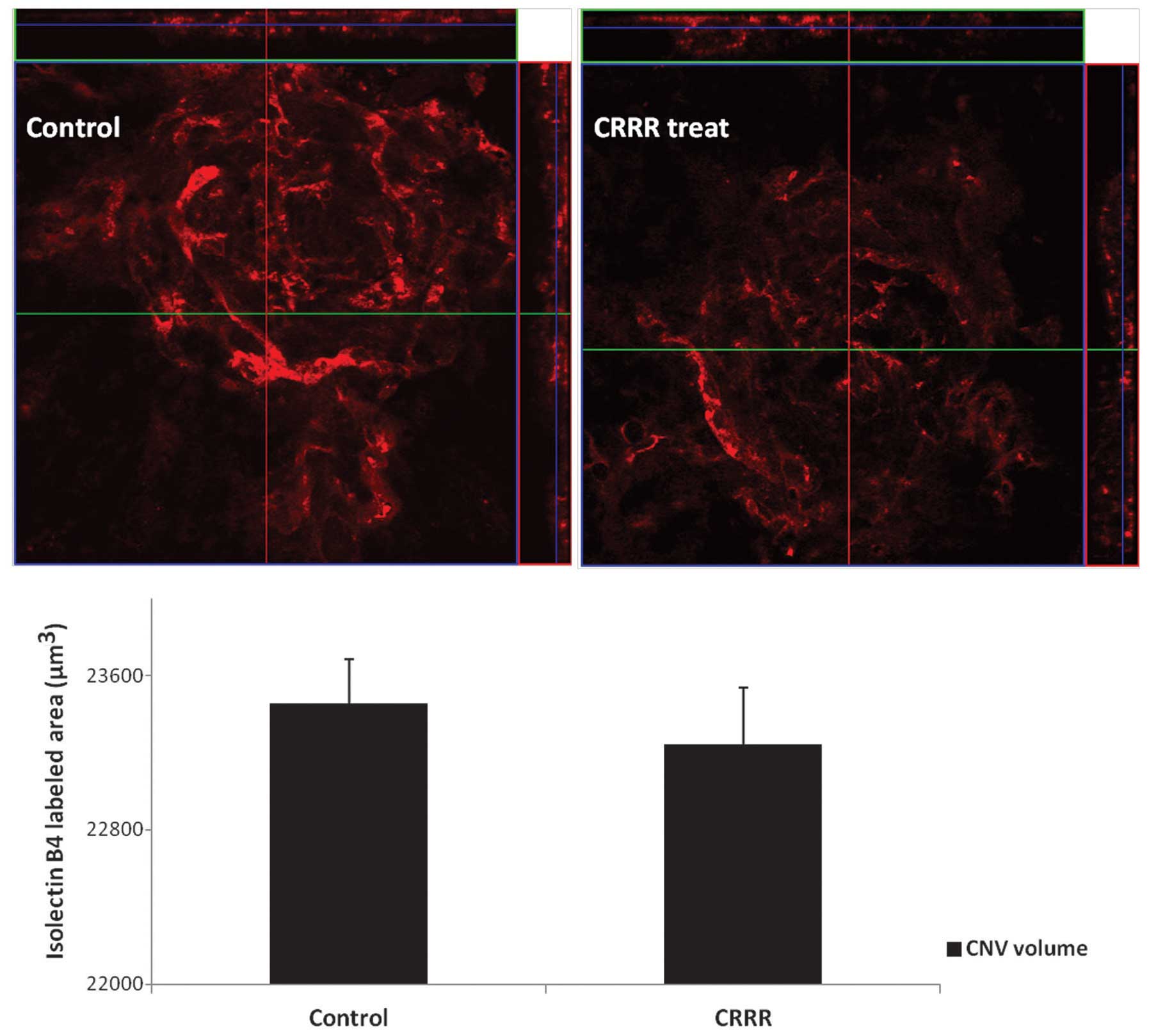
CNV volume in flat mount
The CNV volumes were calculated from the sum of
isolectin B4-labeled signals and were found to be
23457.45±227.42 μm3 in the control group and
23245.43±290.12 μm3 in the CRRR-treated group (P=0.14).
No statistically significant difference was observed between the
two groups (P>0.05; Fig.
2).
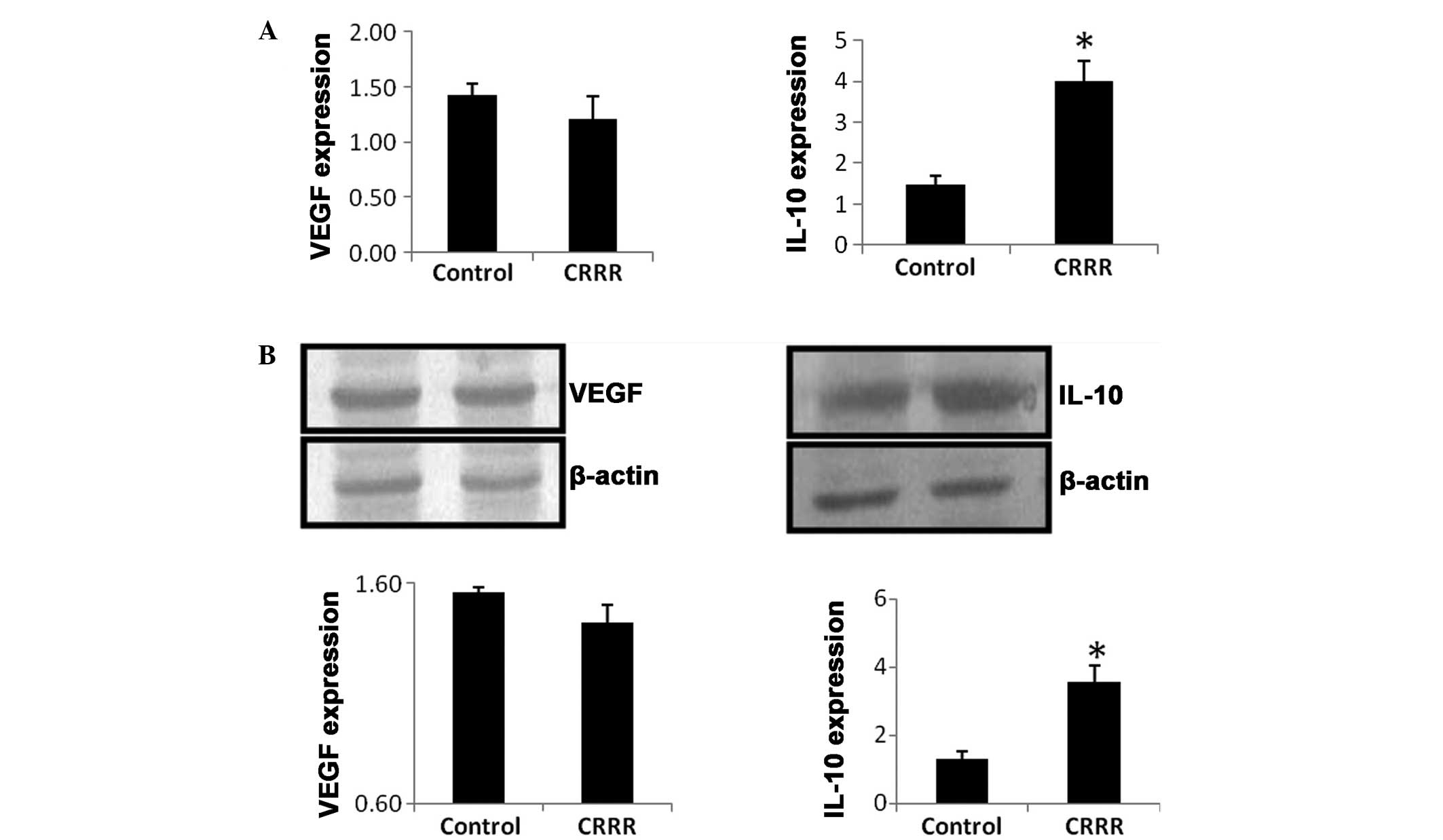
qPCR
At day 7 after laser treatment, the mRNA expression
level of IL-10 was significantly upregulated in the CRRR-treated
group, when compared with the control group (P=0.02). By contrast,
no statistically significant difference was observed in the mRNA
expression level of VEGF between the control and CRRR groups
(P=0.65; Fig. 3A).
Western blot analysis
At day 7 after laser treatment, the protein
expression level of IL-10 was significantly increased in the
CRRR-treated group, when compared with the control group (P=0.03).
However, no statistically significant difference was observed in
the VEGF protein expression level between the control and CRRR
groups (P=0.74; Fig. 3B).
Discussion
Wet AMD accounts for 90% of severe vision loss in
AMD patients (9). The wet AMD
subtype is characterized by the presence of exudate, bleeding,
edema, pigment changes and CNV in the macular fovea. The
development of CNV involves the appearance of hypoxia,
inflammation, angiogenesis, fibrotic changes and endothelial
cell-matrix interactions (10).
The formation of CNV is influenced by a variety of factors, among
which the expression of VEGF is critical; thus, VEGF-A
neutralization has become the standard treatment method for CNV
(11). Anti-inflammatory agents
have also been used to treat CNV. However, corticosteroids are
associated with severe adverse effects, such as elevated
intraocular pressure, cataract development and endophthalmitis
(12,13). Although anti-VEGF agents can
markedly improve the clinical outcome of wet AMD, they are unable
to induce complete regression of the CNV membranes (14–16).
Furthermore, anti-VEGF agents require repeated injections into the
eye at intervals of four to six weeks in order to achieve an
optimal outcome, which may increase the risk of ocular
inflammation, retinal injury and endophthalmitis (14–16).
Since VEGF plays an important role in retinal development and
neuroprotection, anti-VEGF therapy may induce retinal damage
following a long period of administration. Due to the systemic
physiological role of VEGF, previous studies have suggested that
the beneficial effects of VEGF antagonism in the eyes may result in
adverse systemic reactions (17–19).
Therefore, the safety of anti-VEGF treatment requires long-term
observation. A previous study reported that only 30–40% of
individuals experienced vision improvement following anti-VEGF
treatment (20). Thus, VEGF
inhibition or anti-inflammatory treatment alone may not be
sufficient. Combination therapies have been explored in order to
reduce the frequency of intravitreal anti-VEGF injections and the
risk of relevant side-effects due to complete VEGF inactivity and
steroid administration (21,22).
Investigations regarding other therapeutic strategies may prolong
the interval of treatment and provide alternatives to the current
CNV treatment (5,23).
A novel TCM composition devised by Jin et al
(4) (patent no. WO2012079419) is a
prospective alternative therapy for wet AMD. The pharmaceutical
composition consists of a number of TCM components, including
Astragalus membranaceus Bunge, Angelica sinensis,
Poria cocos Wolf, Fritillaria thunbergii, Panax
pseudoginseng, CRRR, pollen Typhae and Curcuma
aromatica Salisb. The compounds in the composition were shown
to be capable of targeting the hallmarks of AMD, including
oxidative stress, RPE cytotoxicity, inflammation and vascular edema
(5). The composition contains raw
materials from herbs that can eliminate inflammation (25–30),
modulate immunity (31–37), promote blood circulation in order
to remove blood stasis (38,39),
prevent bleeding (40,41) and promote the absorption of macular
exudates, edema and hemorrhage, as well as reduce the leakage and
area of CNV (42). Treatment with
the composition was found to enhance and stabilize the vision of
patients with AMD, while the CNV leakage was more significantly
reduced compared with Avastin® treatment (anti-VEGF
agent) (5). Initially, the
composition did not exhibit a favorable clinical outcome, until
Citrus reticulata (included in the original composition) was
replaced with CRRR. Therefore, the present study applied the same
concentration and dose of CRRR, as used in the clinical trial in
order to treat laser-induced CNV in a murine model. The aim of the
present study was to determine the role of CRRR in the composition.
Radix et Rhizoma Rhei (RRR) is the Latin name for Da Huang, a
Chinese herb, which is commonly known as rhubarb (Rheum
palmatum L.). The root and underground stem of rhubarb, known
as the rhizome, are often used to produce medicine, which can be
used in a raw, alcohol-processed or carbonized form (43). RRR manifests a therapeutic function
in the spleen, stomach, large intestine, liver and heart meridians.
The herb mainly comprises derivatives of anthraquinone in a
conjugated form of anthraquinone glycoside or diglycoside, as well
as tannins and their analogues (43). RRR can purge intestinal stagnation,
resolve inflammation, eliminate toxic substances, remove blood
stasis and promote blood circulation (43). This herb has been previously used
to treat conditions, such as constipation, acute intestinal
obstruction, dysentery, bleeding symptoms, eye redness, throat
soreness, abdominal abscess, jaundice, carbuncles and furuncles,
irregular menstruation, traumatic injuries, rosacea,
hyperlipidemia, renal failure, uremia, burns and scalds, and
cholelithiasis (43,44). Processed RRR has a reduced
purgative effect and is typically used for the treatment of red
eyes, sore throat, swollen gums, carbuncles and furuncles. Charred
RRR (CRRR) is used for the treatment of bleeding symptoms and the
prevention of bleeding, as well as the activation of local
circulation (37,43–45).
Therefore, CRRR can improve blood stasis, relieve swelling and
pain, resolve inflammation and promote wound healing.
The present study focused on the effect of CRRR on
CNV leakage and volume reduction. In order to uncover the
underlying mechanisms of the CRRR effect, alterations in the
expression levels of VEGF and IL-10 were determined. IL-10 is an
anti-inflammatory and immunomodulatory cytokine produced by
numerous human cell types, including monocytes, macrophages,
B-lymphocytes and T-helper 2 cells (46). However, the role of IL-10 in CNV
formation remains controversial. Apte et al (47) demonstrated that CNV was reduced in
IL-10 knockout mice, when compared with wild type mice; thus, IL-10
may inhibit the recruitment of macrophages to the CNV area and
promote CNV formation. By contrast, Hasegawa et al (48) indicated that IL-10 had
anti-angiogenic properties in the formation of CNV (48). These results suggest that the
effect of IL-10 on CNV may vary according to the expression level
of IL-10 and the inflammatory microenvironment. In the present
study, the intraocular expression level of IL-10 was found to be
increased following treatment with CRRR. However, no other
statistically significant differences were observed between the
CRRR-treated and control group. Therefore, CRRR may exert
anti-inflammatory and immunomodulatory effects in AMD patients. The
effect of CRRR on bleeding symptoms may also play a role in the
treatment of wet AMD. However, since lesions with hemorrhage were
excluded from the current study, the effect of CRRR on CNV in the
laser-induced CNV model may not mimic wet AMD efficiently. In the
previous clinical trials, CRRR was used to treat patients with wet
AMD synergistically with seven other components in the composition
(4). Therefore, application of
CRRR alone may not be sufficient to result in the regression of
CNV. The differences between laser-induced CNV in mice and human
CNV must also be considered. Furthermore, the experiments of the
present study were performed in a murine model of acute
inflammation; therefore, a chronic inflammatory model should be
established in future studies.
TCM typically uses multiple herb combinations to
treat and relieve the symptoms of various diseases. TCM therapies
are attractive to patients since TCM tends to result in fewer and
less severe side-effects compared with pure single drugs.
Therefore, the multicomponent and synergistic nature of TCM should
be considered in pharmaceutical development. Traditional herbs have
been a major source of modern single-drug developments (49). The extractions used in target
herbal medicine may result in the identification of promising novel
active natural products, whereas synthetic analogues may be
produced through verified pharmacological testing and
bioactivity-directed fractionation and isolation of active
ingredients (50). The active
ingredients of a target drug may then be used in the subsequent
drug development stage. The TCM composition, WO2012079419, consists
of eight herbs that target different pathways and fit the
mechanisms of AMD development. The pharmacological action of this
composition may be due to a particular chemical or the complex
interactions between the composition constituents. Previous studies
have demonstrated that the herbal composition was able to
simultaneously address numerous mechanisms of wet AMD (5). Modern analytical and pharmacological
methods have been used to determine the properties of the herbal
combinations in comparison with a single component. In addition,
qualitative and quantitative analyses were performed to evaluate
and guarantee the safety and efficacy of the composition (49). Modernization is important for the
standardization of TCM and the establishment of qualitative and
quantitative data on its active ingredients and bioactivity. In
addition, the pharmaceutical composition may be prepared into
various pharmaceutically acceptable forms, including decoction,
tablet, capsule, bolus, oral liquid preparation and injection
(50). Therefore, a non-invasive,
efficient and safe therapy for AMD using the discussed TCM
composition appears to be promising. In conclusion, CRRR did not
appear to significantly inhibit CNV in this murine model. The
function of CRRR in the pharmaceutical composition may be due to
the anti-inflammatory and immunomodulatory effects of IL-10, as
well as a synergistic effect with other components of the
composition. A perspective, randomized, double-blind clinical trial
should be performed in future studies to investigate the potential
application of this composition in AMD.
References
|
1
|
Klein R, Peto T, Bird A and Vannewkirk MR:
The epidemiology of age-related macular degeneration. Am J
Opthalmol. 137:486–495. 2004. View Article : Google Scholar
|
|
2
|
Jian L, Panpan Y and Wen X: Current
choroidal neovascularization treatment. Ophthalmologica. 230:55–61.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campagne MVL, LeCouter J, Yaspan BL and Ye
W: Mechanisms of age-related macular degeneration and therapeutic
opportunities. J Pathol. 232:151–164. 2014. View Article : Google Scholar
|
|
4
|
Jin M: Pharmaceutical composition for
treating macular degeneration. International patent WO/2012/079419.
Filed October 13, 2011; issued June 21, 2012.
|
|
5
|
Wang S and Cunnusamy K: Pharmaceutical
composition for treating macular degeneration (W02012079419).
Expert Opin Ther Pat. 23:269–272. 2013. View Article : Google Scholar :
|
|
6
|
Marneros AG, She H, Zambarakji H,
Hashizume H, Connolly EJ, Kim I, Gragoudas ES, Miller JW and Olsen
BR: Endogenous endostatin inhibits choroidal neovascularization.
FASEB J. 21:3809–3818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumara N, Kamei M, Tsujikawa M, et al:
Low-dose lipopolysaccharide pretreatment suppresses choroidal
neovascularization via IL-10 induction. PLoS One. 7:e398902012.
View Article : Google Scholar
|
|
8
|
Andrews NC and Faller DV: A rapid
micropreparation technique for extraction of DNA-binding proteins
from limiting numbers of mammalian cells. Nucleic Acids Res.
19:24991991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferris FL III, Fine SL and Hyman L:
Age-related macular degeneration and blindness due to neovascular
maculopathy. Arch Ophthalmol. 102:1640–1642. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veritti D, Sarao V and Lanzetta P:
Neovascular age-related macular degeneration. Ophthalmologica.
227:11–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Huang D, Xia X, Wang Z, Luo L and
Wen R: CCR3 and choroidal neovascularization. PLoS One.
6:e171062011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arias l, Garcia-Arumi J, Ramon JM, et al:
Photodynamic therapy with intravitreal triamcinolone in
predominantly classic choroidal neovascularization: one-year
results of a randomized study. Opthalmology. 113:2243–2250. 2006.
View Article : Google Scholar
|
|
13
|
Challa JK, Gillies MC, Penfold PL, et al:
Exudative macular degeneration and intravitreal tramcinolone: 18
month follow up. Aust N Z J Opthalmol. 26:277–281. 1998. View Article : Google Scholar
|
|
14
|
Regillo CD, Brown DM, Abraham P, Yue H,
Ianchulev T, Schneider S and Shams N: Randomized, double-masked,
sham-controlled trial of ranibizumab for neovascular age-related
macular degeneration: PIER Study year 1. Am J Ophthalmol.
145:239–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishijima K, Ng YS, Zhong L, Bradley J,
Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP
and Shima DT: Vascular endothelial growth factor-A is a survival
factor for retinal neurons and a critical neuroprotectant during
the adaptive response to ischemic injury. Am J Pathol. 171:53–67.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson GS, Ju M, Shih SC, Xu X, McMahon
G, Caldwell RB and Smith LE: Nonvascular role for VEGF: VEGFR-1, 2
activity is critical for neural retinal development. FASEB J.
15:1215–1217. 2001.PubMed/NCBI
|
|
17
|
Ueta T, Yanagi Y, Tamaki Y and Yamaguchi
T: Cerebrovascular accidents in ranibizumab. Ophthalmology.
116:3622009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campbell RJ, Bell CM, Paterson JM,
Bronskill SE, Moineddin R, Whitehead M and Gill SS: Stroke rates
after introduction of vascular endothelial growth factor inhibitors
for macular degeneration: a time series analysis. Ophthalmology.
119:1604–1608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horsley MB, Mandava N, Maycotte MA and
Kahook MY: Retinal nerve fiber layer thickness in patients
receiving chronic anti-vascular endothelial growth factor therapy.
Am J Ophthalmol. 150:558–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou QB, Anderson C, Zhang H, Li X, Inglis
F, Jayagopal A and Wang SS: Repression of choroidal
neovascularization through actin cytoskeleton pathways by
mircoRNA-24. Mol Ther. 22:378–389. 2014. View Article : Google Scholar :
|
|
21
|
Schaal S, Kaplan HJ and Tezel TH: Is there
tachyphylaxis to intravitreal anti-vascular endothelial growth
factor pharmacotherapy in age related macular degeneration?
Ophthalmology. 115:2199–2205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dhalla MS, Shah GK, Blinder KJ, et al:
Combined photodynamic therapy with verteporfin and intravitreal
bevacizumab for choroidal neovascularization in age-related macular
degeneration. Retina. 26:988–993. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ebrahim S, Peyman GA and Lee PJ:
Applications of liposomes in ophthalmology. Surv Ophthalmol.
50:167–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko JK and Chik CW: The protective action
of radix Astragalus membranaceus against hapten-induced colitis
through modulation of cytokines. Cytokine. 47:85–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han C and Guo J: Antibacterial and
anti-inflammatory activity of traditional Chinese herb pairs,
Angelica sinensis and Sophora flavescens. Inflammation. 35:913–919.
2012. View Article : Google Scholar
|
|
26
|
Chang SH, Choi Y, Park JA, et al:
Anti-inflammatory effects of BT-201, an n-butanol extract of Panax
notoginseng, observed in vitro and in a collagen-induced arthritis
model. Clin Nutr. 26:785–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuchs SM, Heinemann C, Schliemann-Willers
S, et al: Assessment of anti-inflammatory activity of Poria cocos
in sodium lauryl sulphate-induced irritant contact dermatitis. Skin
Res Technol. 12:223–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho IH, Lee MJ, Kim JH, Han NY, Shin KW,
Sohn Y and Jung HS: Fritillaria ussuriensis extract inhibits the
production of inflammatory cytokine and MAPKs in mast cells. Biosci
Biotechnol Biochem. 75:1440–1445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mandal MN, Patlolla JM, Zheng L, et al:
Curcumin protects retinal cells from light-and oxidant
stress-induced cell death. Free Radic Biol Med. 46:672–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HS, Lee MJ, Kim H, et al: Curcumin
inhibits TNFalpha-induced lectin-like oxidised LDL receptor-1
(LOX-1) expression and suppresses the inflammatory response in
human umbilical vein endothelial cells (HUVECs) by an antioxidant
mechanism. J Enzyme Inhib Med Chem. 25:720–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang WJ, Li DP, Li JK, et al: Synergistic
antioxidant activities of eight traditional Chinese herb pairs.
Biol Pharm Bull. 32:1021–1026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang X, Zhao Y, Zhou Y, et al: Component
and antioxidant properties of polysaccharide fractions isolated
from Angelica sinensis (OLIV.) DIELS. Biological Pharmaceutical
Bulletin. 30:1884–1890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jang YJ, Kim ME and Ko SY: n-Butanol
extracts of Panax notoginseng suppress LPS-induced MMP-2 expression
in periodontal ligament fibroblasts and inhibit osteoclastogenesis
by suppressing MAPK in LPS-activated RAW264.7 cells. Arch Oral
Biol. 56:1319–1327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi RC, Jiang Z, Xie HQ, et al:
Anti-oxidative effects of the biennial flower of Panax notoginseng
against H2O2-induced cytotoxicity in cultured
PC12 cells. Chin Med. 5:382010. View Article : Google Scholar
|
|
35
|
Kanayama H, Adachi N and Togami M: A new
antitumor polysaccharide from the mycelia of Poria cocos Wolf. Chem
Pharm Bull. 31:1115–1118. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo JS, Jung EY, Kim JH, et al: A modified
preparation (LMK03) of the oriental medicine Jangwonhwan reduces
Abeta (1–42) level in the brain of Tg-APPswe/PS1dE9 mouse model of
Alzheimer disease. J Ethnopharmacol. 130:578–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Xia X, Zhang S, et al: Up-regulation
of Toll-like receptor 4 was suppressed by emodin and baicalin in
the setting of acute pancreatitis. Biomed Pharmacother. 63:120–128.
2009. View Article : Google Scholar
|
|
38
|
Zhang L, Yang Y, Wang Y and Gao X:
Astragalus membranaceus extract promotes neovascularisation by VEGF
pathway in rat model of ischemic injury. Pharmazie. 66:144–150.
2011.PubMed/NCBI
|
|
39
|
Lam HW, Lin HC, Lao SC, et al: The
angiogenic effects of Angelica sinensis extract on HUVEC in vitro
and zebrafish in vivo. J Cell Biochem. 103:195–211. 2008.
View Article : Google Scholar
|
|
40
|
Yeh JC, Cindrova-Davies T, Belleri M, et
al: The natural compound n-butylidenephthalide derived from the
volatile oil of Radix Angelica sinensis inhibits angiogenesis in
vitro and in vivo. Angiogenesis. 14:187–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohkura N, Tauchi C, Nakayama A and Atsumi
G: Pollen Typhae is a rapid hemostyptic. Blood Coagul Fibrinolysis.
23:254–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ding JD, Johnson LV, Herrmann R, et al:
Anti-amyloid therapy protects against retinal pigmented epithelium
damage and vision loss in a model of age-related macular
degeneration. Proc Natl Acad Sci USA. 108:E279–E287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lei ZQ: Chinese herbs/Da Huang. Chinese
Medicine. Chen SY and Gao XY: 1st edition. Shanghai Science and
Technology Publisher; Shanghai: pp. 176–182. 1998, (In
Chinese).
|
|
44
|
Gan T, Liu YD, Wang Y and Yang J:
Traditional Chinese Medicine herbs for stopping bleeding from
haemorrhoids. Cochrane Database Syst Rev.
10:CD0067912010.PubMed/NCBI
|
|
45
|
Kumar A, Dhawan S and Aggarwal BB: Emodin
(3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-κB
activation, IκB degradation and expression of cell surface adhesion
proteins in human vascular endothelial cells. Oncogene. 17:913–918.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saraiva M and O’Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Apte RS, Richter J, Herndon J and Ferguson
TA: Macrophages inhibit neovascularization in a murine model of
age-related macular degeneration. PLoS Med. 3:e3102006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hasegawa E, Oshima Y, Takeda A, Saeki K,
Yoshida H, et al: IL-27 inhibits pathophysiological intraocular
neovascularization due to laser burn. J Leukoc Biol. 91:267–273.
2012. View Article : Google Scholar
|
|
49
|
Tsang KW, Lam CL, Yan C, et al: Coriolus
Versicolor Polysaccharide Peptide slows progression of advanced
non-small cell lung cancer. Respir Med. 97:618–624. 1997.
View Article : Google Scholar
|
|
50
|
Lee KH: Research and future trends in the
pharmaceutical development of medicinal herbs from Chinese
medicine. Public Health Nutr. 3:515–522. 2000. View Article : Google Scholar
|