Introduction
Tendon injury is a frequent problem for recreational
and competitive athletes. Individuals, who live sedentary
lifestyles may also develop tendinopathy in the absence of any
history of increased physical activity (1). An estimated 30–50% of all
sports-associated injuries are caused by a disorder of the tendons
(2). This injury may be caused by
intrinsic or extrinsic factors, either alone or in combination.
Extrinsic factors lead to the majority of acute tendon injuries,
although in overuse syndromes, including tendinopathy,
multifactorial combinations of intrinsic factors, such as
age-associated cell activity changes and extrinsic factors,
including overuse, repetitive strain injury and microtrauma may be
the cause (3).
Previous studies have indicated that
histopathological changes occur with tendinopathy and are
associated with degeneration and disorganization of collagen
fibers, increased cellularity and minimal inflammation (4). Macroscopic changes include thickening
of the tendon, loss of mechanical properties and pain. Previous
studies have demonstrated that several changes occur in response to
overuse, including the production of matrix metalloproteinases,
cytokines, tendon cell apoptosis, chondroid metaplasia of the
tendon, collagen, glycosaminoglycan and expression of protective
factors (5,6).
Currently, the non-surgical therapies available to
patients who suffer from tendinopathies are exercise-based physical
therapy, ultrasound, non-steroidal anti-inflammatory drugs and
steroid or platelet-rich plasma injections (7). However, these therapies offer
symptomatic relief, but do not result in definitive disease
resolution. Through understanding the cellular and molecular
mechanisms of causation and novel therapeutic targets, small
molecules could potentially be identified for drug development.
This may result in the development of more effective treatments,
while minimizing side effects.
Microarray analysis supports the identification of
drug-sensitive genes and the chemical substructures associated with
specific genetic responses. It has become a powerful tool in drug
development (8). In the present
study, microarrays were utilized to identify differentially
expressed genes (DEGs) between normal and degenerating tendon
cells. The functions of DEGs were investigated by annotating to
biological processes and pathways. Several target sites of the
transcription factors and certain regulatory microRNAs were also
screened. This information may assist in elucidating the molecular
mechanism of tendon injuries. In addition, candidate small
molecules were identified for the potential treatment of
tendinopathy.
Materials and methods
Derivation of genetic data
The gene expression profile of GSE26051 (7) was downloaded from a public functional
genomics data repository, the Gene Expression Omnibus (GEO;
www.ncbi.nlm.nih.gov/geo/) database. A total of
46 specimens, including 23 normal samples and 23 tendinopathy
specimens, were available based on the GPL570 platform. This
information was approved by the ethics committee of the Hospital
for Special Surgery (New York City, NY, USA).
DEG analysis
The derived genetic data was analyzed using the
GEOquery (www.bioconductor.org/packages/release/bioc/html/GEOquery.html)
and Limma (www.bioconductor.org/packages/release/bioc/html/limma.html)
packages in the R programming language (v.2.13.0) (9). Geoquery can quickly access the
expression profiling data on the GEO database, while Limma is the
most popular method of statistical analysis to analyze the DEGs
(10). The preprocessed microarray
data were obtained by Geoquery package and then a log2
transformation was performed. The Limma package, a linear
regression model, was applied to compare the normal samples and
tendinopathy samples. Only the genes with P<0.05 were identified
as DEGs.
Gene Ontology (GO) enrichment
analysis
GO analysis has become a common approach for the
functional annotation of large-scale genomic data (11). Gene ontology enrichment analysis
software toolkit (GOEAST; omicslab.genetics.ac.cn/GOEAST/) is an
easy-to-use web-based toolkit, which identifies statistically
overrepresented GO terms within provided gene sets (12). GOEAST was utilized for GO
enrichment analysis to identify the locations of DEGs within
cellular compartments and molecular functions affected by DEGs,
based on the hypergeometric distribution, with the false discovery
rate (FDR) <0.001.
Biological pathway enrichment
analysis
Biological pathways were investigated to examine the
tendinopathy cell changes at the molecular level. All metabolic and
non-metabolic pathways were downloaded from the open WikiPathways
database (www.wikipathways.org.) (13,14)
and WikiPathways cluster analysis was conducted (15,16)
to the DEGs using the gene set analysis toolkit V2 platform. A
count number >2 and P<0.05 were selected as the cut-off
criteria.
Examining potential target sites of
transcription factors and potential regulatory microRNAs
Well-annotated gene sets in the molecular signature
database (MsigDB; www.broadinstitute.org/gsea/msigdb/index.jsp) were
subject to gene set enrichment analysis (GSEA) (17). Subsequently, the GSEA results were
statistically accounted for with the hypergeometric distribution.
The consequences were adjusted for multiple testing using the
Benjamini-Hochberg procedure. Finally, the target sites with
FDR<0.01 were selected as the potential target sites that may
regulate transcription factors. Similarly, the potential regulatory
microRNAs were identified with an FDR<0.05.
Identification of candidate small
molecules
The connectivity map (CMap) database contains data
on 7,056 gene-expression profiles, involving 6,100 small molecule
treatment-control pairs (18). The
DEGs were divided into up- and downregulated groups. Subsequently,
these genes were subjected to GSEA and compared with the DEGs in
the CMap database. Finally, a correlation score for each
perturbagen was calculated, ranging between −1 and +1 (19).
Results
DEG selection
In order to analyze differentially expressed genes
between cells in tendinopathy and normal controls, a publicly
available microarray dataset, GSE26051 was obtained and a classical
t-test, corrected for multiple comparisons was performed. A total
of 419 probes were considered to be differentially expressed in
tendinopathy samples when compared with normal control tendons
(P<0.001), which corresponded to 318 DEGs.
GO enrichment analysis of DEGs
To investigate the functional changes in the
pathological process of tendinopathy, the DEGs were mapped to the
GO database. The project provided three structured networks of
defined terms to describe gene product attributes: biological
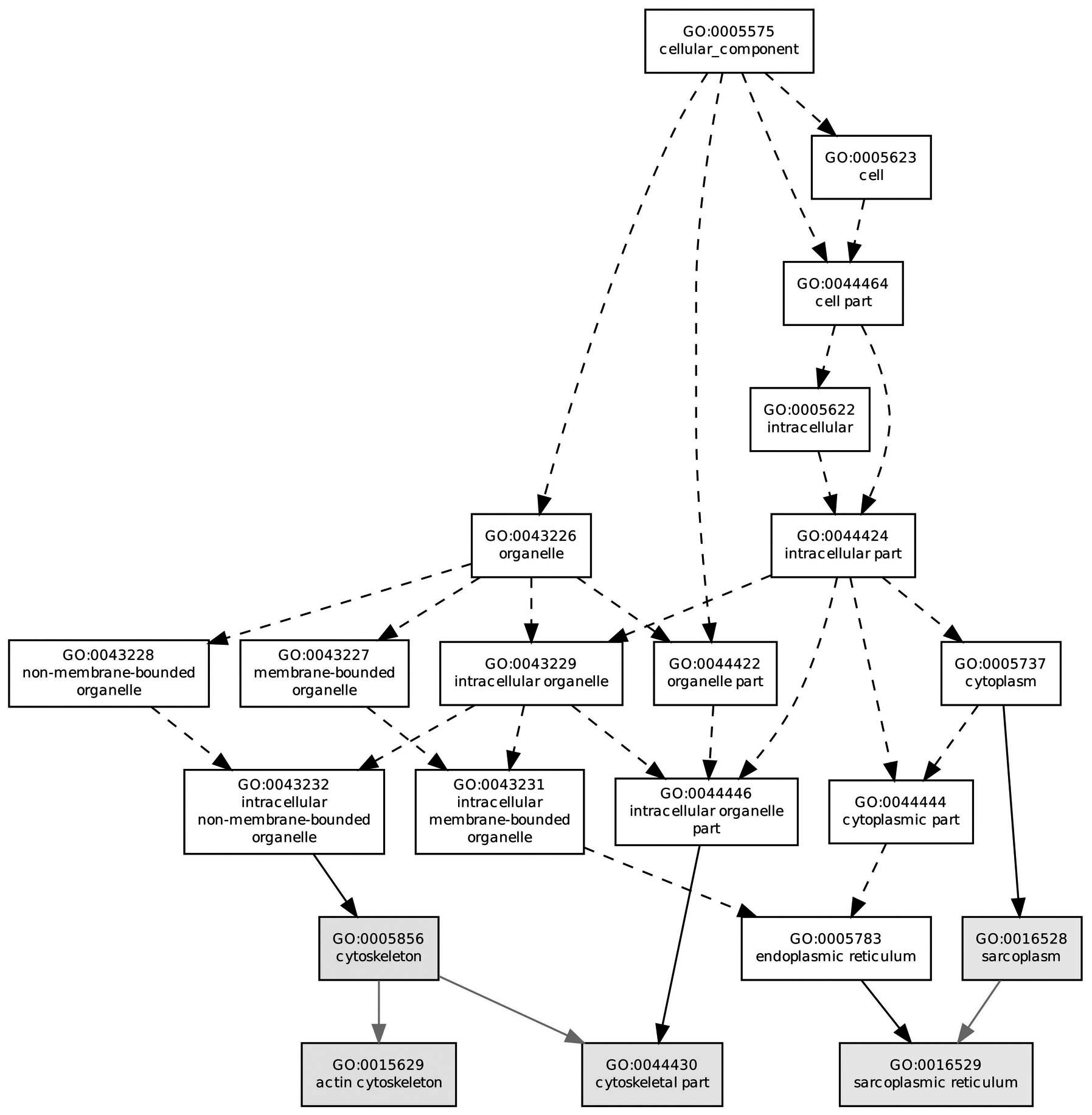
process, molecular function and cellular compartment. Fig. 1 reveals the molecular function in
which the majority of the DEGs were located, such as the
cytoskeleton, actin cytoskeleton and sarcoplasm. In addition,
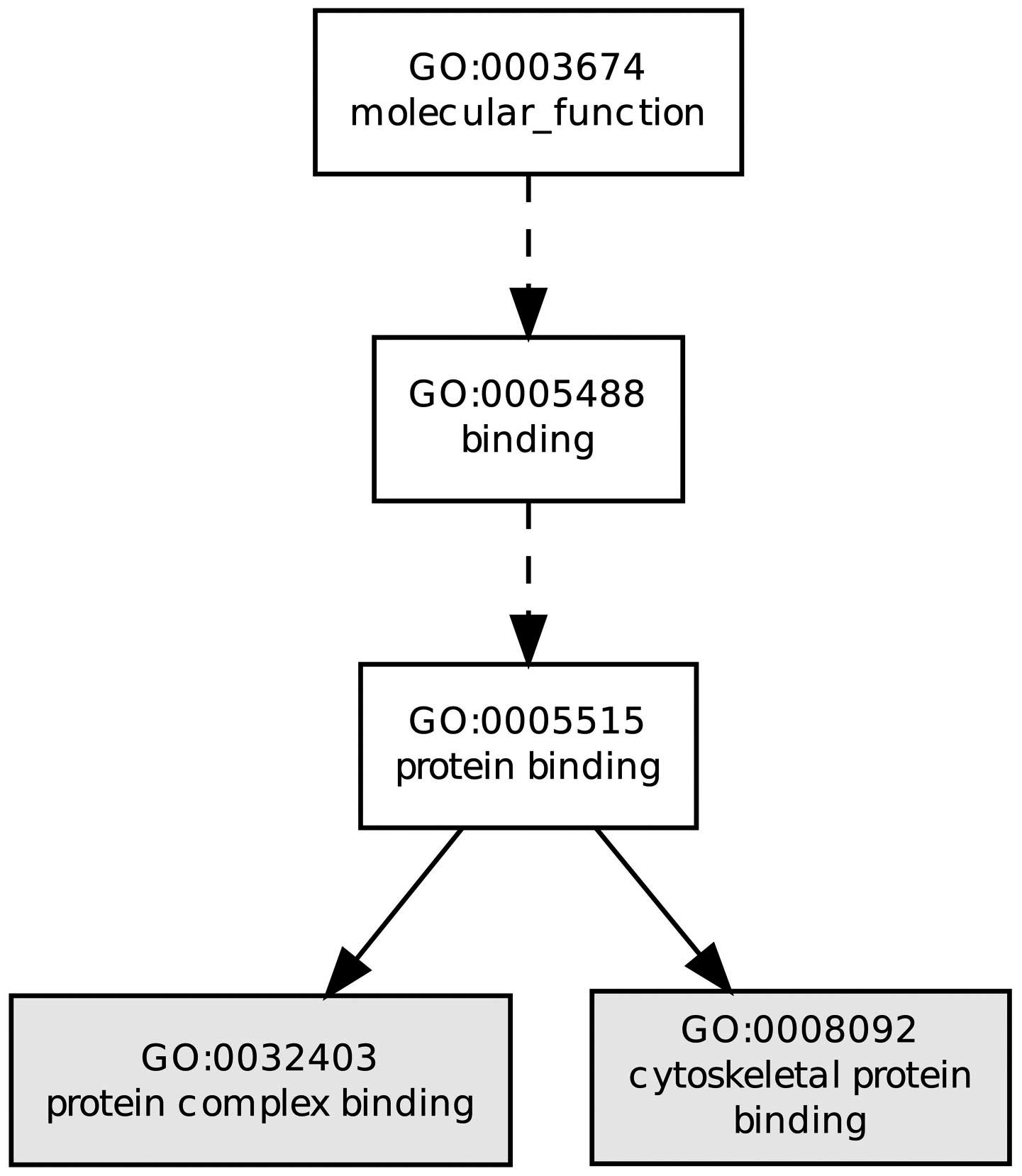
Fig. 2 shows the biological
processes of DEGs, for instance, protein complex binding and
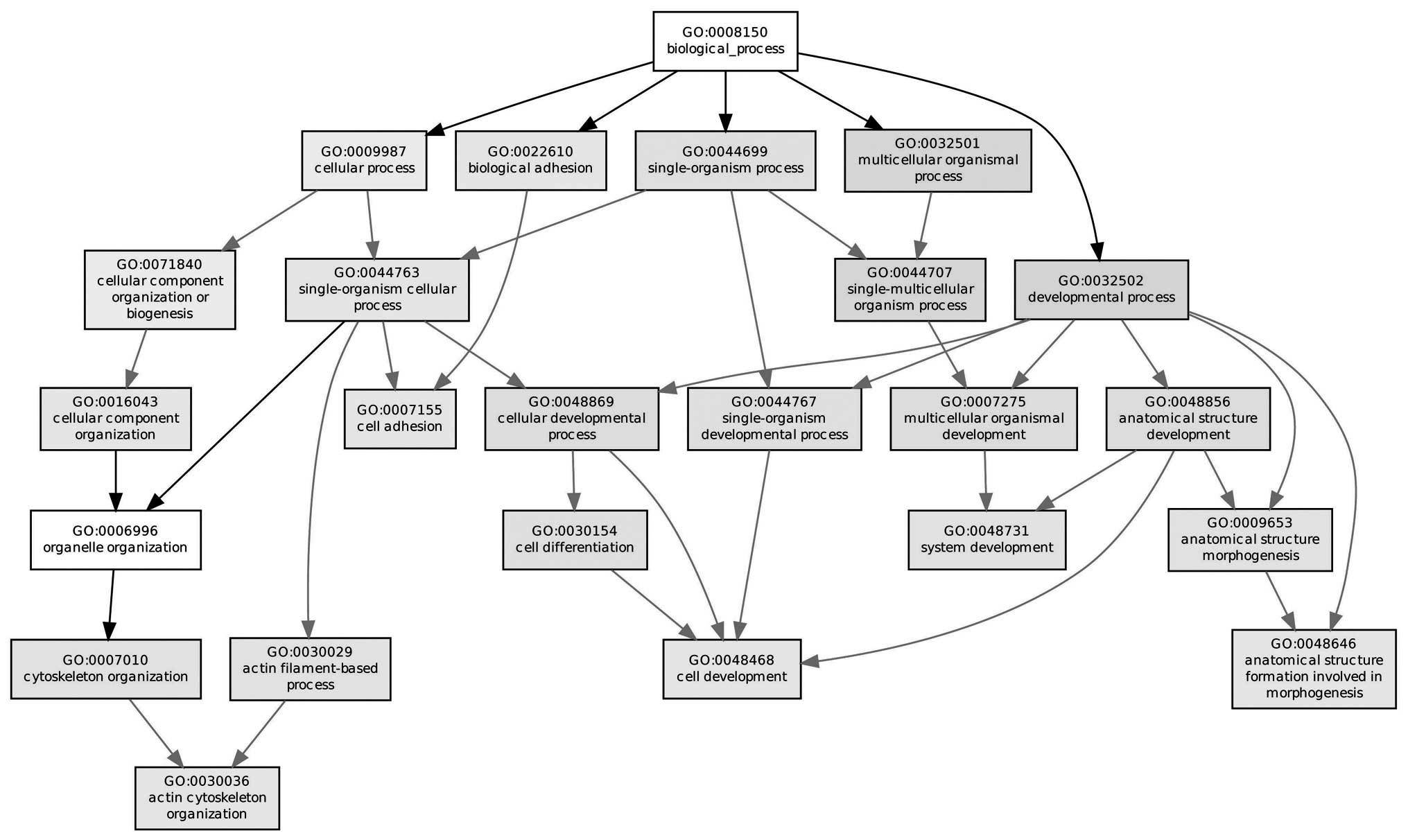
cytoskeletal protein binding. The majority of enriched GO
biological processes of the DEGs between normal and pathological
specimens were associated with a particular cellular compartment
(Fig. 3), for example,
multicellular organismal processes, developmental processes and
single-multicellular organism processes.
Pathway enrichment analysis
To gain further insights into the changes of
biological pathways in cells of tendinopathy, the WikiPathways
cluster analysis was used to identify the significant pathways
associated with DEGs. P<0.05 and counts, which were >2 were
selected as the cut-off criteria. A total of 10 pathways were
identified and the main 8 pathways with a highly significant
correlation are listed in Table I.
The most significant pathway was focal adhesion with P=7.08E-5 and
the genes enriched in focal adhesion were laminin, α4 (LAMA4),
platelet-derived growth factor α (PDGFA), laminin γ1 (LAMC1) and
Src homology 2 transforming protein 1 (SHC1).
 | Table IEnriched biological pathways
(P<0.05). The main eight pathways are listed. |
Table I
Enriched biological pathways
(P<0.05). The main eight pathways are listed.
| Pathway | Genes | P-value |
|---|
| Focal adhesion | LAMA4, PDGFA, LAMC1,
SHC1 | 7.50
×10−0.5 |
| Integrin-mediated
cell adhesion | ITGA5, SORBS1, ILK,
SHC1 | 0.031 |
| Myometrial relaxation
and contraction pathways | YWHAZ, CRHR1, GRK5,
PRKCG | 0.074 |
| Insulin
signaling | PRKAA2, SORBS1, SGK2,
CAP1 | 0.085 |
| Osteoclast | TNFSF11, CTSK | 0.0171 |
| Serotonin receptor
2-> ELK-SRF/GATA4 signaling | HTR2A, ITPR1 | 0.0248 |
| Delta-notch signaling
pathway | YWHAZ, LAMC1,
SHC1 | 0.0342 |
| L-3 Signaling
pathway | YWHAZ, CHEK1,
SHC1, | 0.0582 |
Examining potential target sites
As an important regulatory element, transcription
factors can regulate gene expression. Taking upstream sequences of
the DEGs as the analyzed object, the potential target sites of the
transcription factor were examined. The main 20 target sites with a
highly significantly correlation are listed in Table II. The most significant
transcription factors were lymphoid enhancer-binding factor 1
(LEF1) and OCT1, in which LEF1 may regulate the pantothenate kinase
2 (PANK2) and G protein-coupled receptor kinase 5 (GRK5) by binding
the target sequence CTTTGT.
 | Table IIEnriched potential target sites of
transcription factors. |
Table II
Enriched potential target sites of
transcription factors.
| Target | Genes | P-value |
|---|
|
hsa_CTTTGT_V$LEF1_Q2 | PANK2, SEMA6D, GRK5,
ATOH8 | 2.58
×10−0.5 |
| hsa_V$OCT1_06 | CUGBP2, AGRP, LMO1,
ANK3 | 2.58
×10−0.5 |
| hsa_V$PXR_Q2 | PITX2, HOXD3, SLC6A6,
LMO1 | 0.0001 |
|
hsa_AACTTT_UNKNOWN | PRKAA2, PFKFB1,
SORBS1, TRDN | 0.0001 |
|
hsa_CAGGTG_V$E12_Q6 | EMX1, SORBS1, LMO1,
CDKN1C | 0.0001 |
|
hsa_TGACAGNY_V$MEISI_01 | ELOVL5, DPF3, DNAJA4,
ESRRG | 0.0001 |
| hsa_V$NKX25_02 | PITX2, FOXP2, DPF3,
LRP1B | 0.0004 |
|
hsa_CATTGTYY_V$SOX9_B1 | FOXP2, DPYSL5,
HOXD3, GRK5 | 0.0004 |
| hsa_V$MEISI_01 | FOXP2, DPF3,
SORBS1, EPHB2 | 0.0005 |
| hsa_V$OCT1_02 | FOXP2, PFKFB1,
DNAJA4, TRDN | 0.0005 |
|
hsa_V$POU6F1_01 | PITX2, FOXP2,
CUGBP2, EPHB2 | 0.0005 |
|
hsa_SMTTTTGT_UNKNOWN | DPYSL5, DPF3, LMO1,
RCOR1 | 0.0006 |
|
hsa_V$FREAC3_01 | FOXP2, MMP11,
ATOH8, LEF1 | 0.0006 |
|
hsa_TTGTTT_VFOXO4_01 | SORBS1, GRK5,
ATOH8, MPZL1 | 0.0013 |
| hsa_V$SMAD4_Q6 | ELOVL5, MMP11,
LMO1, CBFA2T3 | 0.0013 |
|
hsa_V$AP1_Q6_01 | LRP1B, EPHB2,
LAMC1, ATP6V1A | 0.0013 |
| hsa_V$WHN_B | FOXP2, LRP1B, LEF1,
ARPC5 | 0.002 |
|
hsa_AAANWWTGC_UNKNOWN | FOXP2, DPYSL5,
EPHB2, BNC2 | 0.0028 |
| hsa_V$PITX2_Q2 | PITX2, FOXP2,
PDGFA, DPYSL5 | 0.0028 |
|
hsa_V$PTF1BETA_Q6 | FOXP2, CRHR1,
CALD1, EEF1A2 | 0.0038 |
|
hsa_YATGNWAAT_V$OCT_C | PITX2, PFKFB1,
SORBS1, LLGL2 | 0.0038 |
|
hsa_TATAAA_V$TATA_01 | PRKAA2, HOXD3,
DNAJA4, SLC2A3 | 0.0038 |
| hsa_V$EN1_01 | FOXP2, NRG1, HOXD3,
LMO1 | 0.0038 |
|
hsa_GCCNNNWTAAR_UNKNOWN | FOXP2, DPF3, ITPR1,
ETV6 | 0.0038 |
| hsa_V$CHX10_01 | PITX2, FOXP2, LMO1,
BACE2 | 0.0038 |
| hsa_V$SMAD3_Q6 | PFKFB1, CHDH,
CBFA2T3, ESRRG | 0.0038 |
| hsa_V$SRF_C | FOXP2, DUSP2, CAP1,
CALD1 | 0.0038 |
|
hsa_GCANCTGNY_V$MYOD_Q6 | DPYSL5, HOXD3,
DPF3, EMX1 | 0.0038 |
|
hsa_V$HP1SITEFACTOR_Q6 | PITX2, FOXP2,
HOXD3, ESRRG | 0.0048 |
|
hsa_WGTTNNNNNAAA_UNKNOWN | CKC25A, DPF3, EMX1,
ATOH8 | 0.0048 |
| hsa_V$TBP_01 | PRKAA2, DNAJA4,
TRDN, ESRRG | 0.0048 |
|
hsa_TGACATY_UNKNOWN | ELOVL5, MICAL2,
DPF3, SORBS1 | 0.0048 |
| hsa_V$ATF1_Q6 | PDGFA, LEOVL5,
ESRRG, CALD1 | 0.0048 |
|
hsa_RTAAACA_V$FREAC2_01 | GRK5, CDKN1C,
ESRRG, BNC2 | 0.0055 |
| hsa_V$DR4_Q2 | ARHGAP24, FOXP2,
LAMA4 | 0.0055 |
|
hsa_TAATTA_V$CHX10_01 | CUGBP2, LMO1,
LAMC1, TRDN | 0.0055 |
| hsa_V$GATA6_01 | PITX2, PFKFB1,
SORBS1, COL4A3 | 0.0055 |
|
hsa_GGGAGGRR_V$MAZ_Q6 | SORBS1, GRK5,
MAPRE3, MTNR1B | 0.0055 |
|
hsa_V$MF2_Q6_01 | FOXP2, PRKAA2,
DNAJA4, MYOG | 0.0055 |
| hsa_VTST1_01 | PITX2, PDGFA,
LAMA4, HOXD3 | 0.0065 |
|
hsa_GGGTGGRR_V$PAX4_03 | DPYSL5, PFKFB1,
ITPKB, ATOHB | 0.0065 |
|
hsa_TGCCAAR_V$NF1_06 | DUSP2, COL4A3,
ESRRG, BNC2 | 0.0074 |
|
hsa_CAGCTG_V$AP4_Q5 | PRKAA2, HOXD3,
SORGS1, GRK5 | 0.0074 |
| hsa_V$AR_Q6 | PITX2, FOXP2,
HOXD3, LMO1 | 0.0085 |
| hsa_V$CEBP_Q2
_01 | PITX2, CDKN1C,
CALD1, BNC2 | 0.0093 |
| hsa_V$ER_Q6 | PRKAA2, ATP6V1A,
ESRRG, CA4 | 0.0093 |
Examining the potential regulatory
microRNA
MicroRNAs are involved in the regulation of numerous
cellular processes by adjusting the stability of mRNA. The
potential regulatory microRNAs were screened out based on the
sequences of DEGs. The main 20 instances with a highly significant
correlation were enumerated in Table
III. The most significant microRNAs were in the miR-499 and
miR-200 family, including miR-200B, miR-200C and miR-429. miR-499
may regulate the CUGBP2 and MYB genes by binding the target
sequence AGTCTTA and the miR-200 family may regulate the LRP1B and
SLC6A6 genes by binding CAGTATT.
 | Table IIIEnriched potential regulatory
microRNAs. |
Table III
Enriched potential regulatory
microRNAs.
| Target
sequence | Potential
microRNA | Genes | P-value |
|---|
| hsa_AGTCTTA | miR-499 | CUGBP2, KLHDC5,
FAM60A | 0.0247 |
| hsa_CAGTATT | miR-200B, miR-200C,
miR-429 | LRP1B, SLC6A6,
LAMC1, | 0.0247 |
| hsa_GAGCCAG | miR-149 | COL4A3, ACLY,
RAP1B | 0.0247 |
| hsa_GTGCAAA | miR-507 | SEMA6D, HECW1,
LEF1 | 0.0296 |
| hsa_ATACTGT | miR-144 | CUGBP2, KPNA1,
ESRRG | 0.0296 |
| hsa_GCAAGGA | miR-502 | PANK2, HOXD3,
LEF1 | 0.0317 |
| hsa_TGCACTT | miR-519C, miR-519B,
miR-519A | ARHGAP24, TNFSF11,
WDR1 | 0.0317 |
| hsa_TTGGGAG | miR-150 | MMP19, NOTCH3,
EPHB2 | 0.0444 |
| hsa_CACTTTG | miR-520G,
miR-520H | DPYSL5, TNFSF11,
KPNA1 | 0.0444 |
| hsa_ATAAGCT | miR-21 | PITX2, ARHGAP24,
CDC25A | 0.049 |
Identification of candidate small
molecules
In order to screen small molecule drugs,
computational bioinformatics analysis of DEGs was performed using
CMap. A total of 20 associated small molecules with a highly
significant correlation are listed in Table IV, including 13
negatively-associated molecules and seven positively-associated
small molecules. Among these molecules, Prestwick-1082 and
Viomycin, with the highest negative correlation had the potential
to treat the tendinopathy.
 | Table IVEnriched significant small
molecules. |
Table IV
Enriched significant small
molecules.
| Connectivity map
name | Enrichment
score | P-value |
|---|
|
Propylthiouracil | 0.91 | 0.00006 |
|
Sulfadimethoxine | −0.867 | 0.00008 |
| Monensin | −0.815 | 0.00012 |
| Viomycin | −0.876 | 0.00052 |
| Nadolol | −0.872 | 0.00056 |
| Cycloserine | −0.857 | 0.00056 |
| Lisuride | −0.782 | 0.00088 |
| Medrysone | 0.728 | 0.00107 |
| Luteolin | 0.832 | 0.00121 |
| Adiphenine | −0.752 | 0.00174 |
|
Diethylstilbestrol | −0.681 | 0.0028 |
|
Alpha-estradiol | 0.433 | 0.00295 |
|
Podophyllotoxin | −0.802 | 0.003 |
|
Etiocholanolone | −0.678 | 0.003 |
| Scopoletin | 0.956 | 0.00344 |
| Omeprazole | 0.795 | 0.00346 |
| Resveratrol | 0.557 | 0.00348 |
|
Fuldrocortisone | −0.591 | 0.00357 |
| Prestwick-1082 | −0.878 | 0.00363 |
| Prestwick-983 | −0.874 | 0.00403 |
Discussion
Tendinopathy is a critical clinical problem as it is
often asymptomatic at onset and during development, and is only
recognized upon rupture of the tendon (20). Therefore, there is an urgent
requirement to investigate the mechanism of tendinopathy and
develop a mechanism to effectively prevent the condition or a
treatment for it. In the present study, bioinformatics methods were
used to investigate the molecular mechanism of tendinopathy and
identify small molecule drugs, which have the potential to treat
this condition. The results revealed that the expression of 318
genes were altered in the human samples of tendinopathy compared
with normal tendons. These genes were mainly involved in pathways
associated with adhesion. Furthermore, it was demonstrated that
Prestwick-1082 and Viomycin may be effective for the treatment of
tendinopathy.
The gene expression analysis, which focussed on
identifying individual genes, which exhibited differences between
two states, although useful, may be unable to detect biological
processes, including metabolic pathways, transcriptional programs
and stress responses, which are distributed across an entire
network of genes and less detectable at the level of individual
genes (17). Current approaches
typically study entire pathways, whether through using singular
enrichment analysis or by gene set enrichment analysis. In the
present study, eight pathways were identified and focal adhesion
was observed to be the most significant pathway in the development
of tendinopathy. Focal adhesions lie at the convergence of integrin
adhesion, signaling and the actin cytoskeleton (21). Genes in the integrin family,
including LAMA4, PDGFA, LAMC1 and SHC1, are closely associated with
focal adhesion. Among this family, the downregulation of LAMA4 may
affect cell survival rate via lamin-integrin interaction (22) and LAMA4-deficient mice have
previously been reported to develop a defect in endothelial cell
viability, followed by cardiac hypertrophy and heart failure
(23). For PDGFA, it may induce
tyrosine phosphorylation of focal adhesion kinase, a member of the
focal adhesion complex family. The PDGFA receptor acts as a high
affinity binding site for several signaling molecules leading to
activation of Ras, followed by activation of Raf, mitogen-activated
protein kinase and extracellular signal-regulated kinase (24). This complex interacts with
extracellular matrix proteins through integrin interactions,
providing a direct sensor to the integrity and composition of the
extracellular environment (25).
Besides, LAMC1 belongs to the Lamins, a family of extracellular
matrix glycoproteins, which are the major noncollagenous components
of basement membranes. LAMC1 has been implicated in a wide variety
of biological processes, including cell adhesion, differentiation,
migration, signaling, neurite outgrowth and metastasis. SHCI has
been reported to be involved in the aging process, a signaling
pathway inducing elevation of extracellular oxidant levels,
cytochrome c release and apoptosis, as well as the oxidative
stress response (26). Consistent
with the present findings, Riley (3) suggested that tendon matrix damage is
the primary event, overwhelming the ability of the resident cell
population to repair structural defects and degradation of the
extracellular matrix may affect the structural properties of the
tendon. Previous studies (27,28)
have also reported that fibronectin is markedly increased following
tendon injury when compared with the levels in the normal tendon
and consequently has been implicated in cell adhesion, migration
and differentiation at the site of injury. Therefore, the present
study indicates that these integrin genes associated with focal
adhesion have crucial roles in tendinopathy development.
Numerous studies have reported an abundance of
transcription factors associated with human disease, thus making
them targets for the investigation of the mechanisms of
tendinopathy. In the present study, LEF1 was identified as one of
the most significant transcription factors, which binds the target
sequence: GTTTGT. A number of genes, including PANK2 and GRK5,
which contain this sequence, can be identified by LEF1. PANK2 is a
mitochondrial enzyme, which catalyzes the first regulatory step of
coenzyme A synthesis and that is processed and active in the
mitochondria (29). Mutations in
PANK2 may lead to a variety of metabolic defects (30). Semaphorin 6D has been found to be
involved in cardiac morphogenesis, cancer and immune responses
(31).
MicroRNAs are small regulatory RNAs, which regulate
the translation and degradation of target mRNAs and are extensively
involved in human disease (32).
The most significant microRNA in the present study was miR-499 and
its targeting sequence was AGTCTTA. The genes, including CUGBP2 and
MYB, which contained this sequence can be regulated by miR-499.
CUGBP2 is an RNA-binding protein, which regulates mRNA translation
and is abundant in the skeletal muscle (33). Ectopic overexpression of this
protein may also induce apoptosis (34). Similar to MYB, it is an important
regulator in the control of cell proliferation, apoptosis and
differentiation, is highly expressed in immature, proliferating
cells and is downregulated as cells become further differentiated.
Tenocyte apoptosis has been observed to occur at an increased
frequency in tendinopathy specimens (7).
There are several important implications of the
present study. The identification of a group of small molecules
with potential therapeutic efficacy for tendinopathy is an
important observation. The data in Table IV show that the small molecules of
Prestwick-1082 (enrichment score=-0.878) and viomycin (enrichment
score=-0.876) were associated with significant negative scores,
which suggest that these small molecules may be used as therapeutic
drugs for tendinopathy.
Viomycin is an RNA-binding peptide antibiotic, which
inhibits prokaryotic protein synthesis and group I intron
self-splicing (35). It has a
marked selectivity for RNAs, which form pseudoknots, a structure
that may function as a ‘tag’ for recognition by this peptide and
also induces interactions between RNA molecules (36). It has demonstrated promise in the
search for drugs, which may be useful for treating tuberculosis
(37). However, to the best of our
knowledge, there are no previous studies investigating the use of
these compounds as systemic therapies for tendinopathy. The present
observations warrant further study and should generate hypotheses
for laboratory, patient or population-based studies. The small
molecule, Prestwick-1082 (enrichment score=-0.878) was associated
with a significant negative score, which suggested that these small
molecules are potential adjuvant drugs to improve the therapeutic
effect in tendinopathy.
In conclusion, the present study has presented novel
insights into the mechanism and treatment of tendinopathy. DEG
profiles were analyzed using a computational bioinformatics
approach. In addition, a group of small molecules were identified,
which can be exploited as adjuvant drugs to improve treatment,
including Prestwick-1082 and viomycin. Although it may be premature
to suggest that these drugs may be ready for psychiatric clinical
trials, it is clearly a direction that warrants additional
consideration.
References
|
1
|
Magra M and Maffulli N: Genetics: does it
play a role in tendinopathy? Clin J Sport Med. 17:231–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Vos RJ, Weir A, Van Schie HT, et al:
Platelet-rich plasma injection for chronic Achilles tendinopathy: a
randomized controlled trial. JAMA. 303:144–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riley G: The pathogenesis of tendinopathy.
A molecular perspective. Rheumatology (Oxford). 43:131–142. 2004.
View Article : Google Scholar
|
|
4
|
Andres BM and Murrell GA: Treatment of
tendinopathy: what works, what does not, and what is on the
horizon. Clin Orthop Relat Res. 466:1539–1554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soslowsky L, Thomopoulos S, Tun S, et al:
Neer award 1999 Overuse activity injures the supraspinatus tendon
in an animal model: A histologic and biomechanical study. J
Shoulder Elbow Surg. 9:79–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ML and Chen CH: Microarray analysis
of differentially expressed genes in rat frontal cortex under
chronic risperidone treatment. Neuropsychopharmacology. 30:268–277.
2005. View Article : Google Scholar
|
|
7
|
Jelinsky SA, Rodeo SA, Li J, Gulotta LV,
Archambault JM and Seeherman HJ: Regulation of gene expression in
human tendinopathy. BMC Musculoskelet Disord. 12:862011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verducci JS, Melfi VF, Lin S, Wang Z, Roy
S and Sen CK: Microarray analysis of gene expression:
considerations in data mining and statistical treatment. Physiol
Genomics. 25:3552006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Team RC. R: A language and environment for
statistical computing. R foundation for Statistical Computing;
2005
|
|
10
|
Diboun I, Wernisch L, Orengo CA and
Koltzenburg M: Microarray analysis after RNA amplification can
detect pronounced differences in gene expression using limma. BMC
genomics. 7:2522006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hulsegge I, Kommadath A and Smits MA:
Globaltest and GOEAST: Two different approaches for gene ontology
analysis. BMC Proc. 3:S4–S10. 2009. View Article : Google Scholar
|
|
12
|
Zheng Q and Wang XJ: GOEAST: a web-based
software toolkit for Gene Ontology enrichment analysis. Nucleic
Acids Res. 36:W358–W363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelder T, Van Iersel MP, Hanspers K, et
al: WikiPathways: building research communities on biological
pathways. Nucleic Acids Res. 40:D1301–D1307. 2012. View Article : Google Scholar :
|
|
14
|
Pico AR, Kelder T, Van Iersel MP, Hanspers
K, Conklin BR and Evelo C: WikiPathways: pathway editing for the
people. PLoS Biol. 6:e1842008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duncan D, Prodduturi N and Zhang B:
WebGestalt2: an updated and expanded version of the web-based gene
set analysis toolkit. BMC Bioinformatics. 11:P102010. View Article : Google Scholar
|
|
16
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
an integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33:W741–W748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subramanian A, Tamayo P, Mootha VK, et al:
Gene set enrichment analysis: a knowledge-based approach for
interpreting genome-wide expression profiles. Proc Natl Acad Sci
USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamb J, Crawford ED, Peck D, et al: The
connectivity map: using gene-expression signatures to connect small
molecules, genes, and disease. Science. 313:1929–1935. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braconi C, Swenson E, Kogure T, Huang N
and Patel T: Targeting the IL-6 dependent phenotype can identify
novel therapies for cholangiocarcinoma. PLoS ONE. 5:e151952010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joseph M, Maresh CM, Mccarthy MB, et al:
Histological and molecular analysis of the biceps tendon long head
post-tenotomy. J Orthop Res. 27:1379–1385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wozniak MA, Modzelewska K, Kwong L and
Keely PJ: Focal adhesion regulation of cell behavior. Biochim
Biophys Acta. 1692:103–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai KN, Chan EC, Tsai TY, et al:
Cytotoxic effect of recombinant mycobacterium tuberculosis
CFP-10/ESAT-6 protein on the crucial pathways of WI-38 cells. J
Biomed Biotechnol. 2009:9170842009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knöll R, Postel R, Wang J, et al:
Laminin-α4 and integrin-linked kinase mutations cause human
cardiomyopathy via simultaneous defects in cardiomyocytes and
endothelial cells. Circulation. 116:515–525. 2007. View Article : Google Scholar
|
|
24
|
Reif S, Lang A, Lindquist JN, et al: The
role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt
signaling in hepatic stellate cell proliferation and type I
collagen expression. J Biol Chem. 278:8083–8090. 2003. View Article : Google Scholar
|
|
25
|
Carloni V, Pinzani M, Giusti S, et al:
Tyrosine phosphorylation of focal adhesion kinase by PDGF is
dependent on ras in human hepatic stellate cells. Hepatology.
31:131–140. 2000. View Article : Google Scholar
|
|
26
|
Abdelwahed A, Bouhlel I, Skandrani I, et
al: Study of antimutagenic and antioxidant activities of Gallic
acid and 1, 2, 3, 4, 6-pentagalloylglucose from Pistacia lentiscus:
Confirmation by microarray expression profiling. Chem Biol
Interact. 165:1–13. 2007. View Article : Google Scholar
|
|
27
|
Devkota AC and Weinhold PS: Prostaglandin
E2, collagenase, and cell death responses depend on cyclical load
magnitude in an explant model of tendinopathy. Connect Tissue Res.
51:306–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riley G: Chronic tendon pathology:
molecular basis and therapeutic implications. Expert Rev Mol Med.
7:1–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poli M, Derosas M, Luscieti S, et al:
Pantothenate kinase-2 (Pank2) silencing causes cell growth
reduction, cell-specific ferroportin upregulation and iron
deregulation. Neurobiol Dis. 39:204–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leoni V, Strittmatter L, Zorzi G, et al:
Metabolic consequences of mitochondrial coenzyme A deficiency in
patients with PANK2 mutations. Mol Genet Metab. 105:463–471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ebert A, Lamont R, Childs S and Mcfarlane
S: Neuronal expression of class 6 semaphorins in zebrafish. Gene
Expr Patterns. 12:117–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fabbri M, Ivan M, Cimmino A, Negrini M and
Calin GA: Regulatory mechanisms of microRNAs involvement in cancer.
Expert Opin Biol Ther. 7:1009–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki H, Takeuchi M, Sugiyama A, et al:
Alternative splicing produces structural and functional changes in
CUGBP2. BMC Biochem. 13:62012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Natarajan G, Ramalingam S, Ramachandran I,
et al: CUGBP2 downregulation by prostaglandin E2 protects colon
cancer cells from radiation-induced mitotic catastrophe. Am J
Physiol Gastrointest Liver Physiol. 294:G1235–G1244. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wank H, Clodi E, Wallis MG and Schroeder
R: The antibiotic viomycin as a model peptide for the origin of the
co-evolution of RNA and proteins. Orig Life Evol Biosph.
29:391–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vos S, Berrisford DJ and Avis JM: Effect
of magnesium ions on the tertiary structure of the hepatitis C
virus IRES and its affinity for the cyclic peptide antibiotic
viomycin. Biochemistry. 41:5383–5396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stanley RE, Blaha G, Grodzicki RL,
Strickler MD and Steitz TA: The structures of the anti-tuberculosis
antibiotics viomycin and capreomycin bound to the 70S ribosome. Nat
Struct Mol Biol. 17:289–293. 2010. View Article : Google Scholar : PubMed/NCBI
|