Introduction
Ultrasound (US) imaging has been a primary choice
for the diagnosis and evaluation of tumors, as it is safe, a
real-time measurement, low-cost and portable. The wide use of US
contrast agents (UCAs), which may enhance the comparison between
lesions and surrounding tissue, has greatly improved the resolution
and sensitivity of clinical US imaging (1). Micro-sized UCAs cannot pass through
the vascular endothelium, so are consequently regarded as blood
pool tracers, nano-scale UCAs however, are able to pass through
gaps in the vascular endothelium into tumor mesenchyma. As a
result, much cancer therapy research has focused on drug-loaded
nanoparticles (NPs). A number of antitumor drugs, including
cisplatin (2), mitoxantrone
(3), DNA and siRNA (4) have been successfully embedded into
NPs, which may provide protection from direct degradation by
nucleases in vivo. In addition, NPs may facilitate drug
uptake into target cells or tissues via an endocytic pathway
(5). Previously, studies have
undertaken investigations to assess the treatment of pancreatic
cancer by drug-loaded NPs (6).
However, the results obtained suggested that the efficiency of
drug-loaded NP uptake into the tumor tissues remained low.
US-targeted microbubble destruction (UTMD) may be an effective
method to facilitate NP uptake into various types of tumor tissue
in vivo, due to an alteration in the permeability of the
vasculature and cell membrane (7–10).
The aims of the current study were as follows: (i) To evaluate the
use of US contrast imaging with a novel NC in vivo and in
vitro; and (ii) to establish the effectiveness of US and UTMD
in promoting the uptake of this paclitaxel-loaded NC (PTX-NC) in
vitro into pancreatic cancer cells to induce cytotoxicity.
Materials and methods
Materials
Poly(lactic-co-glycolic
acid)-monomethoxypoly(ethylene glycol) (PLGA-mPEG) (LA:GA, 8:2;
PEG2000, 10%) was obtained from Shanghai Cancer Institute
(Shanghai, China). Pluronic F68 was obtained from BASF Co., Ltd.
(Shanghai, China). PTX and dichloromethane were obtained from
Tianjin Kaitong Chemical Reagent Co., Ltd. (Tianjin, China).
Rhodamine (Rh) and fluorescein isothiocyanate (FITC) were obtained
from Beijing Biosea Biotechnology Co., Ltd. (Beijing, China).
SonoVue (Bracco, Milan, Italy) is a lipid-coated UCA with sulfur
hexafluoride gas microbubbles (MBs), composed of ~2×108
MBs/ml, and an average diameter of 2.5–6.0 μm. A total of 10 female
New Zealand white rabbits (12 weeks old; average weight,
3128.54±102.32 g) and 30 nude female BALB/c mice (4 weeks old;
average weight, 13.87±1.92 g) were supplied by the First People’s
Hospital Affiliated to Shanghai Jiao tong University (Shanghai,
China), and all animal procedures were performed in accordance with
the research protocol approved by the Animal Care and Use Committee
of the hospital.
Preparation and physicochemical
characteristics of the PTX-PLGA-mPEG NCs
The PTX-mPEG-PLGA NCs were prepared using the
double-emulsion method (11). PTX
solution (1.25 mg in 1.25 ml methanol solution; Shanghai Baoman
Biotechnology Co., Ltd., Shanghai, China) was emulsified in the
PLGA-mPEG solution (25 mg in 1 ml dichloromethane solution) by
sonication (200 W, 5 sec-2 sec-15) using an ultrasonic cell
disrupter (JY92-ZD, Ningbo Xinzhi Biotechnology Co., Ltd., Ningbo,
China). Subsequently, 10 ml F68 aqueous solution (1 mg/ml) was
rapidly added to the first emulsion and sonicated (200 W, 5 sec-2
sec-15). The resultant emulsions were stirred to evaporate the
dichloromethane and were then lyophilized (EPSILON 2–6D; Martin
Christ, Osterode am Harz, Germany). The NC sizes were measured
using an H-7000 Transmission Electron Microscope (Hitachi Ltd.,
Tokyo, Japan). The size distribution and ζ potential of the NPs in
aqueous solution was determined using a Nicomp-380ZLS ζ potential
analyzer, from Particle Sizing Systems, Inc. (Port Richey, FL,
USA). The drug-loading rate was calculated as the ratio of the
amount of PTX encapsulated in NCs to the total amount of NCs (3 mg)
initially used. The detection of drug-loading rate was completed by
the Shanghai Cancer Institute (Shanghai, China).
Cell culture
Human pancreatic cancer cells (Aspc-1; Shanghai
Tumor Institute, Shanghai, China) were incubated in Dulbecco’s
modified Eagle’s medium (Gibco Life Technologies, Grand Island, NY,
USA), then were maintained in 10% fetal bovine serum (Sigma, St.
Louis, MO, USA), penicillin and streptomycin (100 μg/ml; Shanghai
Baoman Biotechnology Co, Ltd.) at 37°C in humidified conditions
with 5% CO2. Subsequently, the pancreatic cancer cells
were seeded into 6- and 96-well plates according to the different
experimental conditions.
US contrast-enhanced imaging analysis of
PTX-PLGA-mPEG NCs (nano-UCA) in vitro
The Philips iE33 xMATRIX US system (Philips
Healthcare, Andover, MA, USA) was used with an L11-3 probe. A total
of 5 ml degassed water was poured into one 5-ml soft tube. Nano-UCA
powder (60 mg) was placed into another 5-ml soft tube and topped up
with degassed water. The lids of the two tubes were then sealed
tightly and the tube containing the nano-UCA solution was vibrated
in order to ensure that the powder was completely dissolved prior
to imaging. The outer surfaces of the soft tubes were covered with
nano-UCA to prevent any air getting between the tubes and the
transducer. US is attenuation in air, therefore, ultrasonic
medicinal coupling gel was smeared between the tube and the
transducer. US contrast images of the nano-UCA solution (12 mg/ml)
were immediately collected and recorded.
US contrast-enhanced imaging analysis of
PTX-PLGA-mPEG NCs (nano-UCA) in vivo
The Acuson Sequoia 512 US system (Siemens, Erlangen,
Germany) and LOGIQ E9 (GE Healthcare, Wauwatosa, WI, USA) were used
with the 15L8W-S and ML6-14 probes, respectively. Degassed water (2
ml) and nano-UCA solution (2 ml; 12 mg/ml) were injected into the
ear veins of the rabbits, while US contrast images of the right
kidney were observed in real-time and recorded. Similarly, 1 ml
degassed water, 1 ml nano-UCA (12 mg/ml) solution and 1 ml SonoVue
suspension were injected into the tail veins of the mice, while US
contrast images of superficial pancreatic tumors in nude mice were
also observed in real-time and recorded. Rabbits were euthanized
via injection of 1,200 mg/kg nembutal injected into the ear vein
and mice were sacrificed by decapitation.
Detection of cellular uptake of PLGA-mPEG
NCs by fluorescence microscopy
Aspc-1 pancreatic cancer cells
(3×105/well) were cultured in two 6-well plates and
incubated for 24 h. A therapeutic US machine (Physiomed
Elektromedizin AG, Schnaittach, Germany) was used at a frequency of
1 MHz, with the optimal US conditions (power, 1 W/cm2;
exposure time, 60 sec; duty cycle, 20%; SonoVue volume ratio, 1:5).
The cells were divided into three groups as follows: The
phosphate-buffered saline (PBS), Rh and Rh-PLGA-mPEG NC group. Each
group was exposed to three environmental conditions: i) Control;
ii) US; and iii) UTMD. The volume of solution in each well was 1
ml, with an equal volume of Rh. Each group was evaluated and imaged
using fluorescence microscopy following 5 h of the respective
treatments.
Detection of cellular uptake of PLGA-mPEG
NCs by flow cytometry
The Aspc-1 cells were divided into four groups: The
controls (Aspc-1 cells only), the FITC-PLGA-mPEG NCs,
FITC-PLGA-mPEG NCs + US and FITC-PLGA-mPEG NCs + UTMD groups. The
cells of each group were seeded at a density of
~5×105–1×106 cells/plate. The groups were
exposed to the same optimal US conditions (power, 1
W/cm2; exposure time, 60 sec; duty cycle, 20%; SonoVue
volume ratio, 1:5). The cells in each plate were washed twice with
PBS subsequent to administration, and promptly harvested by
trypsinization (Aladdin Industrial, Inc., Nashville, TN, USA).
Subsequently, the cells were suspended in 1 ml PBS. In the
FITC-PLGA-mPEG NCs + UTMD group, 200 μl MB solution (SonoVue) was
injected into the 1 ml PBS solution for each plate. Samples were
analyzed 5 h subsequent to administration of NC, using a flow
cytometer (EPICS XL/XL-MCL; Beckman Coulter, Miami, FL, USA).
Cellular cytotoxicity test
The cellular cytotoxicity of the NCs was determined
by MTT assay. Briefly, the Aspc-1 pancreatic cancer cells
(1×105/well) were cultured in 96-well plates and
incubated for 24 h. PBS solution; SonoVue dissolved in sterile
saline solution (Nanjing Bianzhen Biotechnology Co., Ltd., Nanjing,
China); blank PLGA-mPEG NCs; blank PLGA-mPEG NCs + US; blank
PLGA-mPEG NCs + UTMD; PTX-PLGA-mPEG NCs; PTX-PLGA-mPEG NCs + US;
PTX-PLGA-mPEG NCs + UTMD; and PTX were added to the cells at
different concentrations, and incubated for 24 and 48 h at 37°C.
The optimal US conditions (power, 1 W/cm2; exposure
time, 60 sec; duty cycle, 20%; SonoVue volume ratio, 1:5) were
used. Subsequently, 0.2 ml MTT (0.5 mg/ml) was added to the culture
and incubated for an additional 4 h at 37°C. The culture medium was
then removed from the wells and replaced with 0.2 ml dimethyl
sulfoxide. Following agitation of the 96-well plates for 15–20 min
on a swing bed, the absorbance was measured at a wavelength of 450
nm using a Model 680 Microplate Reader from Bio-Rad Laboratories
(Hercules, CA, USA).
Statistical analysis
Student’s t-test was utilized to identify the
significance of differences between the experimental and control
groups using SPSS, version 17.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. All experiments were conducted in triplicate.
Results
Characterization and drug-loading rate of
PTX-PLGA-mPEG NCs
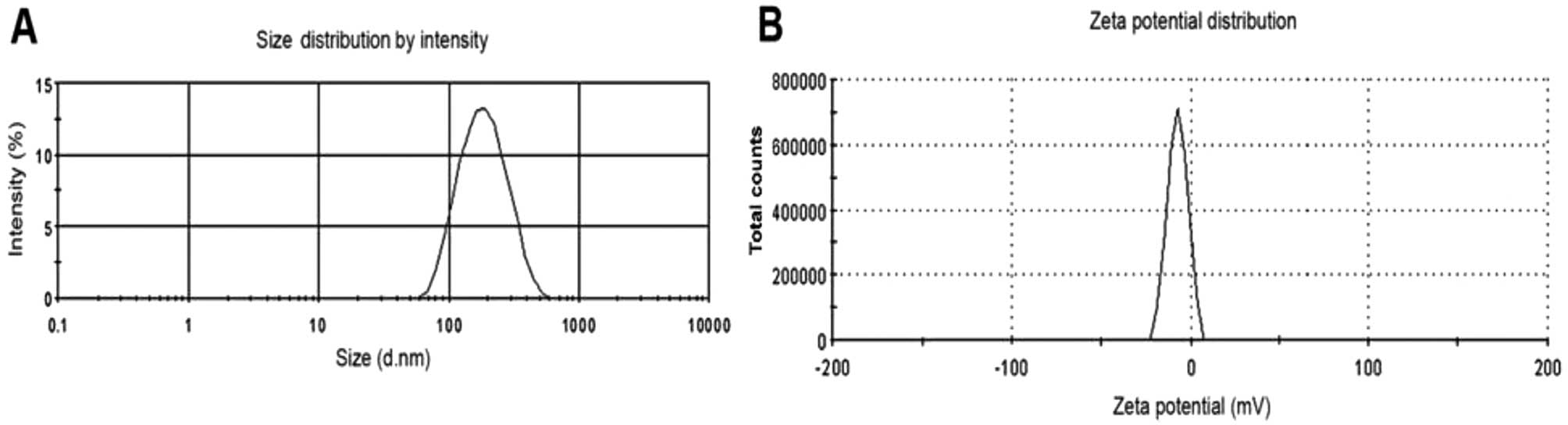
The results from the particle size and ζ potential
analyzer demonstrated that the PTX-PLGA-mPEG NC sizes were between
85.75 and 632.43 nm; the average size was 276.38 nm and the ζ
potential was −6.94 mV (Fig. 1).
Observed by transmission electron microscopy (TEM; Olympus IX53;
Olympus, Tokyo, Japan), the morphology of the PTX-PLGA-mPEG NCs
were spherical with advanced dispersion and no aggregation. The
inside of the PTX-NCs exhibited hollow honeycomb-like holes as
observed in the TEM images shown in Fig. 2. The drug-loading rate of PTX-NCs
was calculated to be 1.6%.
US contrast-enhanced imaging of
PTX-PLGA-mPEG NCs
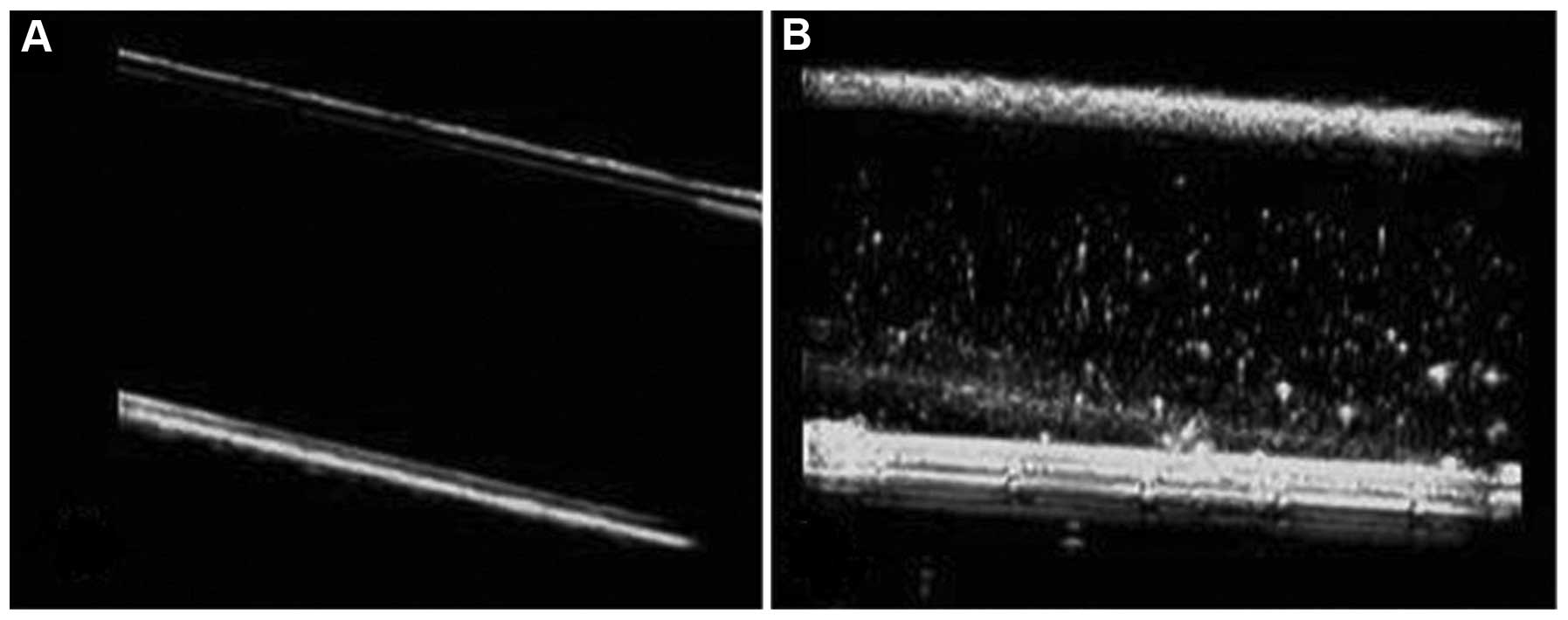
Observed from US contrast-enhanced images, a tube
filled with PTX-PLGA-mPEG NC UCA displayed a strong dotted-echo,
whereas a tube filled with degassed water was observed as black
(Fig. 3). Imaging of the rabbit
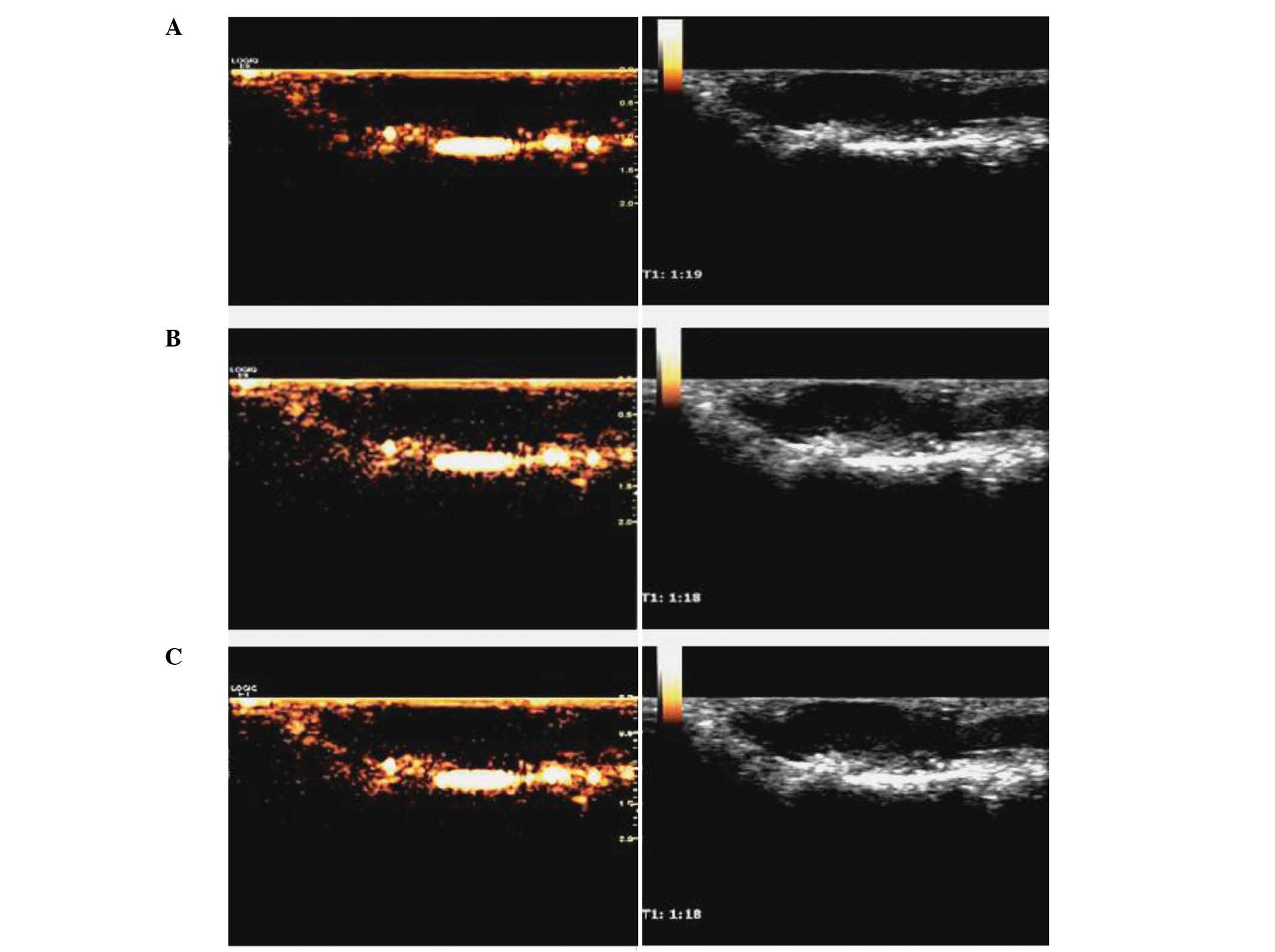
right kidney in vivo following PTX-PLGA-mPEG NC UCA
administration resulted in excellent contrast-enhanced images,
whilst unclear images were observed pre-administration (Fig. 4). However, the contrast-enhanced
images of superficial pancreatic tumors in nude mice following
administration of PTX-PLGA-mPEG NC UCA and SonoVue suspension were
unclear, similar to the level of clarity prior to administration
(Fig. 5).
Detection of cellular uptake of NCs by
fluorescence microscopy
As presented in Fig.
6, greater fluorescence was observed in Aspc-1 cells 5 h
subsequent administration of the Rh-PLGA-mPEG NC solution (panels
N1–3), compared with the control groups [PBS (panels P1–3) and Rh
only (panels R1–3)]. Furthermore, greater fluorescence was observed
in Aspc-1 cells following administration of the Rh-PLGA-mPEG NC
solution under the condition of UTMD (panel N3) compared with US
(panel N2). No marked fluorescence was observed in the control
groups (PBS and Rh only groups) under any of the conditions (panels
P1–3 and R1–3).
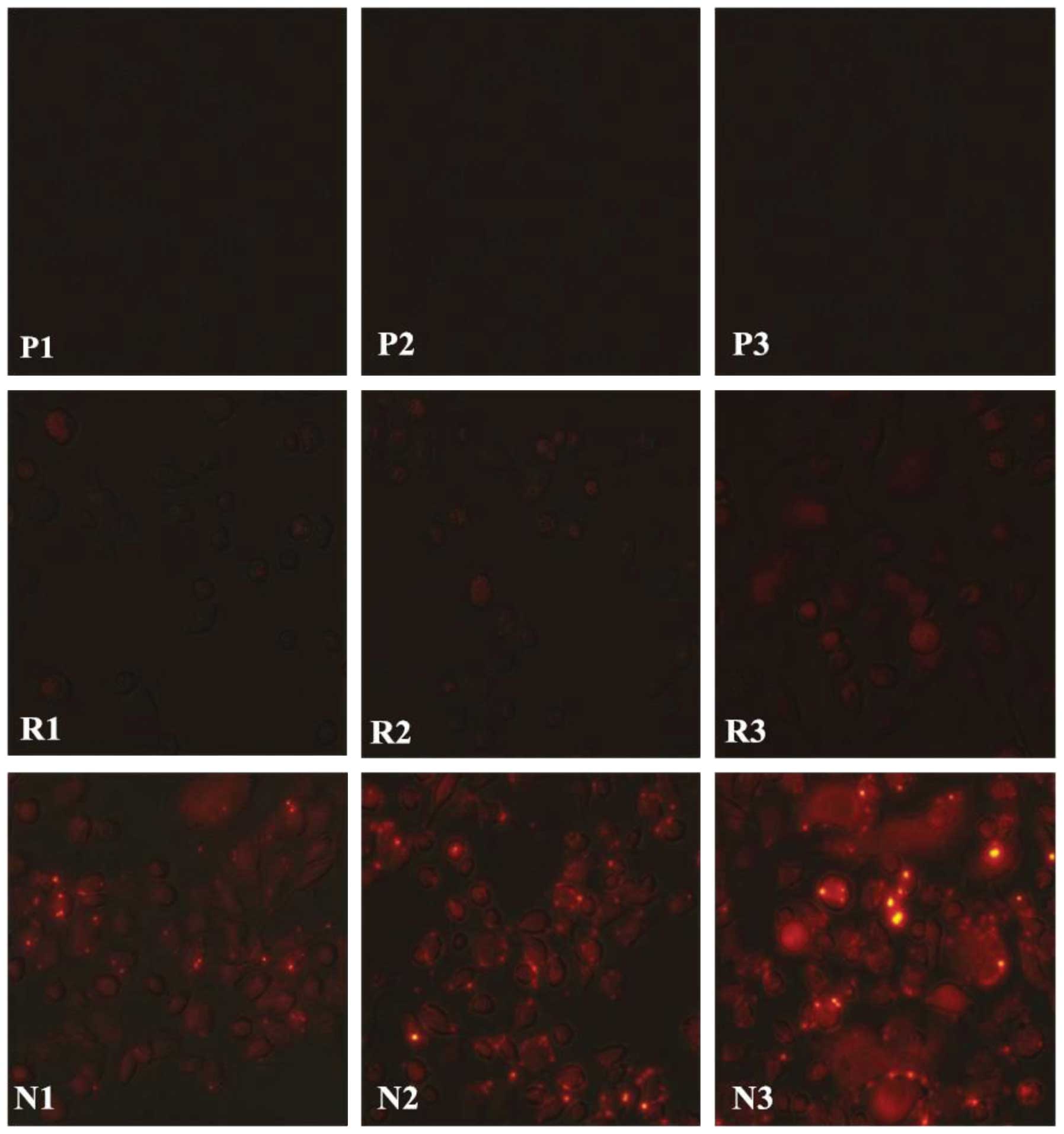 | Figure 6Intracellular uptake of Rh-PLGA-mPEG
NCs following 5 h of different conditions. Representative images
displaying the fluorescence of the 9 groups, observed under the
fluorescence microscope. P, PBS group; R, Rh group; N, Rh-PLGA-mPEG
NCs group. 1, no US; 2, with US; 3, with UTMD. Rh-PLGA-mPEG NCs,
rhodamine-poly(lactic-co-glycolic acid)-monomethoxy poly(ethylene
glycol) nanocapsules; PBS, phosphate-buffered saline; Rh,
rhodamine; US, ultrasound; UTMD, US-targeted microbubble
destruction. |
Quantification of cellular uptake of NCs
by flow cytometry
In the NCs + US and NCs + UTMD groups, the
intracellular uptake rates were significantly greater than in the
group with NCs alone (P<0.05). The NC uptake efficiency was not
significantly higher in the NCs + UTMD group compared with that of
the NCs + US group (Fig. 7)
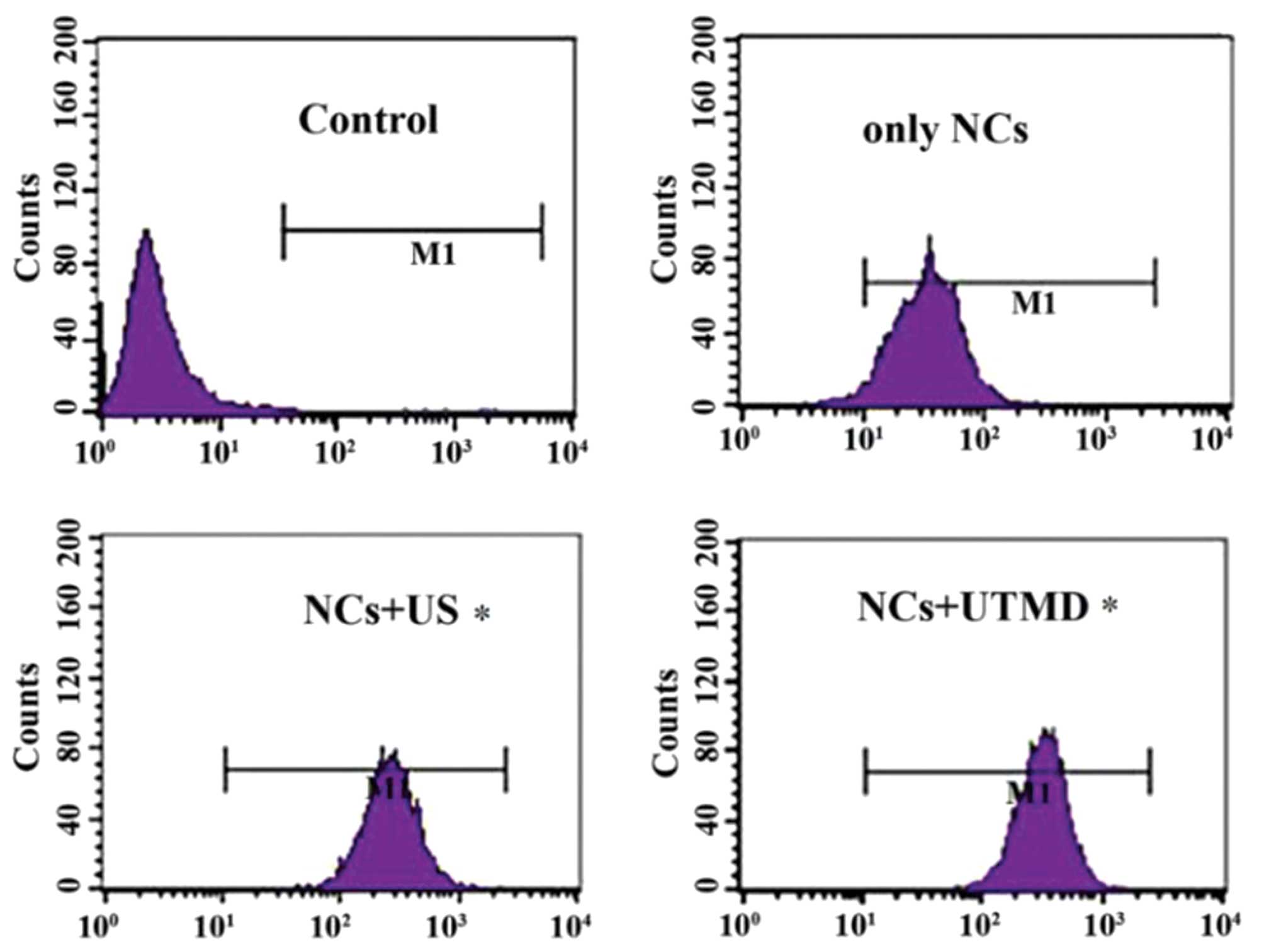 | Figure 7Flow cytometry results indicating the
cell fluorescence of Aspc-1 cells in each group (controls, 0.05%;
NCs only, 23.42%; NCs + US, 77.35%; and NCs + UTMD, 81.47%).
P<0.05 in the NC + US and NC + UTMD, compared with the only NC
group. NCs, nanocapsules; US, ultrasound, UTMD, US-targeted
microbubble destruction; M1, gate. |
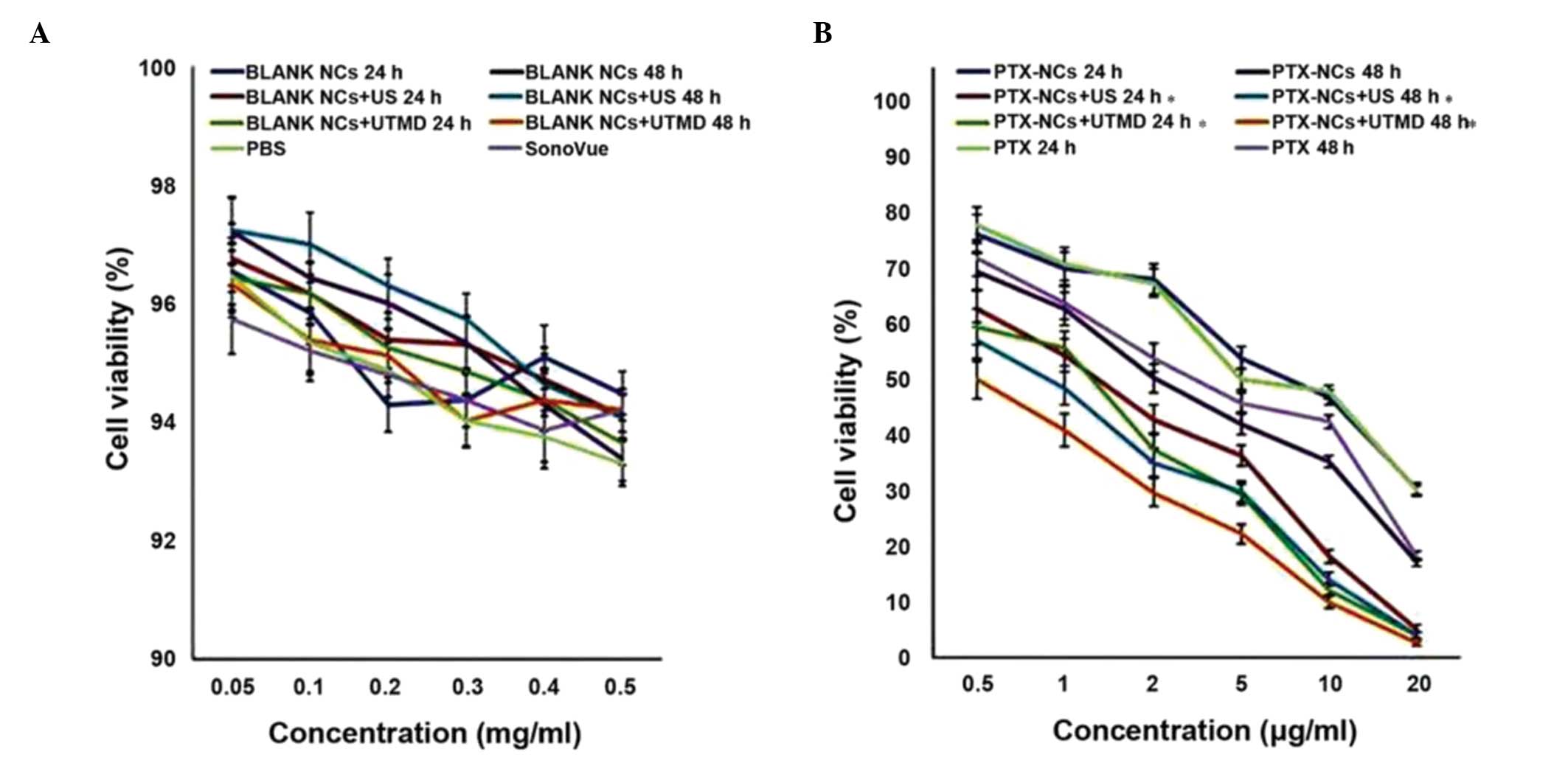
MTT assay
The cell viabilities of Aspc-1 pancreatic cancer
cells following administration of PBS, SonoVue, blank NCs, blank
NCs + US, or blank NCs + UTMD for 24 or 48 h were all between 92
and 96% (Fig. 8A). No significant
differences in cell viability were identified between these groups
(P>0.05). In the PTX-NC groups, it was identified that the NCs
did not significantly affect cell viability in the absence of US or
UTMD compared with PTX treatment alone (P>0.05). However, in the
US or UTMD groups, following incubation for 24 or 48 h, the PTX-NC
cytotoxicities towards the cells were indicated to be significantly
higher than those that underwent treatment with PTX alone
(P<0.05). Furthermore, subsequent to incubation for 24 or 48 h,
the cell viabilities of the pancreatic cancer cells with PTX-NCs
mediated by UTMD were significantly lower than those mediated by US
(P<0.05) (Fig. 8B).
Discussion
Conventional UCAs, such as the lipid-shelled SonoVue
filled with sulfur hexafluoride gas, have been widely used in
clinical practice. These types of UCAs have the ability to produce
useful contrast-enhanced images, but cannot act therapeutically. It
has been demonstrated that drugs and genes are able to adhere to
the surface of the UCAs, or be encapsulated into them to be used
therapeutically (12). The current
study designed a novel nano-UCA that can be used not only in
contrast-enhanced imaging, but also to deliver drugs into tumor
cells.
This novel nano-UCA was made using PLGA-mPEG, a
commonly used biodegradable polyester material, which has been
approved to be nontoxic and harmless by the US Food and Drug
Administration (13). An mPEG
molecule can prolong the body circulation time of drugs and
increase time at the tumor tissue by reducing their recognition by
the reticuloendothelial system, thus an mPEG molecule may be
beneficial in the treatment of tumors in vivo. The
double-emulsion method was used for the preparation of
PTX-PLGA-mPEG NCs, which were observed by TEM to be spherical in
shape, with a diameter ranging between 85.75 and 632.43 nm. During
the course of synthesis, the encapsulated water in the inner
aqueous phase of the NCs was sublimated by lyophilization. This
resulted in small hollow holes with a honeycomb structure,
providing a basis for the US-responsive properties. The properties
of PTX-PLGA-mPEG NCs following lyophilization were stable; this
type of nano-UCA powder may be kept for a long time at a
temperature of −20°C without alteration to its appearance.
Furthermore, the morphology observed by TEM following resolution
revealed good dispersion and no aggregation.
It was also observed that PTX-PLGA-mPEG NCs yielded
effective contrast-enhanced images in vitro and in rabbit
right kidney in vivo. However, contrast images in
superficial pancreatic cancer tumors in nude mice were not
satisfactory with administration of PTX-PLGA-mPEG NCs or with
SonoVue solution. A potential explanation may be as follows: In
general, the vessels of the tumor were divided into two sections;
one section consisted of the original vessels, the endothelial gaps
of which were <100 nm. The other section was composed of newly
formed vessels, the endothelial structure of which was not intact
and did not contain smooth muscle. Due to this, the endothelial
gaps in these newly formed blood vessels were larger; between
380–780 nm (14). As a result of
this, a greater nano-UCA influx into the tumor tissues may have
occurred, compared with micro-UCAs. The pancreatic tumor tissues of
nude mice and humans presented specific pathological and anatomical
similarities, with dense, poorly vascularized connective tissues
immersed in a large volume of fibrous tissues and lymphocytes
(15). Hence, to a certain extent,
nano-UCAs may produce improved contrast-enhanced images in rabbit
kidney and maintain a sufficient blood supply, compared with those
in superficial tumors of nude mouse pancreas. Additionally, the
hollow holes in lyophilized PTX-PLGA-mPEG NCs may be so small that
they lead to strong ultrasonic reflection, and a higher
concentration of the nano-UCA solution would be required for US
contrast imaging in pancreatic tumors.
The present study demonstrated a greater
Rh-PLGA-mPEG NC uptake (red fluorescent signals in Fig. 6) in Aspc-1 cells following
administration of Rh-PLGA-mPEG NCs mediated by US or UTMD (N2 and
3), when comparing intracellular uptake ratios among the PBS, Rh
alone and Rh-PLGA-mPEG groups in US (N1), US (N1) and UTMD (N3)
conditions. Furthermore, stronger red fluorescent signals in Aspc-1
cells were observed following administration of the Rh-PLGA-mPEG
NCs with UTMD (N3) compared with US (N2). Similar results were
observed when measuring NC cellular uptake with flow cytometry. The
cellular uptake efficiencies of the PLGA-mPEG NC groups with US and
UTMD were higher than the uptake of the PLGA-mPEG NCs alone. The
PLGA-mPEG NC cellular uptake efficiency in the PLGA-mPEG NCs + UTMD
group was not significantly higher than in the PLGA-mPEG NCs + US
group. These results indicate that US and UTMD are effective
driving forces that may be favorable methods to increase PLGA-mPEG
NC uptake to Aspc-1 pancreatic cancer cells.
Possible mechanisms for promoting NP uptake into
cells have been studied, but the most efficient mechanism remains
unclear: Micro-circumflex or micro-fluid generation by UTMD
punching transient holes in the cell membrane surface (16); an increase in reactive oxygen
species in cells; cell membrane transport abilities becoming
activated; and an increase in cell membrane temperature during US
(17–21) are a number of possibilities.
Furthermore, a previous study proposed a novel mechanism, that UTMD
may stimulate cellular clathrin-dependent endocytosis (22).
In the MTT assay, the cell viabilities of Aspc-1
pancreatic cancer cells following administration of PBS, SonoVue or
blank PLGA-mPEG NCs with or without US or UTMD for 24 or 48 h were
all above 90%. This demonstrated that the cellular cytotoxicity of
the blank PLGA-mPEG NCs at each concentration tested was
negligible, and also that the optimal US and UTMD conditions had
almost no effect on these Aspc-1 cells in vitro without the
PTX-PLGA-mPEG NCs. However, when combined with PTX-PLGA-mPEG NCs,
the cytotoxicity was greater following US and UTMD than with no US.
In addition, due to more powerful sonoporation, UTMD elicited an
increased NC uptake into the Aspc-1 cells compared with US. Thus,
US and UTMD were demonstrated to be powerful physical techniques,
which may safely and efficiently deliver PTX-PLGA-mPEG NCs into
Aspc-1 cells in vitro, consequently producing an
antitumorigenic effect.
In conclusion, a novel PTX-PLGA-mPEG NC technique,
which combined US contrast imaging and antitumor therapy, was
successfully designed and prepared. UTMD, a promising physical
targeting vehicle, may facilitate improved PTX-PLGA-mPEG NC uptake
into Aspc-1 pancreatic cancer cells and enhanced antitumorigenic
action in vitro. Thus, the combination of nanotechnology and
US may present a novel method for monitoring and treating tumors.
Further study is required to continue the investigation of
US-specific contrast imaging and antitumor treatment, and future
studies should include a variety of tumor types in vivo.
Acknowledgements
The current study was supported by the Department of
Ultrasound, Shanghai First People’s Hospital Affiliated to Shanghai
Jiao tong University School of Medicine (Shanghai, China) and the
National Natural Science Foundation of China. Project approval nos.
81271596 and 81171352.
References
|
1
|
Xing Z, Ke H, Wang J, Zhao B, Yue X, Dai Z
and Liu J: Novel ultrasound contrast agent based on microbubbles
generated from surfactant mixtures of Span 60 and polyoxyethylene
40 stearate. Acta Biomater. 6:3542–3549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen M, Cooper HM, Zhou JZ, et al:
Reduction in the size of layered double hydroxide nanoparticles
enhances the efficiency of siRNA delivery. J Colloid Interface Sci.
390:275–281. 2013. View Article : Google Scholar
|
|
3
|
Du J, Shi QS, Sun Y, et al: Enhanced
delivery of monomethoxypoly(ethylene
glycol)-poly(lactic-co-glycolic acid)-poly l-lysine nanoparticles
loading platelet-derived growth factor BB small interfering RNA by
ultrasound and/or microbubbles to rat retinal pigment epithelium
cells. J Gene Med. 13:312–323. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fields RJ, Cheng CJ, Quijano E, et al:
Surface modified poly(β amino ester)-containing nanoparticles for
plasmid DNA delivery. J Control Release. 164:41–48. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SH, Bae KH, Kim SH, Lee KR and Park
TG: Amine-functionalized gold nanoparticles as non-cytotoxic and
efficient intracellular siRNA delivery carriers. Int J Pharm.
364:94–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arya G, Vandana M, Acharya S and Sahoo SK:
Enhanced antiproliferative activity of Herceptin (HER2)-conjugated
gemcitabine-loaded chitosan nanoparticle in pancreatic cancer
therapy. Nanomedicine. 7:859–870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chappell JC, Song J, Burke CW, Klibanov AL
and Price RJ: Targeted delivery of nanoparticles bearing fibroblast
growth factor-2 by ultrasonic microbubble destruction for
therapeutic arteriogenesis. Small. 4:1769–1777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vancraeynest D, Havaux X, Pouleur AC, et
al: Myocardial delivery of colloid nanoparticles using
ultrasound-targeted microbubble destruction. Eur Heart J.
27:237–245. 2006. View Article : Google Scholar
|
|
9
|
Lin CY, Liu TM, Chen CY, et al:
Quantitative and qualitative investigation into the impact of
focused ultrasound with microbubbles on the triggered release of
nanoparticles from vasculature in mouse tumors. J Control Release.
146:291–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chumakova OV, Liopo AV, Andreev VG, et al:
Composition of PLGA and PEI/DNA nanoparticles improves
ultrasound-mediated gene delivery in solid tumors in vivo. Cancer
Lett. 261:215–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu P, Qin L, Wang Q, et al:
cRGD-functionalized mPEG-PLGA-PLL nanoparticles for imaging and
therapy of breast cancer. Biomaterials. 33:6739–6747. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Miyoshi H and Nakamura M:
Nanomedicine for drug delivery and imaging: a promising avenue for
cancer therapy and diagnosis using targeted functional
nanoparticles. Int J Cancer. 120:2527–2537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Chan HF and Leong KW: Advanced
materials and processing for drug delivery: the past and the
future. Adv Drug Deliv Rev. 65:104–120. 2013. View Article : Google Scholar :
|
|
14
|
Oeffinger BE and Wheatley MA: Development
and characterization of a nano-scale contrast agent. Ultrasonics.
42:343–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei JM, Xu XJ, Wang XY, et al: Potential
relationship between pancreatic histological features and its
diseases. Chin J Hepatobil Surg. 14:414–416. 2008.(In Chinese).
|
|
16
|
Prentice P, Cuschieri A, Dholakia K, et
al: Membrane disruption by optically controlled microbubble
cavitation. Nat Phys. 1:107–110. 2005. View
Article : Google Scholar
|
|
17
|
Tachibana K, Uchida T, Ogawa K, et al:
Induction of cell-membrane porosity by ultrasound. Lancet.
353:14091999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Wamel A, Kooiman K, Harteveld M, et
al: Vibrating microbubbles poking individual cells: drug transfer
into cells via sonoporation. J Control Release. 112:149–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juffermans LJ, Dijkmans PA, Musters RJ, et
al: Transient permeabilization of cell membranes by
ultrasound-exposed microbubbles is related to formation of hydrogen
peroxide. Am J Physiol Heart Circ Physiol. 291:H1595–H1601. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller DL and Gies RA: The interaction of
ultrasonic heating and cavitation in vascular bioeffects on mouse
intestine. Ultrasound Med Biol. 24:123–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schlicher RK, Radhakrishna H, Tolentino
TP, et al: Mechanism of intracellular delivery by acoustic
cavitation. Ultrasound Med Biol. 32:915–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin LF, Li F, Wang HP, Wei F, Qin P and Du
LF: Ultrasound targeted microbubble destruction stimulates cellular
endocytosis in facilitation of adeno-associated virus delivery. Int
J Mol Sci. 14:9737–9750. 2013. View Article : Google Scholar : PubMed/NCBI
|