Introduction
Breast cancer is one of the most common types of
malignant disease affecting females worldwide, causing >450,000
mortalities each year (1). The
current treatment modalities for breast cancer include surgical
resection, adjuvant radiotherapy and advanced chemotherapeutic
agents, including cisplatin, pacliataxel, carboplatin, bevacizumab,
doxorubicin, cyclophosphamide, docetaxel and epirubicin (2). Despite advances in treatment
strategies, mortality from breast cancer remains high. Therefore,
there is a clear requirement for the development of novel
therapeutic agents.
Histone acetyltransferases (HATs) and histone
deacetylases (HDACs) are known to have opposing roles in the
regulation of global gene expression via an epigenetic mechanism
(3). HATs catalyze the acetylation
of lysine residues in histone tails, facilitating and sustaining
gene transcription, while HDACs are responsible for the removal of
acetyl groups from the ɛ-amine of lysine residues of histone tails,
culminating in prevention of gene transcription. HDAC inhibitors
that have the ability to block the activities of HADCs have emerged
as effective anticancer agents (4). Their anticancer effects are
associated with the modulation of various cell behaviors, including
differentiation, cell cycle progression, apoptosis and angiogenesis
(5,6). At the molecular level, HDAC
inhibitors can affect the expression of a large number of genes,
particularly key regulators of apoptosis and the cell cycle such as
p21, p27, Bax, and Bcl-2 (5–7).
Suberoylanilide hydroxamic acid (SAHA) and
trichostatin A (TSA) are two of the most investigated HDAC
inhibitors and have shown cytotoxic effects against a number of
tumor types, including breast cancer (8,9).
Suberoyl bis-hydroxamic acid (SBHA) has a similar structure to SAHA
and TSA, and has been found to prevent tumor growth in several
types of malignancies, such as medullary thyroid cancer (10) and lung cancer (11). You and Park (11) reported that SBHA is capable of
inhibiting the growth of A549 lung cancer cells via
caspase-dependent apoptosis. In addition, in MCF-7 breast cancer
cells, SBHA treatment causes a significant level of apoptosis
(12). However, the antitumor
activity of SBHA in breast cancer cells and its associated
molecular mechanisms are not completely understood. In the current
study, the cytotoxic effect of SBHA against MCF-7 breast cancer
cells was evaluated and its impact on cell cycle progression and
apoptosis was examined.
Materials and methods
Cells and reagents
MCF-7 breast cancer cells were purchased from the
American Type Culture Collection (Manassas, VA, USA). Fetal bovine
serum (FBS), RPMI-1640 medium, penicillin, streptomycin, Hoechst
33258, propidium iodide (PI) and dimethyl sulfoxide (DMSO) were
obtained from Sigma-Aldrich (St. Louis, MO, USA); SBHA was
purchased from Calbiochem (San Diego, CA, USA); Cell Counting kit-8
(CCK-8) was obtained from Dojindo Molecular Technologies (Kumamoto,
Japan); an Annexin-FITC kit was purchased from Beckman Coulter
(Fullerton, CA, USA) and DNase-free RNase was from Roche Applied
Science (Penzburg, Germany). Mouse monoclonal antibodies against
p21 (sc-271532), p27 (sc-1641), Bcl-2 (sc-7382), Bax (sc-20067) and
β-actin (sc-130301) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated goat
anti-mouse IgG antibody was obtained from Rockland (Gilbertsville,
PA, USA).
Cell culture and treatment
MCF-7 cells were cultured in RPMI-1640 medium
supplemented with 10% heat-inactivated FBS, penicillin (100 U/ml)
and streptomycin (100 μg/ml). Cells were maintained in a humidified
incubator with 5% CO2 and 95% air at 37°C and
subcultured every 3–4 days. Twenty-four hours after plating, cells
were treated with different concentrations of SBHA for 72 h and
subjected to cell proliferation, apoptosis and gene expression
analysis. Untreated cells were used as the control.
CCK-8 assay
The effect of SBHA on cell proliferation was
determined with the CCK-8 cell proliferation assay kit. Briefly,
MCF-7 cells were seeded in 96-well plates at a density of
5×103 cells/well and incubated for 24 h. Following
incubation, the cells were exposed to 10, 40, 60, 80, or 100 μM
SBHA for 72 h. Untreated cells served as the control. Cells were
incubated for a further 2 h in the presence of CCK-8 reagent. The
absorbance (optical density) was measured at a wavelength of 450 nm
using a microplate reader (Bio-Rad 3550; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Cell cycle analysis
Following treatment, cells were trypsinized, washed
and fixed with 75% ice-cold ethanol. Cells were pelleted by
centrifugation at 300 × g for 5 min and suspended in DNA staining
solution containing 20 μg/ml PI and 20 μg/ml DNase-free RNase.
After incubation at 37°C for 15–30 min, cells were analyzed on a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Cell cycle distribution was determined and calculated using
CellQuest 5.2 software (BD Biosciences).
Characterization of apoptotic morphology
by Hoechst 33258 staining
Cells were grown on sterile cover slips in 6-well
tissue culture plates. When the cells reached 60–80% confluence,
they were treated with SBHA for 72 h. After washing with
phosphate-buffered saline Invitrogen (Carlsbad, CA, USA), cells
were fixed with 4% paraformaldehyde for 10 min, washed, and stained
with Hoechst 33258 (5 μg/ml) for 5 min at room temperature. Cells
were mounted prior to examination using a DMIRE2 fluorescence
microscope (Leica, Bensheim, Germany).
Apoptosis analysis by Annexin V/PI
staining
Cells were seeded onto 6-well plates and exposed to
different concentrations of SBHA ranging from 20–120 μM for 72 h.
Cells were harvested through trypsinization, washed and centrifuged
at 300 × g for 5 min. The cell pellet was resuspended in 1X binding
buffer. The cell sample solution (100 μl) was incubated with 1 μl
fluorescein isothiocyanate (FITC)-conjugated Annexin V and 5 μl PI
for 15 min at 4°C in the dark. The 1X binding buffer (400 μl) was
added to each sample tube and the samples were analyzed on a
FACSCalibur flow cytometer using CellQuest software.
Western blot analysis
After treatment, cells were lysed in lysis buffer
(10 mmol/l Tris, pH 7.4; 130 mmol/l NaCl, 1% Triton X, 10 mmol/l
NaF, 10 mmol/l NaPi, 10 mmol/l NaPPi, and 1.5 mmol/l EDTA)
supplemented with the protease inhibitor aprotinin (2 mg/l) and
phosphatase inhibitors leupeptin (5 mg/l) and phenylmethylsulfonyl
fluoride (1 mmol/l), which were all purchased from Sigma-Aldrich).
The protein samples were separated on polyacrylamide gels and then
transferred to a nitrocellulose membrane (Bio-Rad Laboratories,
Inc.). After blocking for 45 min in a Tris-buffered solution (TBS)
containing 5% fat-free dried milk and 0.5% Tween-20
(Sigma-Aldrich), the membrane was incubated with individual primary
antibodies anti-p21, anti-p27, anti-Bcl-2, anti-Bax and
anti-β-actin (1:500) overnight at 4°C. The membrane was washed
three times and incubated for 1 h with secondary antibodies at room
temperature. The signals were visualized with the enhanced
chemiluminescence method (Amersham Biosciences, Piscataway, NJ,
USA). Densitometric analysis of western blots was performed using
the Scion Image Beta 4.02 software (SynGene, Cambridge, UK).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical significance was determined using Student’s
t-test or one-way analysis of variance with Tukey’s post hoc test.
Statistical analysis was performed using SPSS 19.0 software
(International Business Machines, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
SBHA impedes cell proliferation in MCF-7
cells
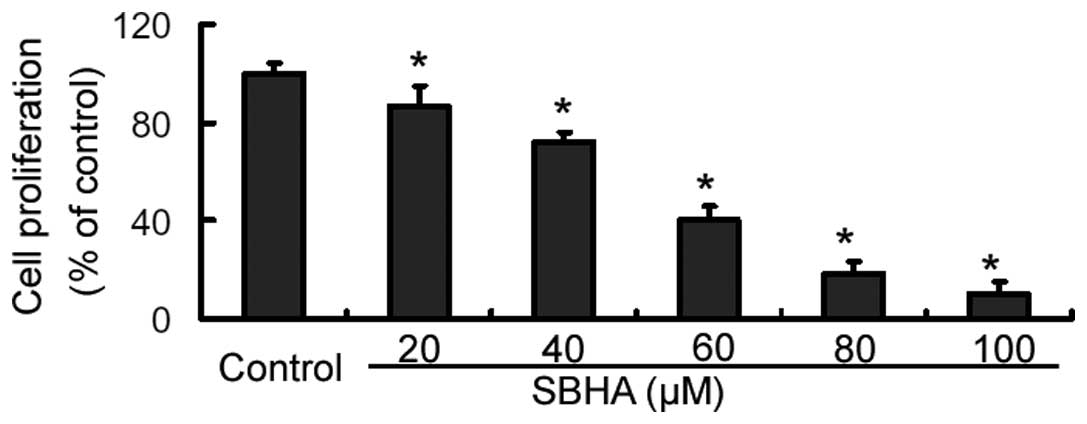
The CCK-8 assay revealed that SBHA treatment for 72
h caused a significant (P<0.05) inhibition of MCF-7 cell
proliferation, compared to that of the untreated cells (Fig. 1). Furthermore, the inhibition
occurred in a concentration-dependent manner. Since the
IC50 value of SBHA in MCF-7 cells was 33.9±2.5 μM, an
approximate concentration of the IC50 (40 μM) was used,
if not stated otherwise.
SBHA induces G0/G1
cell cycle arrest in MCF-7 cells
Flow cytometry revealed that, compared with the
untreated control, SBHA-treated MCF-7 cells showed a significant
(P<0.05) increase in the G0/G1 phase
fraction (79.2±2.5 vs. 38.6±2.0%) and a reduction in the S phase
fraction (20.4±0.8 vs. 58.8±1.4%; Fig.
2A and B). Notably, SBHA appeared to induce apoptosis in MCF-7
cells, as evidenced by the appearance of a sub-G1 fraction
(Fig. 2A). Western blot analysis
demonstrated that SBHA treatment markedly raised the protein levels
of p21 and p27 compared with those of the untreated cells (Fig. 2C).
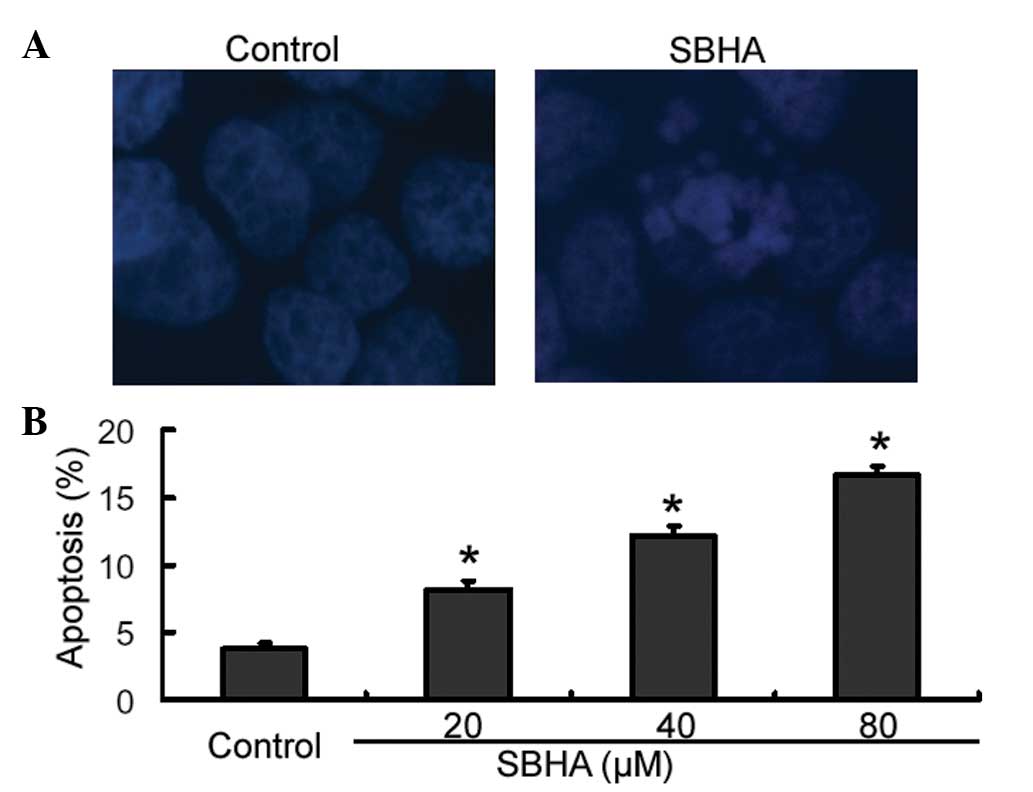
SBHA promotes apoptosis in MCF-7
cells
Cell apoptosis was confirmed using Hoechst 33258
staining. The results showed that SBHA-treated cells displayed cell
shrinkage, chromosomal condensation and nuclear fragmentation,
which are hallmark morphological changes of apoptosis (Fig. 3A). By contrast, untreated control
cells had a normal morphology (Fig.
3A). For further quantification of apoptosis, cells were
stained with Annexin-V and PI and analyzed by flow cytometry. As
shown in Fig. 3B, treatment with
SBHA at different concentrations for 72 h caused significant
concentration-dependent induction of apoptosis in MCF-7 cells
relative to that of the untreated control cells (P<0.05).
SBHA reduces the Bcl-2 expression and
increases the Bax expression
Western blot analysis revealed that there was a
marked reduction in the Bcl-2 protein expression level and a
concomitant elevation in the Bax protein expression level in
SBHA-treated MCF-7 cells, compared with those of the untreated
control (Fig. 4).
Discussion
HDAC inhibitors have been extensively investigated
for their anticancer activities (13). SBHA is a relatively novel HDAC
inhibitor that has demonstrated growth-suppressive effects in
several types of cancer, including medullary thyroid (10) and lung cancer (11). The results of the present study
showed that SBHA impeded the proliferation of MCF-7 breast cancer
cells, with an IC50 value of 33.9±2.5 μM after 72 h
treatment. The antiproliferative potency of SBHA in MCF-7 cells
appears to be less than that of SAHA, which has an IC50
value of 2.4 μM in tamoxifen-resistant MCF-7 cells after 48 h
treatment (9). Induction of cell
cycle arrest is an important mechanism for proliferation
inhibition. Notably, the current study revealed that SBHA treatment
caused a significant cell cycle arrest at the
G0/G1 phase in MCF-7 cells. Furthermore, it
was determined that SBHA-treated MCF-7 cells had a marked elevation
in the expression levels of p21 and p27 proteins, compared with
those of the control cells. p21 and p27 are universal inhibitors of
cyclin-dependent kinases and are thus involved in cell cycle
control (14). Jiang et al
(15) reported that genetic
delivery of p21 and p27 suppresses the proliferation of MCF-7
breast cancer cells in vitro. Induction of p21 and p27
contributes to the growth-inhibitory effect of signal transducer
and activator of transcription 6 overexpression in breast cancer
cells (16). These findings
suggest that the antiproliferative activity of SBHA in breast
cancer cells is, at least partially, mediated through upregulation
of p27 and p21. SBHA-mediated cell cycle arrest has also been
observed in carcinoid cancer cells (17). However, the molecular mechanisms
involved in the induction of p21 and p27 by SBHA remain to be
further defined.
Apoptosis is known as an active suicidal response
that has an important role in tumor biology (18). It is characterized by cellular
shrinkage without loss of plasma membrane integrity, formation of
apoptotic bodies and nuclear condensation, and fragmentation.
Maintenance of plasma membrane integrity during apoptosis prevents
the onset of an inflammatory response that contributes to tumor
progression (19). Therefore,
specific induction of apoptosis represents the preferred strategy
for destroying tumor cells. Notably, the results of the current
study demonstrated that SBHA-treated MCF-7 cells displays apoptotic
morphological changes as determined by Hoechst 33258 staining.
Annexin-V/PI staining analysis further revealed that the
pro-apoptotic effect of SBHA occurred in a concentration-dependent
manner. These results are consistent with a previous study that
demonstrated the induction of MCF-7 cell apoptosis by SBHA via a
p53-dependent pathway (12).
p53-dependent induction of apoptosis is causally
associated with its transcriptional regulation of numerous target
genes (19). Bax is a downstream
target gene of p53 that mediates p53-dependent apoptosis. It has
been documented that Bax deficiency impairs p53-induced apoptosis
in neurons (20). The upregulation
of Bax is implicated in HDAC inhibitor-induced apoptosis in breast
cancer cells (21). For instance,
Wang et al (21) reported
that sirtinol, a class III HDAC inhibitor, induces apoptotic death
in MCF-7 cells through upregulation of Bax. SBHA has also been
documented to enhance the expression of Bax in MCF-7 cells, which
contributes to p53-dependent apoptosis (12). In agreement with this study, the
results of the present study showed that SBHA-treated MCF-7 cells
exhibited a significant increase in the Bax protein level.
Furthermore, SBHA treatment significantly inhibited the expression
of Bcl-2 in MCF-7 cells. Bax is a pro-apoptotic member of the Bcl-2
family. It undergoes mitochondrial intramembranous
homo-oligomerization in response to apoptotic stimuli, which
promotes release of cytochrome c from the mitochondria,
consequently activating the mitochondrial apoptotic pathway
(22). The anti-apoptotic protein
Bcl-2 is predominantly localized in the mitochondria and interacts
with Bax to inhibit its activation (23). The results of the present study
indicate that the pro-apoptotic activity of SBHA is associated with
the modulation of the Bcl-2 family members. Additionally, in A549
lung cancer cells, SBHA-mediated alteration of the Bcl-2 family
proteins has been reported, which leads to caspase-dependent
apoptosis (11).
In conclusion, the current study revealed that SBHA
exerts anticancer effects on breast cancer cells through induction
of G0/G1 cell-cycle arrest and apoptosis. The
modulation of p21, p27 and the Bcl-2 family proteins is involved in
the cytotoxic activity of SBHA. These findings warrant further
investigation of the therapeutic potential of SBHA in animal models
of breast cancer.
Acknowledgements
This study was supported by the Scientific
Foundation of Shanghai Municipal Health Bureau (grant no.
2012–236).
References
|
1
|
Youlden DR, Cramb SM, Dunn NA, Muller JM,
Pyke CM and Baade PD: The descriptive epidemiology of female breast
cancer: an international comparison of screening, incidence,
survival and mortality. Cancer Epidemiol. 36:237–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff SJ: Triple-negative breast cancer:
role of specific chemotherapy agents. Cancer J. 16:53–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuda H, Sano N, Muto S and Horikoshi M:
Simple histone acetylation plays a complex role in the regulation
of gene expression. Brief Funct Genomic Proteomic. 5:190–208. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Federico M and Bagella L: Histone
deacetylase inhibitors in the treatment of hematological
malignancies and solid tumors. J Biomed Biotechnol. 2011:475642011.
View Article : Google Scholar
|
|
5
|
Khan O and La Thangue NB: HDAC inhibitors
in cancer biology: emerging mechanisms and clinical applications.
Immunol Cell Biol. 90:85–94. 2012. View Article : Google Scholar
|
|
6
|
Rikiishi H: Autophagic and apoptotic
effects of HDAC inhibitors on cancer cells. J Biomed Biotechnol.
2011:8302602011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mork CN, Faller DV and Spanjaard RA: A
mechanistic approach to anticancer therapy: targeting the cell
cycle with histone deacetylase inhibitors. Curr Pharm Des.
11:1091–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alao JP, Stavropoulou AV, Lam EW and
Coombes RC: Role of glycogen synthase kinase 3 beta (GSK3beta) in
mediating the cytotoxic effects of the histone deacetylase
inhibitor trichostatin A (TSA) in MCF-7 breast cancer cells. Mol
Cancer. 5:402006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YJ, Won AJ, Lee J, Jung JH, Yoon S,
Lee BM and Kim HS: Molecular mechanism of SAHA on regulation of
autophagic cell death in tamoxifen-resistant MCF-7 breast cancer
cells. Int J Med Sci. 9:881–893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ning L, Jaskula-Sztul R, Kunnimalaiyaan M
and Chen H: Suberoyl bishydroxamic acid activates notch1 signaling
and suppresses tumor progression in an animal model of medullary
thyroid carcinoma. Ann Surg Oncol. 15:2600–2605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
You BR and Park WH: Suberoyl bishydroxamic
acid inhibits the growth of A549 lung cancer cells via
caspase-dependent apoptosis. Mol Cell Biochem. 344:203–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang ZG, Fei F, Chen Y and Jin W:
Suberoyl bis-hydroxamic acid induces p53-dependent apoptosis of
MCF-7 breast cancer cells. Acta Pharmacol Sin. 29:1459–1466. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hrabeta J, Stiborova M, Adam V, Kizek R
and Eckschlager T: Histone deacetylase inhibitors in cancer
therapy. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub 2013. 158:161–169. 2014.
|
|
14
|
Yoon MK, Mitrea DM, Ou L and Kriwacki RW:
Cell cycle regulation by the intrinsically disordered proteins p21
and p27. Biochem Soc Trans. 40:981–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang D, Wang X, Liu X and Li F: Gene
delivery of cyclin-dependent kinase inhibitors p21 (Waf1) and p27
(Kip1) suppresses proliferation of MCF-7 breast cancer cells in
vitro. Breast Cancer. 21:614–623. 2013. View Article : Google Scholar
|
|
16
|
Wei M, Liu B, Gu Q, Su L, Yu Y and Zhu Z:
Stat6 cooperates with Sp1 in controlling breast cancer cell
proliferation by modulating the expression of p21 (Cip1/WAF1) and
p27 (Kip1). Cell Oncol (Dordr). 36:79–93. 2013. View Article : Google Scholar
|
|
17
|
Greenblatt DY, Cayo M, Ning L, et al:
Suberoyl bishydroxamic acid inhibits cellular proliferation by
inducing cell cycle arrest in carcinoid cancer cells. J
Gastrointest Surg. 11:1515–1520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L and Yu J: Role of apoptosis in
colon cancer biology, therapy, and prevention. Curr Colorectal
Cancer Rep. 9:2013. View Article : Google Scholar
|
|
19
|
Beckta JM, Ahmad SF, Yang H and Valerie K:
Revisiting p53 for cancer-specific chemo- and radiotherapy: Ten
years after. Cell Cycle. 13:2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cregan SP, MacLaurin JG, Craig CG,
Robertson GS, Nicholson DW, Park DS and Slack RS: Bax-dependent
caspase-3 activation is a key determinant in p53-induced apoptosis
in neurons. J Neurosci. 19:7860–7869. 1999.PubMed/NCBI
|
|
21
|
Wang J, Kim TH, Ahn MY, et al: Sirtinol, a
class III HDAC inhibitor, induces apoptotic and autophagic cell
death in MCF-7 human breast cancer cells. Int J Oncol.
41:1101–1109. 2012.PubMed/NCBI
|
|
22
|
Danial NN: BCL-2 family proteins: critical
checkpoints of apoptotic cell death. Clin Cancer Res. 13:7254–7263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Dai Y, Pei XY and Grant S: Bim
upregulation by histone deacetylase inhibitors mediates
interactions with the Bcl-2 antagonist ABT-737: evidence for
distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol.
29:6149–6169. 2009. View Article : Google Scholar : PubMed/NCBI
|