Introduction
Ischemic reperfusion (IR) triggers the disturbance
of microcirculation, a primary cause of cardiac failure and
mortality following cardiac surgeries (1,2).
Microcirculatory disturbances include endothelial cell injury,
production of oxygen free radicals, energy supply reduction and
myocardiocyte apoptosis (3).
Tanshinone IIA is an abundant lipophilic abietane
diterpene compound which was isolated from Danshen (Salvia
miltiorrhiza), a Traditional Chinese Medicine used for treating
cardiovascular diseases (4,5). In
addition, tanshinone IIA was reported to balance the superoxide
dismutase content and the malondialdehyde concentration in injured
myocytes through scavenging free acids and reducing lipid
peroxidation, which therefore protected myocytes and vascular
endothelial cells during the IR process (6,7).
Furthermore, tanshinone IIA was found to markedly inhibit
H2O2-induced oxidation in vitro,
significantly inhibit IR-induced cardiomyocyte apoptosis via
attenuation of morphological changes as well as reduce the
percentage of terminal transferase 2′-deoxyuridine 5′-triphosphate
(dUTP) nick end-labeling (TUNEL)-positive myocytes (8). However, it remains to be elucidated
whether other signaling pathways are involved in the protective
effects of tanshinone IIA on IR injury.
The Janus kinase/signal transducer and activator of
transcription (JAK/STAT) signaling pathways regulate gene products
involved in various cellular processes, including survival
(9), apoptosis (10) and cell cycle progression (11); in addition, these pathways have
been reported to have a vital role in inducing cardioprotection
against IR injuries (10,12–15).
Tanshinone IIA was found to inhibit JAK/STAT signaling and thereby
induce apoptosis in cancer cells (16). The aim of the present study was to
evaluate the effect of tanshinone IIA in combination with
JAK2/STAT3 inhibitors on myocardial cells following the induction
of hypoxic/ischemic injury in vitro and in vivo, as
well as to elucidate the role of the JAK2/STAT3 signaling pathway
in mediating the protective effects of tanshinone IIA.
Materials and methods
Cell culture and reagents
The H9c2 embryonic rat heart-derived cells were
purchased from the Chinese Academy of Sciences (Shanghai, China)
cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% v/v fetal bovine serum (FBS) and 100 mg/ml
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. Cells were maintained in serum-free
culture and 100 nM deferoxamine to induce ischemia and hypoxic
conditions. DMEM, FBS, antibiotics and deferoxamine were purchased
from Invitrogen Life Technologies, Carlsbad, CA, USA.
Animals
Male Sprague-Dawley rats (age, six weeks; weight,
200–220 g) were purchased from the Chinese Academy of Sciences
(Shanghai, China). Rats were housed in a specific pathogen-free
laboratory and exposed to a 12-h light/dark cycle at 37°C with free
access to food and water. The rats were randomly divided into four
groups (10 rats per group). Rats in control group were treated with
phosphate-buffered saline, and rats in the experimental groups were
administrated intraperitoneally with Tanshinone IIA, or Tanshinone
IIA in combination with NSC74859 or AG490. Myocardial ischemia was
induced by exposing the heart through a left thoracic incision and
placing a 6-0 silk suture around the left coronary artery.
Following 30 min of ischemia, the slipknot was released to allow
reperfusion. At the end of the experiment, the rats were sacrificed
using anesthetic (sodium pentobarbital, 30 mg/kg body weight;
Akorn, Lake Forest, IL, USA).
Cytotoxicity assay
Cytotoxic effects of tanshinone IIA or JAK/STAT
inhibitors (Sigma-Aldrich, St. Louis, MO, USA) on H9c2 cells were
evaluated using a Cell Counting Kit 8 (CCK-8; Dojindo, Kumamoto,
Japan). Cells were seeded onto 96-well microplates at a density of
2×104 cells per well, preconditioned with
hypoxia/ischemia (hypoxia, 5% O2) for 24 or 48 h and
exposed to various concentrations of tanshinone IIA (0, 2.5, 10 and
40 μM) or JAK/STAT inhibitors AG490 and NSC74859 (50 and 100 μM,
respectively) for 12 h. Cells were then incubated with CCK-8
solution at 37°C for 3 h. Optical density (OD) was measured at 450
nm using a MRX II microplate reader (Dynex Technologies, Inc.,
Chantilly, VA, USA). Cell viability was calculated as the
percentage of viable cells in the drug-treated group versus that of
the untreated control.
Flow cytometric analysis
Following treatment as above, cells
(>1×106) were digested with 0.2% trypsin (BD
Biosciences, Franklin Lakes, NJ, USA) and collected by
centrifugation. Following washing twice with ice-cold
phosphate-buffered saline, 10 μl Annexin V-fluorescein
isothiocyanate and 5 μl propidium iodide (BD Biosciences) were
added to a 85-μl cell suspension and incubated for 15 min in the
dark. Stained cells were then analyzed by flow cytometry using a
FACS Calibur system with Cell Quest software version 5.1 (BD
Biosciences).
Western blot analysis
Following treatment as above, cells were homogenized
in lysis buffer containing 50 mmol/l Tris-HCl (pH 7.3), 150 mmol/l
NaCl, 5 mmol/l EDTA, 1 mmol/l dithiothreitol, 1% Triton X-100 and
1% protease inhibitor cocktail (Sigma-Aldrich). The lysates were
centrifuged for 15 min at 12,000 × g and the resulting supernatant
was transferred to a new tube and stored at −70°C. Protein
concentrations were determined using a Bradford protein assay kit
(Sigma-Aldrich) and proteins were separated using electrophoresis
using 12% SDS-PAGE and transferred to nitrocellulose membranes
(Sigma-Aldrich). The membranes were blocked for 1 h in
Tris-buffered saline and Tween 20 (TBST; pH 7.6; Sigma-Aldrich)
with 5% non-fat dry milk and then incubated overnight at 4°C with
anti-mouse monoclonal antibodies against phospho-JAK2,
phospho-STAT3, JAK2, STAT3 (dilution, 1:500) or β-actin (dilution,
1:1,000) purchased from Cell Signaling Technologies, Inc. (Beverly,
MA, USA) followed by washes with TBST. The membranes were then
probed with appropriate secondary antibodies (dilution, 1:5,000) at
room temperature for 90 min, followed by washes with TBST. Protein
bands were detected using a chemiluminescence kit (Sigma-Aldrich)
and were quantified using the Quantity One software package
(version 4.6.2; Bio-Rad Laboratories, UK). The result of the
control group was defined as 100%.
In situ quantification of apoptotic
cardiomyocytes
A portion of the myocardium from the mid-left
ventricle of the middle slices was fixed in 4% formalin
(Sigma-Aldrich). The apoptotic rate of the myocardial cells was
analyzed using terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) staining using an in situ cell death
detection kit (Roche, Basel, Switzerland). A double-staining
technique was used; TUNEL staining was used to quantify apoptotic
cell nuclei and DAPI staining (Sigma-Aldrich) was used to quantify
the total myocardial cell nuclei. TUNEL-positive cells with green
nuclear staining and all cells with blue nuclear DAPI staining were
counted within five randomly selected fields under high-power
magnification (DM-2500; Leica Microsystems, Wetzlar, Germany). The
index of apoptosis was expressed as the ratio of positively stained
apoptotic myocytes to the total number of myocytes counted
×100%.
Histomorphological analysis
Following experimental preconditioning and
measurement of hemodynamics, the hearts of mice were quickly
removed and dissected into transverse slices from apex to base and
processed for histology. Subsequently, the hearts were fixed in a
10% formalin solution (Sigma-Aldrich) for 24 h, embedded in
paraffin wax (Sigma-Aldrich). After deparrafinization with xylene,
the wax was serially sectioned at 7 μm using routine protocols
(17). For the histomorphological
analysis, the sections were stained with hematoxylin-eosin (HE;
Sigma-Aldrich) and examined using a light microscope (BX40;
Olympus, Tokyo, Japan).
Statistical analysis
Values are presented as the mean ± standard error of
the mean. Group comparisons were performed using analysis of
variance with SPSS 13.0 software (SPSS, Inc. Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Tanshinone IIA protects H9c2 rat myoblast
cells from hypoxic ischemia-induced injury and reduced
viability
In order to determine the effect of tanshinone IIA
on H9c2 cells, the H9c2 rat myoblast cell line was preconditioned
with hypoxia/ischemia (24 and 48 h) and treated with various
concentrations (0, 2.5, 10 or 40 μM) of tanshinone IIA for 12 h. A
CCK8 assay was then performed (Fig. 1A
and B). Hypoxic/ischemic injury significantly reduced the
viability of H9c2 cells; however, following treatment with
tanshinone IIA, cell viability was markedly increased in a
dose-dependent manner compared with that of the untreated group,
with the most potent protective effects observed with 10 μM
tanshinone IIA. Furthermore, the protective effects of tanshinone
IIA were more prominent following 48 h hypoxic/ischemia
preconditioning compared to those in the group preconditioned for
24 h.
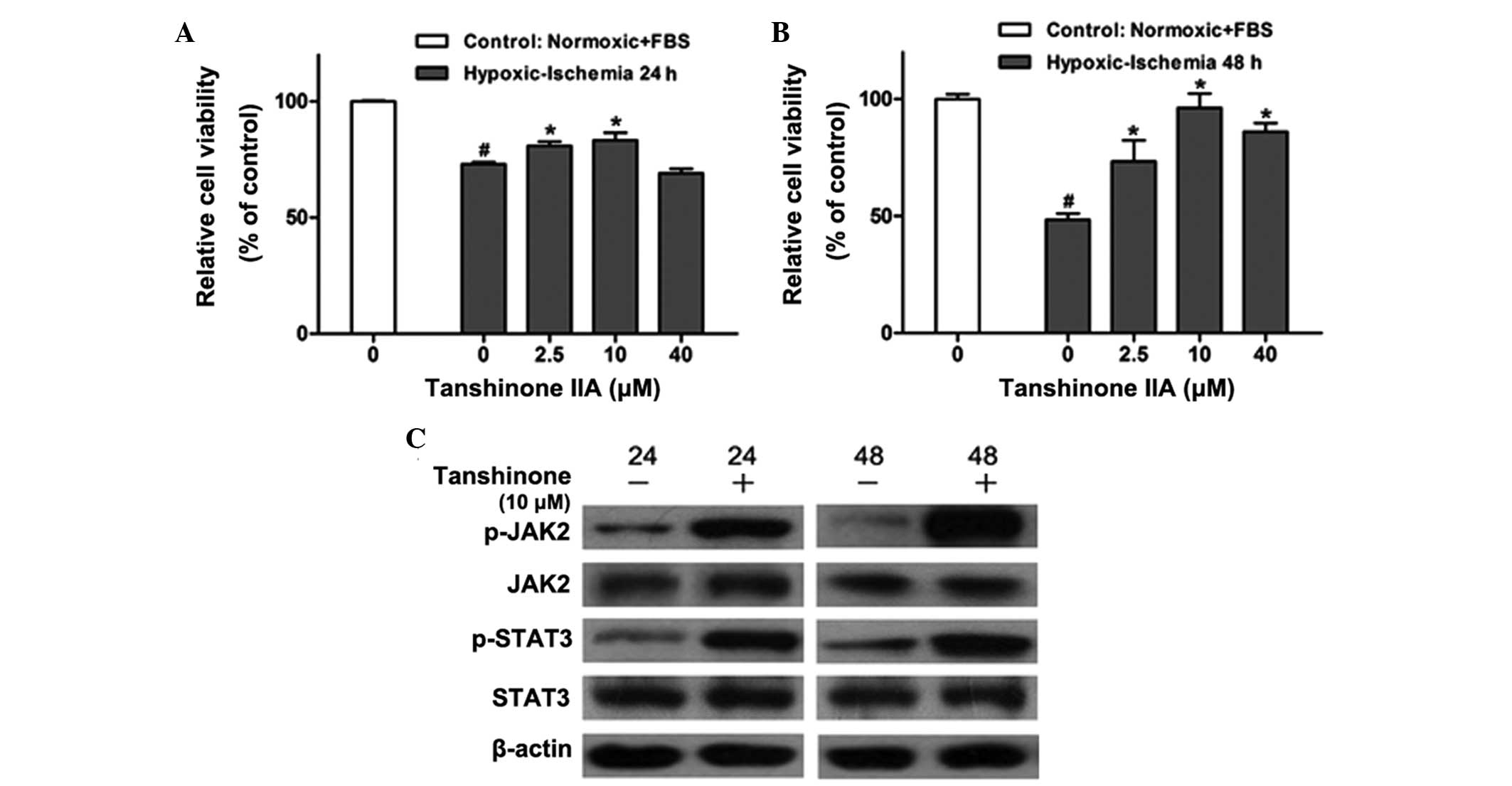 | Figure 1Effects of tanshinone IIA on cell
viability and activation of the JAK/STAT pathway in the H9c2 rat
myoblast cell line following hypoxic-ischemia injury. CCK-8 assays
were used to determine the dose-dependent effects of tanshinone IIA
(0, 2.5, 10 and 40 μM) on the viability of the H9c2 cell line with
or without hypoxic-ischemia pre-treatment for (A) 24 h and (B) 48 h
as determined by the CCK-8 assay. #P<0.05 vs.
control, *P<0.05 vs. 0 μM tanshinone IIA. (C) Western
blot analysis of JAK2, p-JAK2, STAT3 and p-STAT3 proteins in
control group and H9c2 cells treated with 10 μM tanshinone IIA
following hypoxic-ischemia pre-treatment for 24 and 48 h. Each
experiment was performed in triplicate. Values are expressed as the
mean ± standard deviation. β-actin was used as an internal control.
CCK-8, cell counting kit 8; FBS, fetal bovine serum; JAK2, Janus
kinase 2; STAT3, signal transducer and activator of transcription
3; p-, phosphorylated. |
Tanshinone IIA activates the JAK2/STAT3
pathway in H9c2 cells
The JAK-STAT pathway is activated by stress
(9,18), including hypoxic/ischemic injury,
which was first confirmed in a rodent model study (19). In order to verify whether the
protective mechanisms of tanshinone IIA proceeded via the
JAK2/STAT3 signaling pathway, the effects of tanshinone IIA on JAK2
and STAT3 activation were examined using western blot analysis. As
shown in Fig. 1C, 10 μM tanshinone
IIA significantly upregulated the phosphorylation of JAK2 and STAT3
following 24 and 48 h of hypoxia/ischemia treatment, which was
consistent with the results of the CCK-8 assay above.
JAK2 inhibitors promote the myocardial
protective effects of tanshinone IIA from hypoxic-ischemic
injury
In order to further determine whether the JAK2/STAT3
signaling pathway was responsible for the protective effect of
tanshinone IIA, the JAK2 inhibitor AG490 (20) and the STAT3 inhibitor NSC74859
(17) were used to treat H9c2
cells alone (50 and 100 μM, respectively) or in combination with 10
μM tanshinone IIA. As shown in Fig.
2, the viability of H9c2 cells was significantly reduced to
73±1.2% in the 24-h hypoxic ischemia-preconditioned group (Fig. 2A and B) and 44±2.3% in the 48-h
hypoxic ischemia-preconditioned (Fig.
2C and D) compared to that of the normoxic and FBS groups
(P<0.05). Tanshinone IIA reversed the injury and slightly
restored cell viability to 80±1.1% and 49±1.4% in the groups
pre-conditioned with 24 and 48 h hypoxic ischemia, respectively,
which was significantly increased compared to that of the control
group (P<0.05). Conversely, AG490 and NSC74859 reduced cell
viability by 8±1.4% (Fig. 2A and
C) and 13±2.7% (Fig. 2B and D)
in the groups preconditioned with 24 and 48 h hypoxic ischemia,
respectively, compared to that of the control group (P<0.05). Of
note, co-administration of the JAK inhibitor AG490 with tanshinone
IIA restored cell viability by 5.2±1.9% (Fig. 2A and C) in comparison with the
tanshinone IIA only treatment group (P<0.05); however, no
significant effects were observed following co-administration of
tanshinone IIA with the STAT3 inhibitor NSC74859 (Fig. 2B and D). In addition, tanshinone
IIA treatment significantly increased the phosphorylation level of
JAK2 and STAT3. The level of p-JAK2 was inhibited following
treatment with NSC74859 alone or NSC74859 combined with tanshinone
IIA (Fig. 2E). As shown in
Fig. 2F, AG490 suppressed the
activation of JAK2 and STAT3; however, co-administration of
tanshinone IIA and AG490 restored the phosphorylation level of JAK2
and STAT3. This therefore demonstrated that contrary to the
expected result, the co-application of AG490 and tanshinone IIA did
not eliminate the protective effect of tanshinone IIA, but further
restored myocardial cell viability following the induction of
hypoxic ischemia; furthermore, these results indicated that AG490
may affect the protective function of tanshinone IIA via inhibition
of the JAK/STAT pathway.
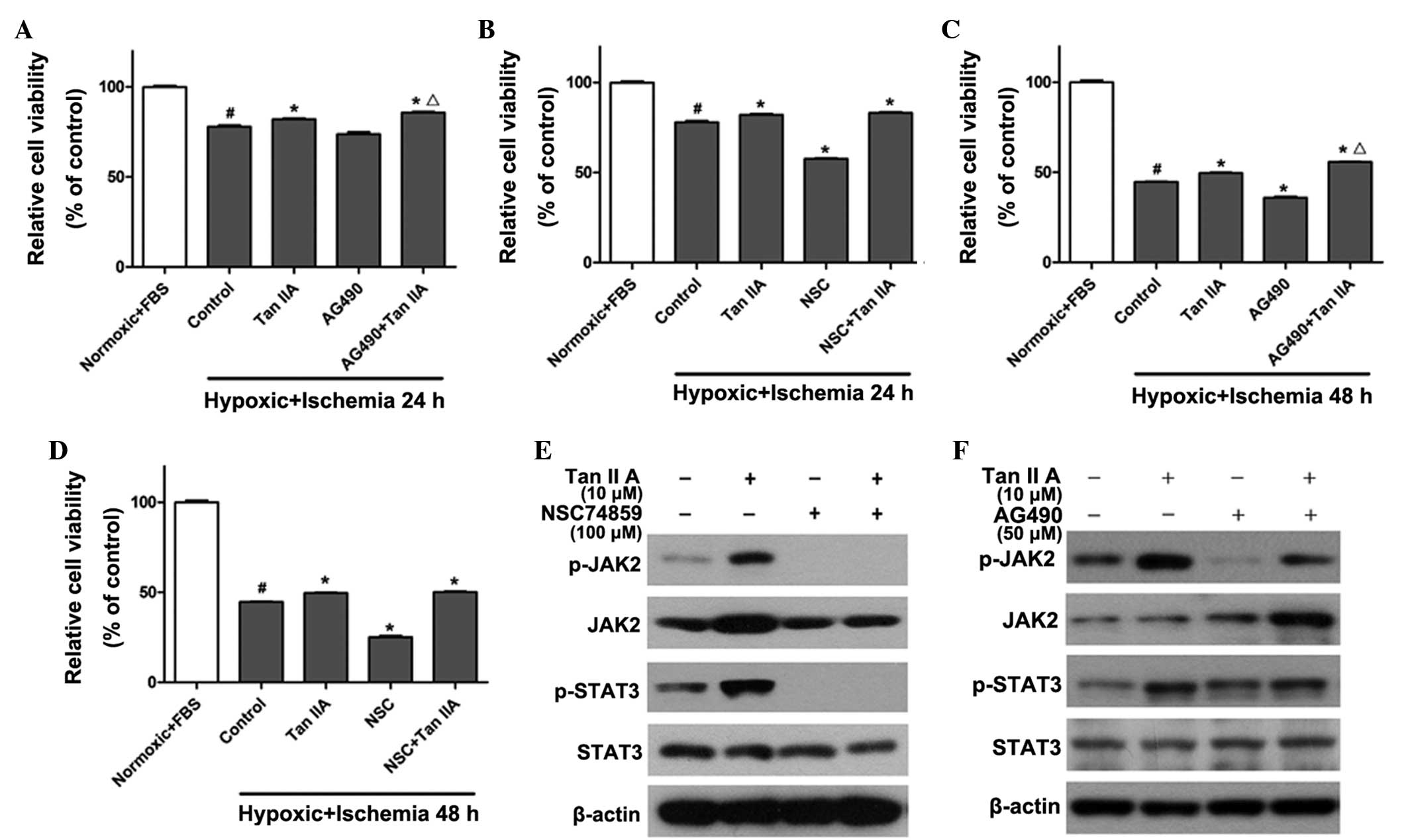 | Figure 2JAK2/STAT3 inhibitors reduce viability
of H9c2 cells and promote the myocardial protective effects of
tanshinone IIA against hypoxic ischemia. CCK-8 assays were used to
determine cell viability in H9c2 cells treated with 10 μM
tanshinone IIA alone or in combination with (A and C) JAK2
inhibitor AG490 or (B and D) STAT3 inhibitor NSC74859 following
hypoxic-ischemia treatment for 24 or 48 h, respectively.
#P<0.05 vs. normoxic + FBS group,
*P<0.05 vs. control group, ΔP<0.05 vs.
Tan IIA group. (E and F) Western blot analysis of the protein
expression levels of JAK2, p-JAK2, STAT3 and p-STAT3 following
treatment with 10 μM tanshinone IIA alone or in combination with
(E) 100 μM NSC74859 or (F) 50 μM AG490 following hypoxic-ischemia
treatment for 48 h. Each experiment was performed in triplicate.
Values are expressed as the mean ± standard deviation. β-actin was
used as an internal control. FBS, fetal bovine serum; Tan IIA,
tanshinone IIA treatment; NSC, NSC74859 treatment; JAK2, Janus
kinase 2; STAT3, signal transducer and activator of transcription
3; p-, phosphorylated; CCK-8, cell counting kit 8. |
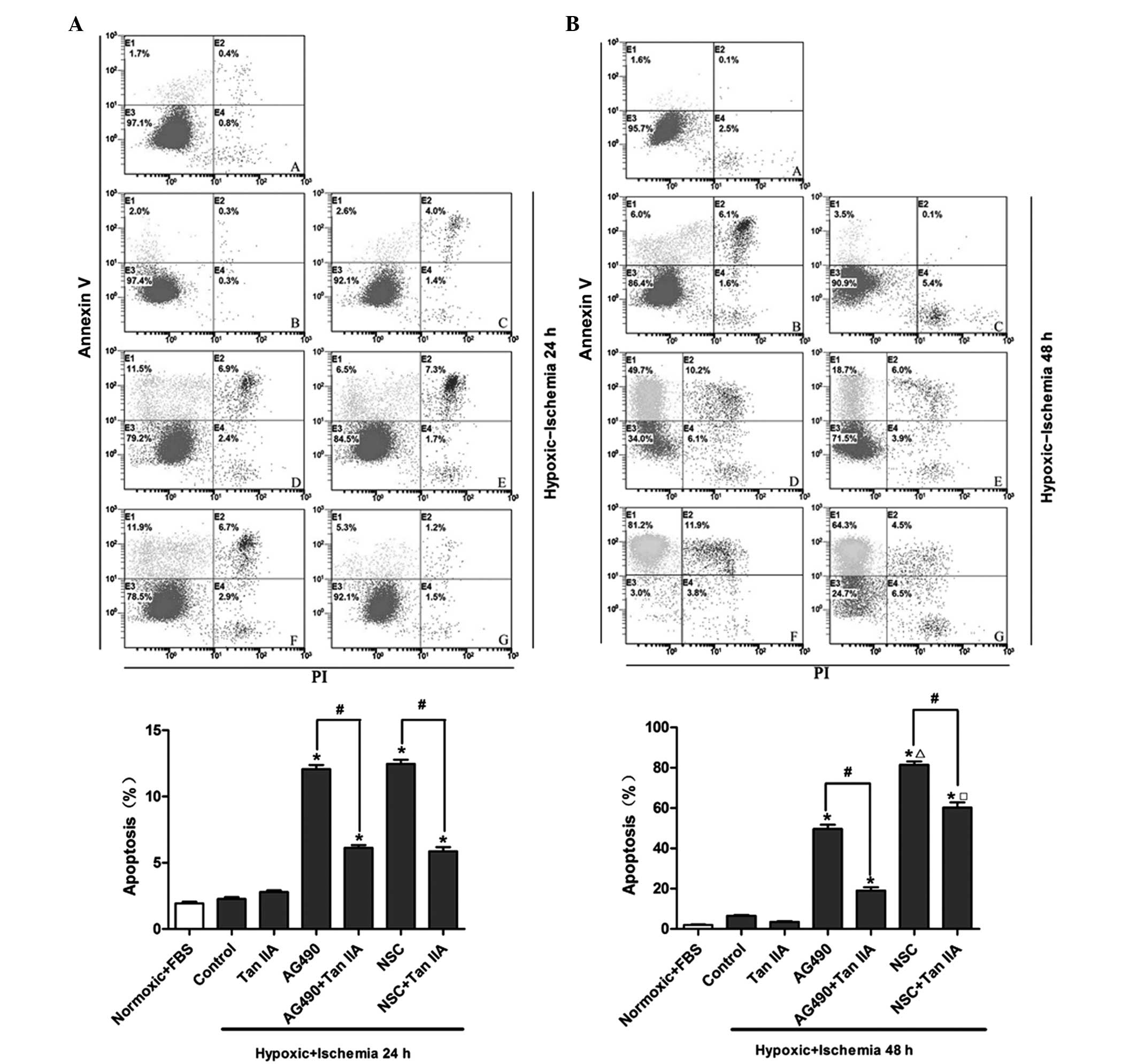
Tanshinone IIA reverses the augmentation
effect of JAK2/STAT3 inhibitors on apoptosis in H92C cells
Flow cytometric analysis was performed in order to
investigate the effects of tanshinone IIA and JAK2/STAT3 inhibitors
on hypoxic ischemia-induced cardiomyocyte apoptosis following 24
(Fig. 3A) and 48 h (Fig. 3B) of hypoxic ischemia
preconditioning. Following treatment with the JAK inhibitor AG490,
the percentage of hypoxic ischemia-induced apoptotic cells
significantly increased from 2.0% to 11.5% and from 6.0 to 49.7%
following 24 and 48 h of hypoxic ischemia preconditioning,
respectively (P<0.05). Tanshinone IIA significantly reduced the
exacerbation of hypoxic ischemia-induced apoptosis mediated by
AG490 from 11.5 to 6.5% and from 49.7 to 18.7% following 24 and 48
h of hypoxic ischemia preconditioning, respectively (P<0.05).
Similarly, the STAT3 inhibitor NSC74859 significantly increased the
percentage of hypoxic ischemia-induced apoptotic cells from 2.0% to
11.9% and from 6.0 to 81.2% following 24 and 48 h of hypoxic
ischemia preconditioning, respectively (P<0.05); however,
co-administration of tanshinone IIA with NSC74859 markedly reduced
the exacerbation of hypoxic ischemia-induced apoptosis from 11.5 to
5.3% and 81.2 to 64.3% following 24 and 48 h of hypoxic ischemia
preconditioning, respectively (P<0.05).
Effect of tanshinone IIA on
apoptosis
In order to verify the combined effects of
tanshinone IIA and JAK/STAT inhibitors in vivo, tanshinone
IIA and JAK/STAT inhibitors were administrated to normal mice
following hypoxic/ischemia treatment. TUNEL-staining was used to
identify apoptotic cells in the myocardium of control and treated
mice (Fig. 4A). The number of
TUNEL-positive cells observed was reduced in mice treated with
tanshinone IIA; this effect was markedly enhanced in mice treated
tanshinone IIA and AG490 combined (P<0.05). In addition,
co-treatment with NSC74859 slightly decreased the percentage of
TUNEL-positive cells compared with that in the tanshinone IIA
group.
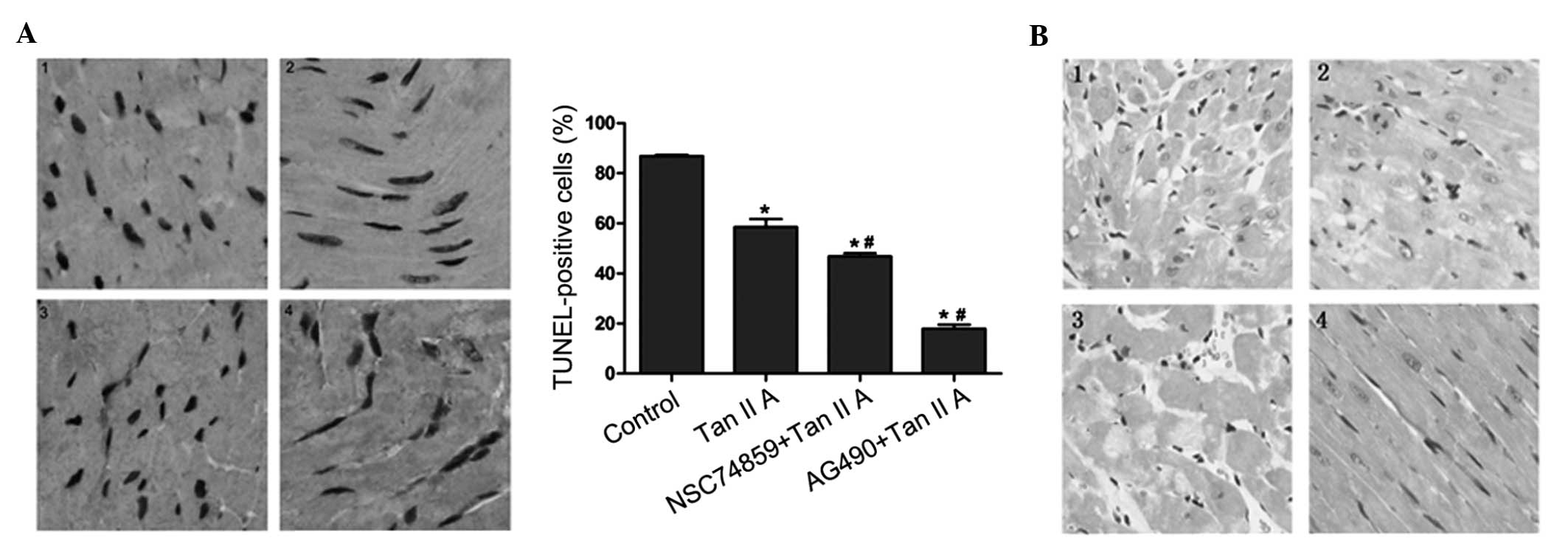 | Figure 4Representative slides for TUNEL and HE
analyses of myocardial tissue of mice following hypoxic-ischemia
preconditioning and treatment with 10 μM Tan IIA alone or in
combination with JAK2/STAT3 inhibitors. (A) Left: TUNEL staining in
cardiomyocytes, nuclei with brown staining indicate TUNEL-positive
cells (magnification, ×200). Right: Quantitative results of TUNEL
staining for each group. *P<0.05 vs. control group,
#P<0.05 vs. Tan IIA group. Values are expressed as
the mean ± standard deviation. (B) HE-stained myocardial samples
(magnification, ×200). Each experiment was performed in triplicate.
1, control group; 2, Tan IIA treatment group; 3, Tan IIA and JAK2
inhibitor AG490 treatment group; 4, Tan IIA and STAT3 inhibitor
NSC74859 treatment group. TUNEL, terminal deoxynucleotidyl
transferase 2′-deoxyuridine 5′-triphosphate nick end labeling; HE,
hematoxylin-eosin; Tan IIA, tanshinone IIA; JAK2, Janus kinase 2;
STAT3, signal transducer and activator of transcription 3. |
JAK2 inhibitor enhances the
ishemia/reperfusion injury alleviating effect of tanshinone IIA in
vivo
In order to demonstrate the pathological effects of
tanshinone IIA and JAK/STAT inhibitors on ischemia/reperfusion
in vivo, mice underwent hypoxic-ischemia preconditioning and
treatment with the respective drugs, then histological sections of
the myocardium were obtained and stained with HE (Fig. 4B). Staining revealed that
ischemia/reperfusion injury was markedly alleviated in the
tanshinone IIA group compared with that in the control group; in
addition, these beneficial effects were observed to be enhanced by
co-treatment with AG490. However, no difference was observed
between the ischemia/reperfusion injury in the tanshinone IIA group
and the group threated with tanshinone IIA and NSC74859
combined.
Discussion
According to Traditional Chinese Medicine (TCM),
tanshinone IIA, a derivative of phenanthrenequinone, enhances blood
circulation and exhibits protective effects on the heart (4,21–23).
Hypoxia/ischemia induces the disturbance of microcirculation, which
leads to numerous adverse effects, including endothelial cell
injury, energy supply reduction and myocardiocyte apoptosis
(3). Previous studies have
reported that tanshinone IIA markedly inhibited
H2O2-induced cardiomyocyte apoptosis
(24) as well as serum withdrawal-
or ethanol- induced apoptosis in cultured PC12 cells (25). The results of the present study
demonstrated that tanshinone IIA protected myocardial cells from
hypoxia/ischemia-induced injury, as shown by CCK-8 assays and flow
cytometric analysis; in addition, the effects of tanshinone IIA
treatment were more marked following prolonged hypoxia/ischemia
pretreatment from 24 to 48 h.
The JAK-STAT pathway has previously been confirmed
to be involved in cardiac apoptosis (26); as was first reported by a study on
rat models of myocardial infarction, activation of the JAK1/STAT3
pathway limits apoptosis (27).
The results of the present study suggested that tanshinone IIA
treatment increased phosphorylation of JAK/STAT3, which may be the
mechanism by which tanshinone IIA exerts its cardiomyocyte
protective and antiapoptotic effects. Previous studies have
demonstrated that activated STAT3 has an essential role in ischemic
injury (3,4); in addition, in these studies, the
nonspecific JAK2 inhibitor AG490 and the specific STAT3 inhibitor
NSC 74859 were administered in order to attenuate JAK/STAT3
activation prior to ischemic insult. AG-490 was reported to
suppress the phosphorylation of STAT3, which resulted in increased
caspase-3 activity and B cell lymphoma-associated X protein
expression, concomitant with an increase in TUNEL-positive
myocytes; this therefore indicated that JAK/STAT signaling may have
antiapoptotic effects (28).
Similarly, NSC74859 was reported to partially inhibit STAT3
dimerization and decrease target gene activation to reduce cell
survival (29). In concurrence
with these previous studies, the results of the present study
demonstrated that AG490 and NSC74859 enhanced apoptosis and reduced
cell viability following hypoxic/ischemic injury; in addition,
tanshinone IIA reversed the exacerbation of
hypoxia/ischemia-induced apoptosis mediated by JAK/STAT3
inhibitors.
Contrary to the expected results, co-administration
of tanshinone IIA and JAK/STAT3 inhibitors augmented the myocardial
protective function of tanshinone IIA against hypoxic/ischemic
injury in H9c2 cells in vitro as well as in mouse models
in vivo. In addition, AG490 exhibited a more potent
synergistic effect with tanshinone IIA than NSC74859. These results
therefore suggested that the JAK/STAT3 signaling pathway had an
important biphasic effect on the cardiac protection by tanshinone
IIA from ischemia/reperfusion injury. One possible explanation for
this is that JAK2 may activate STAT1 as well as STAT3; it has been
previously reported that ischemia/reperfusion injury activated
STAT1, which exerted a proapoptotic effect, whereas STAT3 had an
antiapoptotic effect and antagonized the effects of STAT1 (30). This putative proapoptotic role of
STAT1 in cardiomyocytes would be consistent with that observed in
non-cardiac cells (31). In
addition, the effects of tanshinone IIA may involve the STAT1 and
STAT3 pathways, and the crosstalk between them determines the
pharmacodynamic effects. It was previously reported that tanshinone
IIA inhibited JAK2/STAT5 signaling, which induced apoptosis in
cancer cells (16); in addition,
AG920 was found to stimulate STAT5 signaling, which protected cells
from apoptosis, therefore forming a negative feedback loop. Another
possible explanation is that AG92, as a nonspecific inhibitor of
JAK, initiated other pathways which were not involved in JAK2/STAT3
signaling. The exact mechanism underlying the effect of tanshinone
IIA on ischemia/reperfusion remains elusive and further studies are
required in order to elucidate the precise mechanisms by which
JAK2/STAT3 inhibitors exert their synergistic action with
tanshinone IIA. However, co-administration of JAK2/STAT3 pathway
inhibitors and tanshinone IIA may be a potential novel treatment
option for myocardial ischemia reperfusion in future clinical
therapy.
In conclusion, the results of the present study
demonstrated that JAK2/STAT3 inhibitors enhanced the protective
effect of tanshinone IIA on cardiac myocytes following
hypoxia/ischemia-induced injury, suggesting that JAK2/STAT3
inhibitors have the potential to be used for treating
ischemia-reperfusion injury in combination with tanshinone IIA.
Acknowledgements
The present study was supported by general project
funds of the Administration of Traditional Chinese Medicine of
Zhejiang province (no. 2012ZA015).
References
|
1
|
Liao X, Wang L, Yang C, et al:
Cyclooxygenase mediates cardioprotection of angiotensin-(1–7)
against ischemia/reperfusion-induced injury through the inhibition
of oxidative stress. Mol Med Rep. 4:1145–1150. 2011.PubMed/NCBI
|
|
2
|
Ramzy D, Rao V and Weisel RD: Clinical
applicability of preconditioning and postconditioning: the
cardiothoracic surgeons’s view. Cardiovasc Res. 70:174–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang N, Min X, Li D, He P and Zhao L:
Geranylgeranylacetone protects against myocardial ischemia and
reperfusion injury by inhibiting high-mobility group box 1 protein
in rats. Mol Med Rep. 5:521–524. 2012.
|
|
4
|
Zhou L, Zuo Z and Chow MS: Danshen: an
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharm. 45:1345–1359. 2005. View Article : Google Scholar
|
|
5
|
Shang QH, Xu H and Huang L: Tanshinone
IIA: a promising natural cardioprotective agent. Evid Based
Complement Alternat Med. 7:1120–1126. 2012.
|
|
6
|
Luo Y, Xu DQ, Dong HY, Zhang B, Liu Y, Niu
W, Dong MQ and Li ZC: Tanshinone IIA inhibits hypoxia-induced
pulmonary artery smooth muscle cell proliferation via
Akt/Skp2/p27-associated pathway. PLoS One. 8:e567742013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu J, Huang H, Liu J, Pi R, Chen J and Liu
P: Tanshinone IIA protects cardiac myocytes against oxidative
stresstriggered damage and apoptosis. Eur J Pharmacol. 568:213–221.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou G, Jiang W, Zhao Y, et al: Sodium
tanshinone IIA sulfonatemediates electron transfer reaction in rat
heartmitochondria. Biochem Pharmacol. 65:51–57. 2003. View Article : Google Scholar
|
|
9
|
Koppikar P, Bhagwat N, Kilpivaara O, et
al: Heterodimeric JAK-STAT activation as a mechanism of persistence
to JAK2 inhibitor therapy. Nature. 89:155–159. 2012. View Article : Google Scholar
|
|
10
|
Zhang S, Liu X, Goldstein S, et al: Role
of the JAK/STAT signaling pathway in the pathogenesis of acute
myocardial infarction in rats and its effect on NF-κB expression.
Mol Med Rep. 7:93–98. 2013.
|
|
11
|
Lange CM, Gouttenoire J, Duong FH, et al:
Vitamin D receptor and Jak-STAT signaling crosstalk results in
calcitriol-mediated increase of hepatocellular response to IFN-α. J
Immunol. 192:6037–6044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zgheib C, Kurdi M, Zouein FA, et al:
Acyloxy nitroso compounds inhibit LIF signaling in endothelial
cells and cardiac myocytes: evidence that STAT3 signaling is
redox-sensitive. PLoS One. 7:e433132012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolli R, Dawn B and Xuan YT: Role of the
JAK-STAT pathway in protection against myocardial
ischemia/reperfusion injury. Trends Cardiovasc Med. 13:72–79. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu HC, Qin HY, He F, et al: Canonical
notch pathway protects hepatocytes from ischemia/reperfusion injury
in mice by repressing reactive oxygen species production through
JAK2/STAT3 signaling. Hepatology. 54:979–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith RM, Suleman N, Lacerda L, Opie LH,
Akira S, Chien KR and Sack MN: Genetic depletion of cardiac myocyte
STAT3 abolishes classical preconditioning. Cardiovasc Res.
63:611–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung JH, Kwon TR, Jeong SJ, et al:
Apoptosis induced by Tanshinone IIA and Cryptotanshinone is
mediated by distinct JAK/STAT3/5 and SHP1/2 signaling in chronic
myeloid leukemia K562 cells. Evid Based Complement Alternat Med.
2013:8056392013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siddiquee K, Zhang S, Guida WC, et al:
Selective chemical probe inhibitor of Stat3, identified through
structure-based virtual screening, induces antitumor activity. Proc
Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mertens C and Darnell JE Jr: SnapShot:
JAK-STAT signaling. Cell. 131:612–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Negoro S, Kunisada K, Tone E, et al:
Activation of JAK/STAT pathway transduces cytoprotective signal in
rat acute myocardial infarction. Cardiovasc Res. 47:797–805. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grandage VL, Everington T, Linch DC and
Khwaja A: Gö6976 is a potent inhibitor of the JAK 2 and FLT3
tyrosine kinases with significant activity in primary acute myeloid
leukaemia cells. Br J Haematol. 135:303–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan P, Liu IM, Li YX, Yu WJ and Cheng JT:
Antihypertension induced by tanshinone II A isolated from the roots
of salvia miltiorrhiza. Evid Based Complement Alternat Med.
12:234–239. 2011.
|
|
22
|
Li YS, Wang ZH and Wang J: Effect of
tanshinone IIA on angiotensin receptor in hypertrophic myocardium
of rats with pressure over-loading. Chin J Integr Med. 28:632–636.
2008.(In Chinese).
|
|
23
|
Feng J and Zheng Z: Effect of sodium
tanshinone IIA sulfonate on cardiac myocyte hypertrophy and its
underlying mechanism. Chin J Integr Med. 14:197–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang R, Liu A, Ma X, et al: Sodium
tanshinone IIA sulfonate protects cardiomyocytes against oxidative
stress-mediated apoptosis through inhibiting JNK activation. J
Cardiovasc Pharmacol. 51:396–401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng XF, Zou XJ, Peng B, et al: Inhibition
of ethanol-induced toxicity by tanshinone IIA in PC12 cells. Acta
Pharmacol Sin. 27:659–664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borensztejn A, Boissoneau E, Fernandez G,
Agnès F and Pret AM: JAK/STAT autocontrol of ligand-producing cell
number through apoptosis. Development. 140:195–204. 2013.
View Article : Google Scholar
|
|
27
|
Obana M, Maeda M, Takeda K, et al:
Therapeutic activation of signal transducer and activator of
transcription 3 by interleukin-11 ameliorates cardiac fibrosis
after myocardial infarction. Circulation. 121:684–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Negoro S, Kunisada K, Tone E, et al:
Activation of JAK/STAT pathway transduces cytoprotective signal in
rat acute myocardial infarction. Cardiovasc Res. 47:797–805. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siddiquee K, Zhang S, Guida WC, et al:
Selective chemical probe inhibitor of Stat3, identified through
structure-based virtual screening, induces antitumor activity. Proc
Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stephanou A, Brar BK, Scarabelli TM, et
al: Ischemia-induced STAT-1 expression and activation play a
critical role in cardiomyocyteapoptosis. J Biol Chem.
275:10002–10008. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar A, Kumar A, Michael P, et al: Human
serum from patients with septic shock activates transcription
factors STAT1, IRF1, and NF-κB and induces apoptosis in human
cardiac myocytes. J Biol Chem. 280:42619–42626. 2005. View Article : Google Scholar : PubMed/NCBI
|