Introduction
Schizophrenia is characterized by positive symptoms,
including delusions and hallucinations, negative symptoms such as
avolation and flat affect, and cognitive impairments (1). The cognitive symptoms of
schizophrenia often precede the occurrence of psychosis (2), and their treatment is considered an
improved predictor of therapeutic outcome (3), in particular, cognitive dysfunction
often remains resistant to treatment with current antipsychotics,
even following remission of the psychosis (4). Consequently, developing novel
compounds that demonstrate increased efficacy against cognitive
dysfunction of schizophrenia is urgently required (5).
Warm-supplementing kidney yang (WSKY) is a herbal
prescription that has been used for the treatment in psychiatric
conditions in Traditional Chinese Medicine (TCM). According to TCM,
schizophrenia is a syndrome caused by a yin-yang imbalance
characterized by spleen and stomach weakness and inadequate kidney
yin. Herbal prescriptions, such as WSKY or strong kidney yin, are
considered to improve deficits associated with schizophrenia.
Previously, it was reported that WSKY capsules in addition to
risperidone significantly enhanced cognitive function, social
function (6) and improved the
quality of life (7) compared with
the placebo added to risperidone. The present study focused on the
mechanisms underlying WSKY’s effects on cognition enhancement.
Brain-derived neurotrophic factor (BDNF) and its
receptors and downstream cascade have been associated not only with
neurodevelopment and neuroprotection, but also with synapse
regulation, learning and memory (8). BDNF has a critical role in synaptic
plasticity through N-methyl-d-aspartate (NMDA) receptor
activation in the hippocampus (9).
Hippocampal long term potentiation (LTP), which involves the
strengthening of synapses as the result of binding of BDNF to the
tropomyosin-related kinase B (TrkB) receptor, is responsible for
learning and memory (10), and
impairment as the result of lack of BDNF was found to be restored
by reintroduction of BDNF. Furthermore, cognitive impairment
observed in schizophrenia suggests that BDNF may be a potential
biomarker candidate as its effect has been implicated in learning
and memory (11).
In the present study, the Morris water maze (MWM)
test was utilized to identify cognition improvement of WSKY in a
rat model of schizophrenia. The expression of BDNF-related
signaling molecules, including, brain-derived neurotrophic factor
(BDNF), phosphorylated extracellular signal-regulated kinase (pERK)
and phosphorylated cAMP response element binding protein (pCREB)
expression in rat hippocampus was observed using western blotting
analysis following administration of WSKY in a chronic manner. The
results of the present study indicated that WSKY has potential
therapeutic implications for cognitive impairment in schizophrenia
and psychiatric diseases involving abnormal expression of BDNF.
Materials and methods
Animals
Male Sprague-Dawley rats (weighing 300–350 g,
corresponding to ~2 months of age) were purchased from Wuhan
University, Center of Experimental Animal (Wuhan, China). The rats
were housed at four animals per cage and were provided with food
and water ad libitum and maintained under a 12-h light/dark
cycle (light on 07:30–19:30) at constant temperature (23±1°C) and
humidity (60±10%). A 60-min habituation period to the experimental
room on the first day and 30 min on the following days preceded the
behavioral experiments. The number of animals used in each group
for behavioral tests is described in Table I. Animal treatment and maintenance
were conducted in accordance with the Principles of Laboratory
Animal Care (NIH publication no. 85-23, revised 1985) and the
Animal Care and the use guidelines of Wuhan University (Wuhan,
China). The present study was approved by the ethics committee of
Wuhan University.
 | Table INumbers of animals used in each
group. |
Table I
Numbers of animals used in each
group.
| Test | Group and drug dose
(mg/kg) | n |
|---|
| MWM test (with MWM
training) | Control | 6 |
| Vehicle + MK-801
(0.05) | 8 |
| WSKY (25) + MK-801
(0.05) | 6 |
| WSKY (100) + MK-801
(0.05) | 8 |
| WSKY (250) + MK-801
(0.05) | 7 |
| WSKY (500) + MK-801
(0.05) | 8 |
| Western blot analysis
(without MWM training) | Control | 6 |
| WSKY (25) | 7 |
| WSKY (100) | 6 |
| WSKY (250) | 6 |
| WSKY (500) | 6 |
Materials
MK-801 and rabbit polyclonal anti-BDNF antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The rabbit monoclonal anti-phosphorylated ERK, rabbit
monoclonal anti-ERK, rabbit monoclonal anti-phosphorylated CREB and
rabbit monoclonal anti-CREB antibodies were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). All of the other
materials were of the highest grades available and were obtained
from commercial sources.
Preparation of WSKY
WSKY consisted of 13 traditional Chinese herbs
(Table II). All of the
ingredients were purchased from Kang Sheng Pharmaceutical Co., Ltd.
(Wuhan, China) and carefully authenticated by Dr. Ben-Hong Zhou
(Department of Pharmacy, Renmin Hospital of Wuhan University,
Wuhan, China). The voucher specimens were deposited at the
Herbarium of the Renmin Hospital of Wuhan University: Aconitum
Carmichaeli Debx WHUOPS2010-12, Herba Epimedii WHUOPS2010-10, Radix
Morinda Officinalis WHUOPS2010-32, Cortex Cinnamomoi WHUOPS2010-24,
Rhizoma Zingiberis WHUOPS2010-34, Radix Rehmanniae WHUOPS2010-28,
Radix Glycyrrhizae WHUOPS2010-16, Radix Astragali WHUOPS2010-8,
Pericarpium Citri Reticulatae WHUOPS2010-15, Fructus Amomi
WHUOPS2010-7, Carapax Et Plastrum Testudinis WHUOPS2010-30, Radix
Codonopsis Pilosulae WHUOPS2010-9 and Rhizoma Curculigins
WHUOPS2010-33 (purchased from Kang Sheng Pharmaceutical Co. Ltd.,
Wuhan, China). The procedure of preparation of WSKY was performed
in accordance with the method used in a previous study by our group
(6). The volatile oil of
Pericarpium Citri Reticulatae, Cortex Cinnamomoi and Rhizoma
Zingiberis were extracted by steam distillation. Dregs of these
three components were added to the other components for water
extraction and ethanol precipitation extraction. Icariin [National
Institute for the Control of Pharmaceutical and Biological Products
(NICPBP), Beijing, China] was detected as an indicator of
orthogonal test to determine the optimal conditions (Table III). According to these optimal
conditions, all of the ingredients were firstly soaked in 11 times
their volume of distilled water and boiled for 1 h. Following
filtration, the residue was added to distilled water ten times its
volume again and boiled for 1 h. The two filtrates were combined
and concentrated to a liquid ratio of 1:1, 70% alcohol
precipitation extraction, then concentrated with a rotary
evaporator and lyophilized (ALPHA1–4; Martin Christ
Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) to dry
powder. The volatile oil was sprayed evenly onto the dry the
extract powder. The final dried extract was 32.4% (w/w) of the
weight of the raw herbs. For quality control of WSKY extraction,
icariin, as a standard of quality control, was purchased from the
NICPBP, and a high-performance liquid chromatography system
(Agilent 1100; Agilent Technologies, Santa Clara, CA, USA) equipped
with a diode array detector and a Venusil MP-C18 chromatographic
column (4.6×250 mm, 5 μm; Bonna-Agela Technologies, Inc.,
Wilmington, DE, USA) was used for standardization of the herbal
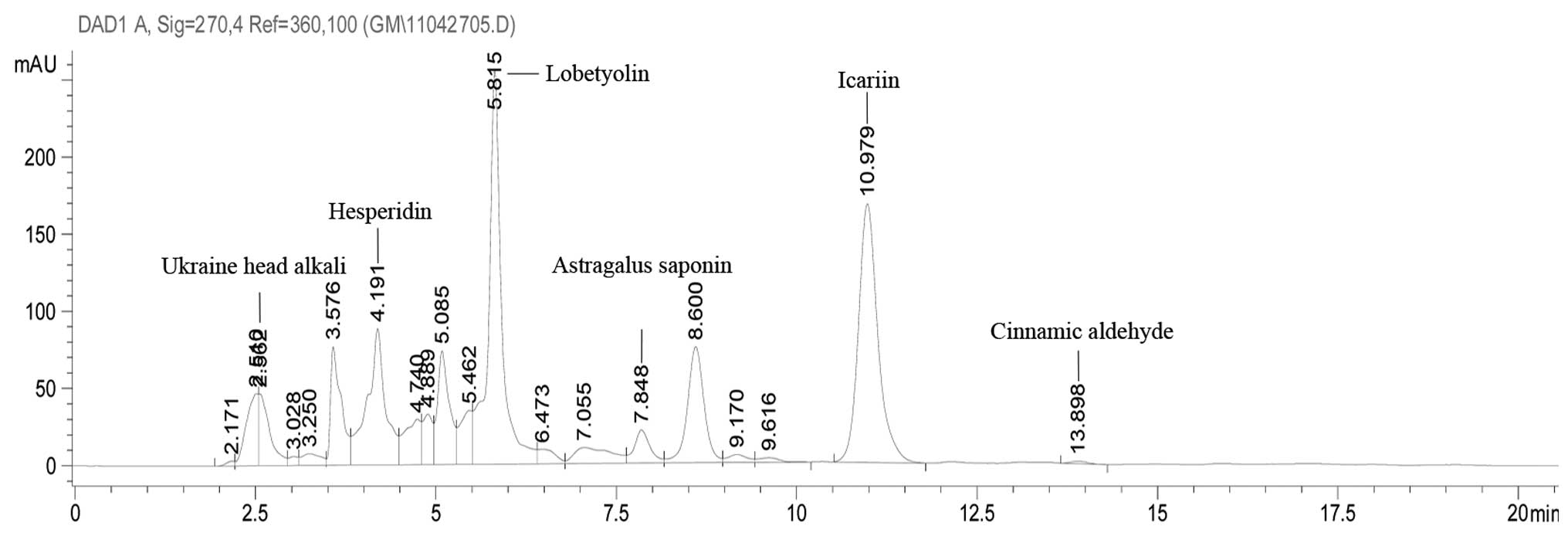
extract (Fig. 1).
 | Table IIIngredients and botanical/zoological
origins of warm-supplementing kidney yang formula. |
Table II
Ingredients and botanical/zoological
origins of warm-supplementing kidney yang formula.
| Pharmacological
name |
Botanical/zoological source/family | Part used | Amount (g) |
|---|
| Aconitum
carmichaeli Debx | Aconitum
carmichaeli Debx. | Daughter root
(processed) | 9 |
| Radix Morinda
officinalis | Morinda
officinalis F.C. How | Dried root and
rhizome | 3 |
| Herba Epimedii | Epimedium
brevicornu Maxim. | Dried body | 9 |
| Rhizoma
Curculigins | Curculigo
orchioides Gaertn. | Dried root and
rhizome | 9 |
| Cortex
Cinnamomoi | Cinnamomum
cassia (Nees & T. Nees) J. Presl | Dried tender
branch | 6 |
| Rhizoma
Zingiberis | Zingiber
officinale Rosc | Dried root and
rhizome | 6 |
| Radix Codonopsis
Pilosulae | Codonopsis
pilosula (Franch.) Nannf | Dried root and
rhizome | 6 |
| Radix
Astragali | Astragalus
abbreviatus Kar. & Kir. | Dried root and
rhizome | 9 |
| Radix
Rehmanniae | Rehmannia
glutinosa (Gaertn.) DC | Root
(processed) | 15 |
| Pericarpium Citri
Reticulatae | Citrus
reticulata Blanco | Dried mature
peel | 9 |
| Fructus Amomi | Amomum
villosum Lour | Dried fruit | 3 |
| Radix
Glycyrrhizae | Glycyrrhiza
uralensis Fisch | Dried root and
rhizome | 3 |
| Carapax Et Plastrum
Testudinis | Chinemys
reevesii (Gray) | Carapace
(processed) | 15 |
 | Table IIIResults of orthogonal test. |
Table III
Results of orthogonal test.
| Experiment
number | Water volume
(fold) | Heat-extraction
(h) | Ethanol
precipitation (%) | Ethanol
precipitation (h) | Icariin content
(mg/g) | Extraction rate
(%) |
|---|
| 1 | 8 | 1 | 60 | 12 | 0.22 | 18.64 |
| 2 | 8 | 1.5 | 70 | 24 | 0.20 | 18.99 |
| 3 | 8 | 2 | 80 | 48 | 0.19 | 15.28 |
| 4 | 10 | 1 | 70 | 48 | 0.26 | 21.87 |
| 5 | 10 | 1.5 | 80 | 12 | 0.19 | 16.91 |
| 6 | 10 | 2 | 60 | 24 | 0.15 | 21.36 |
| 7 | 12 | 1 | 80 | 24 | 0.27 | 14.77 |
| 8 | 12 | 1.5 | 60 | 48 | 0.13 | 19.39 |
| 9 | 12 | 2 | 70 | 12 | 0.22 | 22.39 |
Drug administration
WSKY or MK-801 was dissolved in 0.9% saline
solution. In a pilot study, it was observed that a 14-day
consecutive injection of MK-801 at 0.05 mg/kg induced impairment of
the spatial memory in rats, as was observed in other studies
(12). Based on these results, the
effective dose of MK-801 (0.05 mg/kg) was adopted for further
experiments. In addition, the effective dose of WSKY (25, 100, 250
or 500 mg/kg, W/W) that was selected was roughly translated from
the dosage of the WSKY capsule, according to a previous study based
on the human-animal dose translation equation (13). The rats were orally administered
WSKY for 14 days (Fig. 2B). During
the behavioral sessions, the rats were subjected to WSKY treatment
2 h prior to the behavioral tests, and MK-801 (0.05 mg/kg, i.p.)
was administered 30 min prior to the behavioral tests. The control
group was orally administered 0.9% saline solution 2 h prior to the
behavioral tests.
 | Figure 2Effect of WSKY on the SLM of
MK-801-treated rats in the MWM test. (A) The apparatus of the MWM
test included a 180 cm diameter pool, a 15 cm diameter hidden
platform and extra visual cues. (B) Four training trails of the MWM
test per day were conducted for five consecutive sessions, probe
trail was performed 24 h following the last training session. (C)
The mean escape latency of each group to locate the hidden platform
was recorded and analyzed during the MWM test. MK-801-injected rats
demonstrated significant spatial reference memory function
impairment during training days compared with the control group,
while WSKY treatment at dose of 25, 100 and 250 mg/kg ameliorated
the SLM impairment induced by MK-801 injection; however, WSKY
treatment at dose of 500 mg/kg did not. (D) There was no
significant difference in the swimming speed in each group. (E)
WSKY improved the SLM of MK-801-treated rats in the probe trail.
WSKY treatment at doses of 100 and 250 mg/kg led to a significant
increase in the time spent in the target quadrant and (F) 250 mg/kg
treatment led to a significant increase in the number of platform
crossings when compared with the MK-801 treated group. Values are
presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01 vs. the control
group; #P<0.05, ##P<0.01 vs. the
MK-801-treated group. WSKY, warm-supplementing kidney yang; SLM,
spatial learning and memory; MWM, Morris water maze. |
MWM test
The MWM test was conducted as previously described
(12). The water maze consisted of
a circular, 180 cm diameter and 45 cm deep, black tank filled with
water at 22°C to a depth of 28 cm. The testing room was maintained
at 22°C and contained fixed extra-visual cues. A circular, 15 cm
diameter escape platform composed of black plexiglas was placed in
the pool 1 cm below the water surface.
The pool was divided into four equally sized
quadrants (northeast, southeast, southeast and southwest). The
platform was constantly placed in the ‘Northeast’ (Fig. 2A) halfway between the center and
edge for all hidden platform testing. The animals were always
examined in the same order at approximately the same time each day.
On day one, the first trial was started from the starting point
‘South’. The starting points varied over the days of training, and
they were rotated clockwise, one-quarter of a turn per trial.
Assessment of behavioral performance was conducted
with the assistance of EthoVision™ (Noldus Information Technology,
Wageningen, The Netherlands) The rats received the first trial,
four trials per day, over 14 consecutive days (Fig. 2B). For each trial, the rats were
placed into the water gently facing the wall. The rats were then
permitted to swim until they reached the escape platform in a
maximum of 60 sec. Once on the platform, the rats remained there
for 30 sec prior to being removed for an inter-trial interval of 15
min. Probe trials were conducted 24 h following the last hidden
platform training. Each animal was allowed to swim for 30 sec
starting from the Southwest position.
Western blot analysis
For the preparation of western blot samples, the
rats without behavioral training were sacrificed 1 h following the
last administration of WSKY, and their isolated hippocampal tissues
were analyzed by 4–10% SDS-PAGE (Beyotime Institute of
Biotechnology, Haimen, China) and blotted onto polyvinylidene
fluoride membranes. The western blots were incubated for 1 h with
5% skimmed milk, and then with rabbit anti-pERK1/2 (1:2,500
dilution), rabbit anti-ERK1/2 (1:5,000 dilution), rabbit anti-pCREB
(1:2,500 dilution), rabbit anti-CREB (1:5,000 dilution) or rabbit
anti-BDNF (1:1,000 dilution) antibodies overnight at 4°C with
gentle agitation. The secondary antibodies were added and incubated
with gentle rocking for 45 min at room temperature prior to washing
of the membranes as described above. To analyze the relative
protein quantity, β-actin was used as a loading control.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. P<0.05 was considered to indicate a statistically
significant difference between values. For the data from the MWM
task, the treatment differences in the escape latency or average
swim speed were analyzed using repeated measures analysis of
variance (ANOVA) with a least significant difference (LSD) test as
a post-hoc test. The time spent in the target quadrant (%) and the
number of platform crossings during the probe test was analyzed by
one-way ANOVA with LSD test as a post-hoc test for each group. The
data obtained from the western blot analysis of hippocampal tissues
from rats were analyzed by one-way ANOVA with LSD test as a
post-hoc test. Statistical analysis was performed using SPSS 17.0
for Windows (IBM, Armonk, NY, USA).
Results
Effect of WSKY on spatial learning and
memory in the MWM test
MWM is a test for assessing spatial learning and
memory (SLM) to locate a submerged escape platform (14). The present study examined whether
WSKY ameliorated the MK-801-induced SLM impairment by using the MWM
test (Fig. 2). Spatial learning
was assessed by measuring the time taken to escape from the water
to a hidden platform (escape latency).
The experimental schedule is demonstrated in
Fig. 2B. Repeated-measure ANOVA
revealed a significant delay effect on escape latency
(F4,167=600.837; P<0.01), indicating that, across the
groups, the rats improved their spatial learning effectively with
increased training. The MK-801-injected rats demonstrated
significant spatial reference memory function impairment during the
training days compared with the control group
(F1,23=502.759, P<0.001), while the performance of
MK-801-treated rats was significantly improved by consecutive
training (F4,115=37.14, P<0.01). There was also a
significant treatment effect on the escape latency; WSKY treatment
of schizophrenic animals at doses of 25, 100 and 250 mg/kg
demonstrated significant SLM performance improvement compared with
the MK-801-treated group (WSKY 25 mg/kg group,
F1,27=10.248, P<0.05; WSKY 100 mg/kg group,
F1,27=206.159, P<0.01; WSKY 250 mg/kg group,
F1,27=273.908, P<0.01, respectively, compared with
the MK-801 group), which indicated that WSKY ameliorated the SLM
impairment induced by MK-801 injection. Notably, WSKY at a dose of
500 mg/kg demonstrated no significant difference in training
compared with that in the MK-801 group (F1,27=0.622,
P>0.05; Fig. 2C). However,
there was no significant difference in the swimming speed between
the groups (Fig. 2D). This result
was different from that of a previous study (12), which is possibly associated with
the different visual cues settings of the MWM test used in the
present study.
Furthermore, the effects of WSKY on spatial memory
formation were investigated. The probe trials were conducted to
assess the spatial memory at 24 h following the final training. As
demonstrated in Fig. 2E, the 100
and 250 mg/kg WSKY-treated groups spent more time in the target
quadrant of the pool in which the hidden platform was previously
located than the MK-801 group (WSKY 100 mg/kg: 29.1±4.9 vs. MK-801:
21.5±2.5, P=0.039, P<0.01; WSKY 250 mg/kg: 32.31±3.65 vs.
MK-801: 21.5±2.5, P=0.002, P<0.01). In addition, the 250 mg/kg
WSKY treatment group demonstrated a significantly increased number
of platform crossings when compared with the MK-801 treated group
(WSKY 250 mg/kg, 2.33±0.58 vs. MK-801, 0.67±0.58; P<0.01;
Fig. 2F). This indicated that WSKY
improved the SLM of rats treated with MK-801.
Effect of WSKY on BDNF expression in the
hippocampus
BDNF has a key role in participating in the
processes of synaptic plasticity and learning by enhancing
long-term potentiation (LTP) (8),
which is regarded as the cellular basis of learning and memory in
the hippocampus. BDNF levels have previously been positively
associated with cognitive impairment of schizophrenia patients
(15,16). To determine whether BDNF was
involved in the cognitive enhancement by WSKY, the expression of
BDNF in the hippocampal region of rats without MWM test was
detected by western blot analysis. It was identified that treatment
of rats with 100, 250 or 500 mg/kg WSKY for 14 days resulted in an
increase in BDNF expression levels (WSKY 100 mg/kg, P<0.001;
WSKY 250 mg/kg, P<0.001; WSKY 500 mg/kg, P<0.05;
respectively, compared with the vehicle-treated group), while the
25 mg/kg WSKY treatment group revealed no significant difference
with the vehicle-treated group (P=0.915, P>0.05). The of BDNF
protein expression levels in the hippocampus demonstrated an
inverted U-shaped dose-response pattern (Fig. 3).
Effect of WSKY on pERK/ERK and pCREB/CREB
in the hippocampus
To investigate whether the hippocampal BDNF/TrkB
downstream cascade, including activation of ERK (ERK1 and ERK2) and
CREB, was involved with WSKY-induced SLM changes in rats, western
blot analysis was used to measure the phosphorylation levels of
ERK1/2 and CREB in the hippocampi of rats that were not subjected
to behavioral tests. The rats receiving WSKY treatment at doses of
100 or 250 mg/kg for 14 days demonstrated significantly increased
levels of BDNF in the hippocampus (WSKY 100 mg/kg, P<0.001; WSKY
250 mg/kg, P<0.001, compared with the control group,
respectively; Fig. 4). The levels
of phosphorylation of extracellular signal-regulated kinase
(ERK1/2), which is an important component of the mitogen-activated
protein kinase (MAPK)/ERK pathway, were significantly higher in the
hippocampus (WSKY 100 mg/kg, pERK1 P<0.001, pERK2 P<0.001;
WSKY 250 mg/kg, pERK1 P<0.001, pERK2 P<0.001, compared with
the control group, respectively). In addition, the activation of
CREB was analyzed, which is phosphorylated at Ser-133 by ERK
following ERK phosphorylation, and has a critical role in LTP and
memory formation. The 14-day 250 mg/kg WSKY treatment produced
increased levels of pCREB in the hippocampus (WSKY 100 mg/kg, pCREB
P<0.05; WSKY 100 mg/kg, pCREB P>0.05, compared with the
control group, respectively; Fig.
4). These results suggested that WSKY improved the cognitive
performance in the MWM test and may therefore be involved in the
upregulation of BDNF expression and activation of its downstream
cascade.
 | Figure 4Effect of WSKY on hippocampal BDNF and
BDNF downstream signaling components of rats without MWM test.
Western blot analysis of BDNF, pERK, ERK, pCREB and CREB levels.
Following treatment with WSKY, BDNF was significantly increased in
the hippocampus compared with the control group. In addition, WSKY
treatment increased levels of hippocampal pERK1/2, and its
downstream signaling molecule pCREB. Quantification of BDNF data
was normalized against β-actin, whereas pERK and pCREB were
normalized against each unphosphorylated form and expressed as a
percentage of the control (baseline). The blots represent typical
results from three independent experiments. Densitometric data are
presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01 vs. the control
group; #P<0.05, ##P<0.01 vs. the WSKY
100 mg/kg group (each group, n=6). WSKY, warm-supplementing kidney
yang; BDNF, brain derived neurotrophic factor; MWM, Morris water
maze; pCREB, phosphorylated cAMP response element binding protein;
ERK, extracellular signal-regulated kinase. |
Discussion
The cognitive deficits of schizophrenia, which are
considered a core manifestation and an important predictor of the
functional outcome, may be understood in the context of the
molecular and cellular mechanisms of learning and memory, in which
BDNF has a key role through the regulation of synaptic plasticity
(17). Therefore, BDNF may be
assumed to be a potential biomarker candidate, as it is implicated
in cognitive impairment in schizophrenia. In the present study, the
distinct effects of different doses of WKSY on cognitive
performance in a chronic MK-801-induced rat model of schizophrenia
were investigated. Numerous studies have demonstrated that acute or
chronic treatment with NMDA antagonists, including phencyclidine
(PCP) and MK-801, induced NMDA receptor (NMDAR) hypofunction and
produced behavior in healthy patients which resembled psychotic
symptoms, cognitive deficits and negative symptoms of schizophrenia
(18). In the present study, rats
received a total of 14 consecutive MK-801 injections, as repeated
treatment with MK-801 has been suggested as a better animal model
of schizophrenia than a single dose (19,20).
Chronic MK-801 treatment impairs diverse factors in the working
memory of rats, including attention, speed of processing, visual
learning, long-term memory, reasoning and problem solving skills
(21). In the acquisition phase,
it was identified that MK-801 treatment increased the escape
latency compared with that of the control group, and this
impairment was rescued by a 14-day administration of WSKY doses of
25, 100 and 250 mg/kg; however, without changing the swimming speed
of the animals. In the probe test, 250 mg/kg WSKY treatment
increased the time spent in the target quadrant and the number of
platform crossings. This indicated that 250 mg/kg WSKY treatment
ameliorated the MK-801-induced SLM impairment in the MWM test by
improving murine ability of learning and enhancing consolidation of
recognition memory.
The present study also investigated effects of WSKY
on BDNF expression in the hippocampus of rat without training. NMDA
antagonists, including PCP and MK-801, have been shown to induce
cognitive impairment via inhibition of BDNF secretion and
activation (22,23). Therefore, the present study
examined whether the underlying mechanism of the enhancement of SLM
by WSKY was associated with the upregulation of BDNF in the
hippocampal region. Hippocampal tissues were collected immediately
following the last administration of WSKY. It was identified that a
14-day administration of WSKY at doses of 100, 250 or 500 mg/kg
significantly stimulated endogenous BDNF expression in the
hippocampal region when compared with the MK-801-treated group. It
is known that activation of TrkB by BDNF stimulates intracellular
signaling cascades involved in plasticity, including the ERK/MAPK
pathway and the PI3K/Akt pathway (24). Callaghan and Kelly (25) reported that the consolidation of
recognition memory was associated with increased release of BDNF in
the dendate gyrus (DG) and perirhinal cortex, which was associated
with significant increases in ERK activation and c-fos expression
in the hippocampal DG, and PI3K activation and c-fos expression in
the perirhinal cortex in rats. Therefore, the present study aimed
to confirm whether WKSY induced increases in levels of hippocampal
BDNF expression, to clarify whether it activates the TrkB
downstream signaling cascade. The expression levels of pERK and
pCREB, which are important molecules involved in the BDNF/TrkB
signaling pathway, were assessed by western blot analysis. It was
identified that 250 mg/kg WSKY treatment significantly upregulated
the phosphorylation of ERK1/2 as well as the phosphorylation of
CREB in the hippocampus when compared with the vehicle-treated
group. The ERK signaling pathway is involved in neuroplasticity and
memory formation (26,27), and the activation of the CREB
signaling pathway in the hippocampus has an important role in
spatial memory formation (28).
The results suggested that WSKY may improve cognition via
activation of the BDNF/TrkB downstream signaling cascade.
Of note, in the WSKY-treated groups, the effect of
memory enhancement in the MWM test and the hippocampal BDNF protein
expression levels both demonstrated an inverted U-shaped
dose-response curve fashion. It has been suggested that aconitum
alkaloids, including aconitine, lappaconitine and
6-benzoylheteratisine, three chemical components of Radix Aconiti
Lateralis, an ingredient of the WSKY formula, which is associated
with Na+ channels, have been shown to exert inhibitory
effects on neuronal activity in rat hippocampal slices in
vitro at specific doses (29,30).
It is therefore hypothesized that the inverted U-shaped
dose-response curve of WSKY may be due to the inhibitory effect of
aconitum alkaloids at high doses of WSKY. Further studies are
required to clarify these issues.
In the present study, the effect of WSKY on
schizophrenia-associated cognitive impairment by modulating BDNF
expression and the BDNF/ERK/CREB pathway in hippocampus were
elucidated, and it was identified that WSKY may be a potential
cognitive enhancer for a number of psychiatric diseases, including
schizophrenia. However, as BDNF has an important and unique role in
regulating a wide range of functions at different neural
development stages, and human epidemiology provides compelling
evidence that exposure of the neonate, from the gestation or the
perinatal period, to environmental adversities, including abnormal
BDNF expression increase the risk of developing schizophrenia
(31,32). Considering the neurodevelopmental
hypothesis of schizophrenia, BDNF has been proposed to be involved,
at least in part, in the pathogenesis of this disease. Therefore,
further studies are required to clarify the effect of WSKY on BDNF
expression using neurodevelopmental schizophrenic animal models,
including the disruption of neurogenesis during a critical
gestational period, neonatal ventral hippocampal lesions and
post-weaning social isolation. In addition, it is necessary to
specify the effective components in WSKY to clear its mechanisms of
action and develop more effective cognitive remediation for
psychiatric diseases.
In conclusion, the results of the present study
indicated that WSKY enhanced cognitive performance in schizophrenia
by upregulating BDNF/ERK/CREB signaling in the hippocampus, and
WSKY has potential therapeutic implications for cognitive
impairment of schizophrenia.
Acknowledgements
The present study was supported by two grants from
the Key Technology Research of Major Mental Illness Prevention and
Treatment for the Barriers to the Recognition and Prevention of
Depression and Anxiety in General Hospital (grant no.
2012BAIO1B00), and the Chinese Medicine research projects of Hubei
Provincial Health Department, China (grant no. 2012Z-Y37). The
authors also acknowledge the technical support from the HuBei
Provincial Key Library of Digestive Disease and the Central
Laboratory of Renmin Hospital of Wuhan University.
Abbreviations:
|
WSKY
|
warm-supplementing kidney yang
|
References
|
1
|
Taylor MA and Abrams R: Cognitive
impairment in schizophrenia. Am J Psychiatry. 141:196–201. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reichenberg A, Weiser M, Caspi A, et al:
Premorbid intellectual functioning and risk of schizophrenia and
spectrum disorders. J Clin Exp Neuropsychol. 28:193–207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurtz MM, Moberg PJ, Gur RC and Gur RE:
Approaches to cognitive remediation of neuropsychological deficits
in schizophrenia: a review and meta-analysis. Neuropsychol Rev.
11:197–210. 2001. View Article : Google Scholar
|
|
4
|
Lieberman JA, Stroup TS, McEvoy JP, et al:
Effectiveness of antipsychotic drugs in patients with chronic
schizophrenia. N Engl J Med. 353:1209–1223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marder SR and Fenton W: Measurement and
Treatment Research to Improve Cognition in Schizophrenia: NIMH
MATRICS initiative to support the development of agents for
improving cognition in schizophrenia. Schizophr Res. 72:5–9. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ZH, Wang GH, Wang XP, et al: Effects
of warm-supplementing kidney yang (WSKY) capsule added on
risperidone on cognition in chronic schizophrenic patients: a
randomized, double-blind, placebo-controlled, multi-center clinical
trial. Hum Psychopharmacol. 23:465–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen ZH, Wang GH, Wang XP, et al: Effect
of Warm-Supplementing Kidney Yang (WSKY) added to risperidone on
quality of life in patients with schizophrenia: a randomized
controlled trial. Clin Rehabil. 23:963–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YS and Silva AJ: The molecular and
cellular biology of enhanced cognition. Nat Rev Neurosci.
10:126–140. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lipsky RH and Marini AM: Brain-derived
neurotrophic factor in neuronal survival and behavior-related
plasticity. Ann NY Acad Sci. 1122:130–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buckley PF, Pillai A and Howell KR:
Brain-derived neurotrophic factor: findings in schizophrenia. Curr
Opin Psychiatry. 24:122–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Favalli G, Li J, Belmonte-de-Abreu P, Wong
AH and Daskalakis ZJ: The role of BDNF in the pathophysiology and
treatment of schizophrenia. J Psychiatr Res. 46:1–11. 2012.
View Article : Google Scholar
|
|
12
|
Ahlander M, Misane I, Schött PA and Ogren
SO: A behavioral analysis of the spatial learning deficit induced
by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat.
Neuropsychopharmacology. 21:414–426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar
|
|
14
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niitsu T, Shirayama Y, Matsuzawa D, et al:
Associations of serum brain-derived neurotrophic factor with
cognitive impairments and negative symptoms in schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 35:1836–1840. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asevedo E, Gadelha A, Noto C, et al:
Impact of peripheral levels of chemokines, BDNF and oxidative
markers on cognition in individuals with schizophrenia. J Psychiatr
Res. 47:1376–1382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinotti G, Di Iorio G, Marini S, et al:
Nerve growth factor and brain-derived neurotrophic factor
concentrations in schizophrenia: a review. J Biol Regul Homeost
Agents. 26:347–356. 2012.PubMed/NCBI
|
|
18
|
Gilmour G, Dix S, Fellini L, et al: NMDA
receptors, cognition and schizophrenia - testing the validity of
the NMDA receptor hypofunction hypothesis. Neuropharmacology.
62:1401–1412. 2012. View Article : Google Scholar
|
|
19
|
Mandillo S, Rinaldi A, Oliverio A and Mele
A: Repeated administration of phencyclidine, amphetamine and MK-801
selectively impairs spatial learning in mice: a possible model of
psychotomimetic drug-induced cognitive deficits. Behav Pharmacol.
14:533–544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li JT, Su YA, Guo CM, et al: Persisting
cognitive deficits induced by low-dose, subchronic treatment with
MK-801 in adolescent rats. Eur J Pharmacol. 652:65–72. 2011.
View Article : Google Scholar
|
|
21
|
Mouri A, Nagai T, Ibi D and Yamada K:
Animal models of schizophrenia for molecular and pharmacological
intervention and potential candidate molecules. Neurobiol Dis.
53:61–74. 2013. View Article : Google Scholar
|
|
22
|
Castren E, da Penha Berzaghi M, Lindholm D
and Thoenen H: Differential effects of MK-801 on brain-derived
neurotrophic factor mRNA levels in different regions of the rat
brain. Exp Neurol. 122:244–252. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adachi N, Numakawa T, Kumamaru E, et al:
Phencyclidine-induced decrease of synaptic connectivity via
inhibition of BDNF secretion in cultured cortical neurons. Cereb
Cortex. 23:847–858. 2013. View Article : Google Scholar
|
|
24
|
Bekinschtein P, Cammarota M, Igaz LM, et
al: Persistence of long-term memory storage requires a late protein
synthesis-and BDNF- dependent phase in the hippocampus. Neuron.
53:261–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Callaghan CK and Kelly ÁM: Differential
BDNF signaling in dentate gyrus and perirhinal cortex during
consolidation of recognition memory in the rat. Hippocampus.
22:2127–2135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ying SW, Futter M, Rosenblum K, et al:
Brain-derived neurotrophic factor induces long-term potentiation in
intact adult hippocampus: requirement for ERK activation coupled to
CREB and upregulation of Arc synthesis. J Neurosci. 22:1532–1540.
2002.PubMed/NCBI
|
|
27
|
Mazzucchelli C and Brambilla R:
Ras-related and MAPK signalling in neuronal plasticity and memory
formation. Cell Mol Life Sci. 57:604–611. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pláteník JJ, Kuramoto N and Yoneda Y:
Molecular mechanisms associated with long-term consolidation of the
NMDA signals. Life Sci. 67:335–364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ameri A, Gleitz J and Peters T: Inhibition
of neuronal activity in rat hippocampal slices by Aconitum
alkaloids. Brain Res. 738:154–157. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ameri A, Gleitz J and Peters T: Aconitine
inhibits epileptiform activity in rat hippocampal slices. Naunyn
Schmiedebergs Arch Pharmacol. 354:80–85. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neill JC, Barnes S, Cook S, et al: Animal
models of cognitive dysfunction and negative symptoms of
schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther.
128:419–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rapoport JL, Addington AM, Frangou S and
Psych MR: The neurodevelopmental model of schizophrenia: update
2005. Mol Psychiatry. 10:434–449. 2005. View Article : Google Scholar : PubMed/NCBI
|