Introduction
Gangliosides are sialic acid-containing
glycosphingolipids, which are generally localized on the outer
leaflet of the plasma membrane due to the insertion of their
hydrophobic ceramide portion into the membrane, with their
oligosaccharide moiety being exposed on the cell surface.
Therefore, gangliosides are able to interact with other components
of the membrane, including receptors, cellular antigens, adhesion
molecules and the extracellular matrix (1). Additionally, gangliosides are
involved in the regulation of various processes, including cell
proliferation and differentiation, apoptosis, embryogenesis and
oncogenesis (2). Each cell type
exhibits a distinct pattern of ganglioside content, which is
altered by neoplasia (3–5) and tumor cells or oncogene-transformed
cells exhibit a different ganglioside composition compared with
non-transformed cells. Among the gangliosides, GM3 is the simplest
member and is a common precursor for the more complex gangliosides
(6). The involvement of GM3 in the
malignant activity of tumor cells, including cell proliferation,
adhesion, invasion and metastasis, has been an area of particular
interest (7–10). However, the precise mechanism
remains to be elucidated. GM3 exerts its actions in a tumor-type
specific manner and exerts either inhibitory actions or enhancing
actions on tumor growth, migration and metastasis in different
types of tumor. It also suppresses the proliferative and invasive
potential of various types of cell (11–15).
However, an inverse correlation is observed between the
proliferative and metastatic properties of cells and their GM3
content in certain types of tumor (16,17).
This indicates that GM3 may exert different or opposing actions in
different types of tumor and, until recently, the underlying
mechanism of GM3 cell-type specificity remained to be
elucidated.
To elucidate the underlying mechanism by which GM3
exerted these opposing actions on Hepa1–6 cell migration, the
effects of GM3 on the tyrosine residue phosphorylation of EGF
receptor (EGFR) and HGF receptor (HGFR) were examined and the
activity of certain signaling pathways, which are essential for the
modulation of tumor cell growth, invasion, migration and metastasis
were investigated.
Materials and methods
Antibodies
The following antibodies were used:
Anti-phosphorylated(p)-EGFR (Tyr-1173) immunoglobulin rabbit
polyclonal IgG, anti-p-EGFR (Tyr-845) rabbit polyclonal IgG,
anti-p-EGFR (Tyr-1086) rabbit polyclonal IgG, anti-p-EGFR
(Tyr-1068) rabbit polyclonal IgG, anti-p-HFGR (Met; Tyr1313) rabbit
polyclonal IgG, ant-p-Met (Tyr-1349) rabbit polyclonal IgG,
ant-p-Met (Tyr-1365) rabbit polyclonal IgG, anti-Akt1/2/3 rabbit
polyclonal IgG, anti-P-Akt1/2/3 (Ser473) rabbit polyclonal IgG,
anti-P-Akt1/2/3 (Thr308) rabbit polyclonal IgG,
anti-phospholipaseCγ (PLCγ)1 rabbit polyclonal IgG, anti-p-PLCγ1
(Tyr-783) rabbit polyclonal IgG, anti-p-p44/42 mitogen-activated
protein kinase (MAPK) rabbit polyclonal IgG (Erk1/2;
Thr-202/Tyr-204), anti-β-actin mouse polyclonal IgG, goat
anti-rabbit polyclonal horseradish peroxidase-conjugated IgG,
rabbit anti-goat polyclonal horseradish peroxidase-conjugated IgG,
goat anti-mouse polyclonal horseradish peroxidase-conjugated IgG
and horseradish peroxidase-labeled streptavidin (all from Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Anti-GM3 IgM was
obtained from Wako Pure Chemical Industries Ltd (Osaka, Japan) The
dilution used was 1:500 for all antibodies.
Reagents
LY294002 was obtained from Sigma-Aldrich (St. Louis,
MO, USA), EGF was obtained from ProSpec-Tany TechnoGene, Ltd. (East
Brunswick, NJ, USA), Lipofectamine™2000 and opti-MEM™ were obtained
from Invitrogen Life Technologies, Carlsbad, CA, USA, Protease
inhibitor cocktail was obtained from Sigma-Aldrich and GM3 and GM2
were purchased from Sigma-Aldrich.
Cells and cell culture
Mouse hepatocellular carcinoma Hepa1–6 cells were
grown in 10-cm cell culture dishes or in multi-well plates in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin
at 37°C in a 5% CO2 atmosphere for 48 h.
RNAi GM3 synthase plasmid construction
and selection
A negative control and four types of GM3 synthase
short hairpin (sh)RNA plasmids were constructed, as follows:
PGPU6/green fluorescent protein (GFP)/Neo-shNC (negative control),
PGPU6/GFP/Neo-St3ga15-mus-469, PGPU6/GFP/Neo-St3 ga15-mus-613,
PGPU6/GFP/Neo-St3ga15-mus-772 and PGPU6/GFP/Neo-St3ga15-mus-832. To
select an effective shRNA plasmid that inhibited GM3 synthase, the
Hepa1–6 cells were transfected with the GM3 synthase shRNA plasmids
using Lipofectamine™ 2000 in opti-MEM™. The ratio of DNA (μg) to
lipofectamine 2000 (μl) was 1:3. The effective GM3 synthase shRNA
plasmid was selected using reverse transcription polymerase chain
reaction (RT-PCR).
Total RNA was isolated from cells using Trizol
(Invitrogen Life Technologies), and RT-PCR was conducted with Taq
polymerase, according to the manufacturer’s instructions
(Boehringer-Mannheim, Indianapolis, IN, USA). The cycling
parameters were implemented as follows: denaturation at 94°C for 30
sec, annealing at 58–61°C for 1 min (temperature dependant on
primer specifications), and elongation at 72°C for 1 min. The
number of cycles varied between 25 and 35, depending on the
quantity of mRNA. The identity of the PCR products was confirmed
through sequencing. The relative quantitative analysis was
normalized to that of the endogenous reference gene,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The mouse GM3
synthase forward primer used was 5′-AGCTGCCAGAGGTGATGAGT-3′ and its
reverse primer was 5′-TCAAGTGGCTTCAAGCAATG-3′. The mouse GAPDH
forward primer used was 5′-TACTTATGCCGATGTCGTTGT-3′ and its reverse
primer was 5′-CCAGCCTCGTCCCGTAGA-3′. The
PGPU6/GFP/Neo-St3ga15-mus-772 exhibited effective inhibition and
was used in the subsequent investigation (GenePharma Co., Ltd.,
Shanghai, China).
Modulating ganglioside expression in the
Hepa1–6 cells
To decrease the expression of GM3 in the Hepa1–6
cell, the cells were transfected with the GM3 synthase shRNA
plasmid at 37°C in a 5% CO2 atmosphere. After 48 h
incubation, the cells were harvested and the inhibition of
ganglioside synthesis was monitored by high performance thin layer
chromatography (HPTLC). For the GM3 synthase shRNA, the cells were
transfected with PGPU6/GFP/Neo-St3ga15-mus-772 using lipofectamine™
2000 in opti-MEM medium. After 48 h transfection, the cells were
harvested and the expression of GM3 synthase was monitored by
RT-qPCR. To increase the expression of ganglioside in the Hepa1–6
cell, the cells were treated with 50 μM GM3 or 50 μM GM2 for 48 h
prior to harvesting.
Analysis of ganglioside expression in
Hepa1–6 cell by HPTLC
HPTLC analysis was performed, as described
previously (18). Briefly, the
cells were grown in 10 cm dishes until ~90% confluent. The cells
were then trypsinized and washed three times with
phosphate-buffered saline (PBS; Sangon Biotech, Shanghai, China).
The total lipids were extracted twice from the cell pellet using
chloroform/methanol (1:1). The extracts were then combined and
dried under a stream of N2. The gangliosides were
purified by partitioning the dried total lipid in di-isopropyl
ether/1-butanol/17 mM aqueous NaCl followed by sephadex G-50 gel
filtration and lyophilization. The individual gangliosides were
separated onto silica gel 60 HPTLC plates (Merck KGaA, Darmstadt)
with a solvent system of chloroform/methanol/0.25% aqueous
CaCl2·2H2O (60:40:9; Sangon Biotech). The gangliosides were
visualized as purple bands by spraying them with resorcinol-HCI
reagent and heating the plate at 120°C.
In vitro cell migration assay
The cell migration assays were performed using a
Boyden chamber (Corning Costar, Cambridge, MA, USA) with 8 μm pore
polycarbonate filters (BD Biosciences, Franklin Lakes, NJ, USA).
The Hepa1–6 cells were treated with GM3 and transfected with the
GM3 synthase shRNA plasmid, as described above. To inhibit the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway with LY294002, the
Hepa1–6 cells treated, as above, were exposed to 15 μM LY294002 for
4 h. The cells were then harvested and used for migration
assays.
Following incubation of the cells in serum-free
medium overnight, the cells (1×105) were resuspended in
300 μl serum-free medium containing 50 ng/ml EGF or 100 ng/ml HGF
and the desired agents and placed in the top compartment of the
chamber. Subsequently, 250 μl 10% FBS medium was placed in the
bottom chamber. After 36 h incubation at 37°C in a 5%
CO2 incubator, the cells on the top membrane surface
were mechanically removed. The cells that migrated to the lower
surface of the membrane were fixed and stained with 0.1% DAPI.
Images of the cells were captured and the stained cells from five
randomly selected fields were counted under a microscope (Nikon
Corporation, Shanghai, China).
Western blot analysis
The cells treated, as described above, were seeded
into 12-well plates (Falcon, BD Biosciences) and incubated
overnight in serum-free medium containing the desired agents, as
described in the figure legends. The medium was changed and the
cells were stimulated with 50 ng/ml EGF or 100 ng/ml HGF for 10 min
at room temperature or left unstimulated as a control. The cells
were harvested and lysed in 200 μl radioimmunoprecipitation assay
buffer containing 1% Triton X-100, 150 mM NaCl, 25 mM Tris (pH
7.5), 0.5% sodium deoxycholate, 0.1% SDS, 5 mM pyrophosphate and 50
mM NaF with 1 mM Na3VO4, 1 mM DTT, 1%
protease inhibitor cocktail and 1% phosphatase inhibitor cocktail
per well. The lysate was subjected to SDS-PAGE using 10% gel.
Following electrophoresis, the proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes at 100 mA for 2 h. The
non-specific binding sites on the PVDF membranes were inhibited
using 3% (w/v) bovine serum albumin. The target protein bands in
the PVDF membranes were revealed by immunoblotting with the primary
antibodies given in the ‘antibodies’ section of ‘Materials and
methods’ and detected using enhanced chemiluminescence reagents
(Amersham Pharmacia Biotech, Amersham, UK).
Results
Suppression of GM3 synthesis by RNAi
targeting GM3 synthase in Hepa1–6 cells
To decrease the expression of GM3 in the Hepa1–6
cells, the cells were transfected with GM3 synthase shRNA plasmids.
The RT-qPCR results revealed that, among the four GM3 synthase
shRNA plasmids, the PGPU6/GFP/Neo-St3ga15-mus-772 plasmid was the
most effective at inhibiting the expression of GM3 synthase
(Fig. 1A). The inhibitory effect
was monitored by HPTLC (Fig.
1B).
Effect of GM3 on in vitro motility and
migration of Hepa1–6 cell stimulated with EGF and HGF
The effects of GM3 on the motility and migration of
the EGF- or HGF-stimulated Hepa1–6 cells were investigated. A
decrease in GM3 content by the GM3 synthase shRNA plasmid promoted
EGF-stimulated cell migration (Fig. 2a
and b) and inhibited HGF-stimulated migration (Fig. 2e and f). Conversely, an increase in
GM3 content, caused by the addition of exogenous GM3 in the culture
medium, inhibited EGF-stimulated cell migration (Fig. 2a and c) and promoted HGF-stimulated
migration (Fig. 2e and g). The
addition of exogenous GM2 had no effect on the migration of the
Hepa1–6cells treated with EGF and HGF (Fig. 2a, d and e, h). These results
indicated that GM3, but not GM2, inhibited EGF-stimulated cell
migration and promoted the HGF-stimulated migration in
vitro.
Effect of GM3 on the phosphorylation of
EGFR and HGFR
To elucidate the precise molecular mechanism by
which GM3 affected EGF- and HGF-stimulated migration of the Hepa1–6
cells, the present study examined the effect of GM3 on the
phosphorylation of EGFR and HGFR. Under control conditions, no
background phosphorylation at either Tyr-1173 or Tyr-845 was
observed. However, a decrease in GM3 content, by treating cells
with the GM3 synthase shRNA plasmid, significantly elevated the
EGF-stimulated phosphorylation of EGFR at Tyr-1173 and reduced the
EGF-stimulated phosphorylation of Tyr-845 (Fig. 3Aa). An increase in GM3 content, by
treating cells with exogenous GM3, markedly inhibited the
EGF-stimulated phosphorylation of EGFR at the Tyr-1173 and elevated
the phosphorylation of Tyr-845 (Fig.
3Ab). The phosphorylation of EGFR at Tyr-1068 and Tyr-1086 were
not detected (data not shown). The results demonstrated that GM3
suppressed the EGF-stimulated phosphorylation of EGFR at Tyr-1173,
but promoted the phosphorylation of Tyr-845. Tyr-1173 is located at
the c-terminus of EGFR and is a major EGFR autophosphorylation
site. Phosphorylated Tyr-1173 serves as a docking site for
SH2-domain containing signaling molecules and leads to the
activation of the downstream signaling pathways. Tyr-845 is
distinct from others located at the EGFR c-terminus. It is located
in the kinase domain of the EGF receptor and can be
transphosphorylated by src kinase (19). The significance of Tyr-845
phosphorylation remains to be elucidated, however, it may be
involved in the modulation of tyrosine kinase activity of the
receptor. These results suggested that the mechanism for the
GM3-suppressed phosphorylation of EGFR in Hepa1–6 cells is
complicated.
By contrast, a decrease in GM3 content, by treating
cells with a GM3 synthase shRNA plasmid, significantly reduced the
HGF-stimulated phosphorylation at Tyr-1313 and Tyr-1365 (Fig. 3Ba). An increase in GM3 content, by
treating cells with exogenous GM3, markedly promoted the
HGF-stimulated phosphorylation of cMet at Tyr-1313 and Tyr-1365
(Fig. 3Bb). The phosphorylation of
Tyr-1349 was not detected (data not shown). GM3 promoted the
HGF-stimulated phosphorylation of HGFR at Tyr-1313 and
Tyr-1365.
These results indicated that GM3 suppressed the
EGF-stimulated phosphorylation and activation of EGFR, but promoted
the HGF-stimulated phosphorylation and activation of cMet, which
may explain why GM3 exerted opposing affects on the motility and
migration of the EGF- and HGF-stimulated Hepa1–6 cells.
Effect of GM3 on the activity of
signaling pathways in Hepa1–6 cells
It is well known that the autophosphorylation of
tyrosine kinase receptors is an essential step for their
activation, which results in phosphorylation at specific tyrosine
residues on the intracellular domain of the receptors. Certain
cytoplasmic SH2-domain containing signaling molecules, including
PI3K, PLC-γ, growth factor receptor-bound protein 2 and protein
tyrosine phosphatases, bind to specific phosphotyrosine sequences
on the receptor, thereby initiating the intracellular corresponding
signaling pathways (20). To
further investigate the mechanism underlying the different effects
of GM3 on EGF- and HGF-stimulated motility and migration in
vitro, the present study investigated the effects of GM3 on the
activity of intracellular signaling pathways, which are essential
for the modulation of tumor cell invasion and migration. Three of
the main downstream signal transduction pathways activated by GFRs
are the MAPK, PLCγ and PI3K/Akt pathways. The present study
examined the effect of GM3 on the activity of these signaling
pathways. Fig. 4 shows that an
alteration in GM3 content had no significant affect on the
phosphorylation of MAPK (p44/42) in the Hepa1–6 cells when
stimulated by either EGF (Fig. 4Aa and
b) or HGF (Fig. 4Ba and b).
This result suggested that GM3 did not affect the activity of MAPK
signaling in the Hepa1–6 cells. The effect of GM3 on the activity
of the PLCγ signaling pathway was also assessed. It is understood
that the phosphorylation and translocation of PLCγ from the cytosol
to the plasma membrane are necessary for its activation and
function (13). The present study
attempted to examine the effect of GM3 on the phosphorylation of
PLCγ1, however phosphorylated PLCγ1 was undetected in the control
cells and in the cells treated with EGF or HGF (data not shown).
The localization of PLCγ1 in the cells was further examined,
however, membrane-binding PLCγ1 was not detected in the cells
stimulated with either EGF or HGF (data not shown). As shown in
Fig. 4, GM3 promoted the
expression of PLCγ1 in the cells stimulated with EGF (Fig. 4Ac and d) and inhibited the
expression of PLCγ1 in the cells stimulated with HGF (Fig. 4Bc and d). These data indicated that
GM3 did not affect the activity of the PLCγ1 signaling pathway. The
phosphorylation of Akt was also examined. In the EGF-treated cells,
suppression of GM3 synthesis by treatment of the cells with a GM3
synthase shRNA plasmid, elevated the phosphorylation of Akt at Ser
473 (Fig. 4Ae), whereas increasing
the GM3 content by treating the cells with exogenous GM3 inhibited
the phosphorylation of Akt at Ser 473 (Fig. 4Af). By contrast, in the HGF-treated
cells, suppression of GM3 synthesis by treatment of the cells with
a GM3 synthase shRNA plasmid inhibited the phosphorylation of Akt
at Ser 473 (Fig. 4Be). An increase
in the GM3 content following treatment with exogenous GM3 promoted
the phosphorylation of Akt at Ser 473 (Fig. 4Bf). These results suggested that
GM3 suppressed the EGF-stimulated activation of the PI3K/Akt
signaling pathway and promoted the HGF-stimulated activation of the
PI3K/Akt signaling pathway, indicating that the PI3K/Akt signaling
pathway may be important in GM3-regulated cell migration.
 | Figure 4(Aa, c, e, g, i, k and Bb, d, f, h, j,
l) Effect of GM3 on the EGF- and HGF-dependent signaling pathways
in the Hepa1–6 cells. The treated Hepa1–6 cells were serum-starved
for 12 h in the presence of the desired reagents and then
stimulated with 50 ng/ml EGF or 100 ng/ml HGF for 10 min. The cells
were then harvested and subjected to western blot analysis with
antibodies, as described in the Materials and methods. All
experiments were performed at least three times. Data are expressed
as the mean ± standard deviation and were analyzed by one-way
analysis of variance (Dunnett’s test). *P<0.05
between the different treatments. EGF, epidermal growth factor;
HGF, hepatocyte growth factor; PLCγ1, phospholipase Cγ1; MAPK,
mitogen-activated protein kinase; p-, phosphorylated; SiGM3S, GM3
synthase shRNA plasmid. |
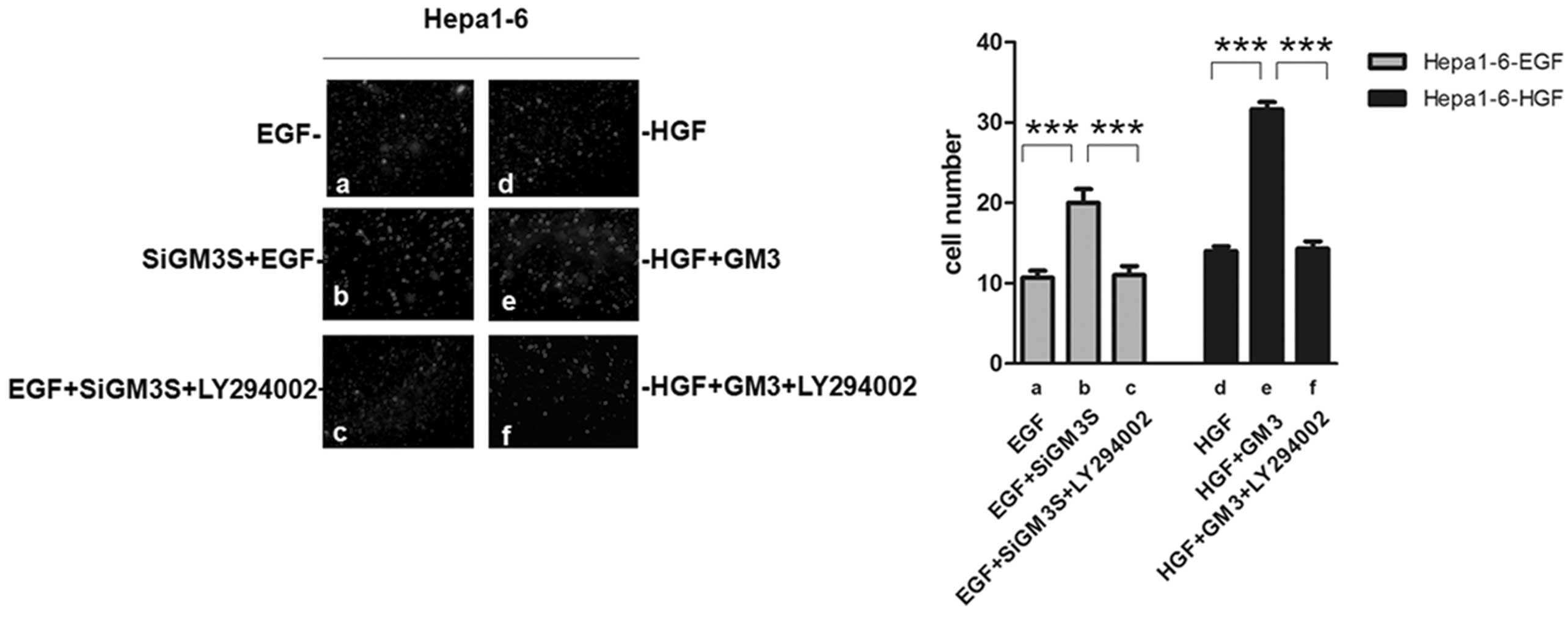
Effect of inhibiting the PI3K/Akt pathway
on cell mobility and migration
To confirm whether Hepa1–6 cell motility and
migration were mainly regulated by the PI3K/Akt signaling pathway,
the activity of PI3K in cells was inhibited using LY294002, an
inhibitor of PI3K. Inhibiting the PI3K/Akt pathway with LY294002
reversed the positive effect following transfection with a GM3
synthase shRNA plasmid (Fig. 5a–c)
on EGF-stimulated migration. The positive effect on HGF-stimulated
migration, caused by the addition of exogenous GM3, was also
reduced by inhibiting the PI3K/Akt pathway (Fig. 5d–f). This finding confirmed that
the PI3K/Akt pathway was involved in the modulation of Hepa1–6 cell
motility and migration in vitro.
Discussion
Several studies have demonstrated that expression of
the ganglioside GM3 is associated with the malignant behavior of
cancer cells, including cell proliferation, adhesion, invasion and
metastasis (21). However, the
precise mechanism of this effect remains to be elucidated. One
major problem is that GM3 exerts different, even opposite, actions
on different types of tumor. Several mechanisms that underline the
actions of GM3 have been reported. GM3 interacts with basal
membrane components, including adhesion molecules, to regulate cell
adhesion and migration and GM3 directly interacts with GFRs,
including EGFR, fibroblast growth factor receptor, platelet-derived
growth factor receptor, vascular endothelial growth factor and
insulin receptor, to modulate the receptor function and
subsequently affect the intracellular signaling pathways (20–23).
GM3 also indirectly regulates GFR activity by affecting the
interaction between GFR and membrane proteins, including integrins,
CD9 and CD82 tetraspanins (24–26)
or by affecting the intracellular Src kinase and protein tyrosine
phosphatase activity (27–29). GM3 also regulates the activation of
non-tyrosine kinase receptors, including G-protein coupled
receptors (30). Thus, the
mechanisms by which GM3 function are particularly complicated. In
different types of tumor or under different physiological or
pathological conditions, the expression levels of molecules
associated with GM3, including growth factors, adhesion molecules
and other membrane lipids differ and, consequently, GM3 exerts its
actions by different mechanisms (31).
As EGF and HGF are important cytokines regulating
various cell processes, including cell proliferation,
differentiation, apoptosis and oncogenesis, Hepa1–6 cells contain
mainly GM3. In the present study, GM2 had no effect on the
migration of Hepa1–6 cells treated with EGF and HGF. GM3 suppressed
the EGF-stimulated autophosphorylation of EGFR at tyr1173 and
subsequently downregulated the PI3K/Akt signaling pathway.
Conversely, GM3 enhanced the HGF-stimulated autophosphorylation of
HGFR at Tyr-1313 and Tyr-1365 and subsequently upregulated the
PI3K/Akt signaling pathway. This finding may, at least in part,
explain why GM3 had opposing effects on the EGF- and HGF-induced
Hepa1–6 cell motility and migration in vitro and may explain
why GM3 exerts its actions in a tissue or tumor-type specific
manner.
Acknowledgements
This study was support by grants from the National
Natural Science Foundation of China (grant no. 81401738) and
Liaoning Province Science and Technology Project (grant no.
2013023041).
References
|
1
|
Huwiler A, Kolter T, Pfeilschifter J and
Sandhoff K: Physiology and pathophysiology of sphingolipid
metabolism and signaling. Biochim Biophys Acta. 1485:63–99. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toledo MS, Suzuki E, Handa K and Hakomori
S: Cell growth regulation through GM3-enriched microdomain
(glycosynapse) in human lung embryonal fibroblast WI38 and its
oncogenic transformant VA13. J Biol Chem. 279:34655–34664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakomori S: Glycosphingolipids in cellular
interaction, differentiation and oncogenesis. Annu Rev Biochem.
50:733–764. 1981. View Article : Google Scholar
|
|
4
|
Regina-Todeschini A and Hakomori SI:
Functional role of glycosphingolipids and gangliosides in control
of cell adhesion, motility, and growth, through glycosynaptic
microdomains. Biochim Biophys Acta. 1780:421–433. 2008. View Article : Google Scholar
|
|
5
|
Hakomori SI: Glycosynaptic microdomains
controlling tumor cell phenotype through alteration of cell growth,
adhesion, and motility. FEBS Lett. 584:1901–1906. 2010. View Article : Google Scholar :
|
|
6
|
Van Echten G and Sandhoff K: Ganglioside
metabolism. Enzymology, topology, and regulation. J Biol Chem.
268:5341–5344. 1993.PubMed/NCBI
|
|
7
|
Iwabuchi K, Yamamura S, Prinetti A, Handa
K and Hakomori S: GM3- enriched microdomain involvedin cell
adhesion and signal transduction through carbohydrate-carbohydrate
interaction in mouse melanoma B16 cells. J Biol Chem.
273:9130–9138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kojima N and Hakomori S: Cell adhesion,
spreading, and motility of GM3-expressing cells based on
glycolipid-glycolipid interaction. J Biol Chem. 266:17552–17558.
1991.PubMed/NCBI
|
|
9
|
Hakomori S: Tumor malignancy defined by
aberrant glycosylation and sphingo (glyco) lipid metabolism. Cancer
Res. 56:5309–5318. 1996.PubMed/NCBI
|
|
10
|
Birklé S, Zeng G, Gao L, Yu RK and Aubry
J: Role of tumor-associated gangliosides in cancer progression.
Biochimie. 85:455–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimoto Y, Izumoto S, Suzuki T, et al:
Ganglioside GM3 inhibits proliferation and invasion of glioma.
Neurooncol. 71:99–106. 2005. View Article : Google Scholar
|
|
12
|
Kawamura S, Ohyama C, Watanabe R, et al:
Glycolipid composition in bladder tumor: a crucial role of GM3
ganglioside in tumor invasion. Int J Cancer. 94:343–347. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XQ, Sun P and Paller AS: Ganglioside
GM3 blocks the activation of epidermal growth factor receptor
induced by integrin at specific tyrosine sites. J Biol Chem.
278:48770–48778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XQ, Sun P, Go L, et al: Ganglioside
GM3 promotes carcinoma cell proliferation via urokinase plasminogen
activator-induced extracellular signal-regulated kinase-independent
p70S6 kinase signaling. J Invest Dermatol. 6:2687–2696. 2006.
View Article : Google Scholar
|
|
15
|
Mitsuzuka K, Handa K, Satoh M, Arai Y and
Hakomori S: A specific microdomain (“glycosynapse 3”) controls
phenotypic conversion and reversion of bladder cancer cells through
GM3-mediated interaction of alpha3beta1 integrin with CD9. J Biol
Chem. 280:35545–35553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saha S and Mohanty KC: Enhancement of
metastatic potential of mouse B16-melanoma cells to lung after
treatment with gangliosides of B-16-melanoma cells of higher
metastatic potential to lung. Indian J Exp Biol. 41:1253–1258.
2003.
|
|
17
|
Gu Y, Zhang J, Mi W, et al: Silencing of
GM3 synthase suppresses lung metastasis of murine breast cancer
cells. Breast Cancer Res. 10:1–12. 2008. View Article : Google Scholar
|
|
18
|
Ladisch S and Gillard B: A solvent
partition method for microscale ganglioside purification. Anal
Biochem. 146:220–231. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Biscardi JS, Maa MC, Tice DA, et al:
c-Src-mediated phosphorylation of the epidermal growth factor
receptor on Tyr845 and Tyr1101 is associated with modulation of
receptor function. J Biol Chem. 274:8335–8343. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bremer EG and Hakomori S: GM3 ganglioside
induces hamster fibroblast growth factor inhibition in chemically
defined medium: ganglioside may regulate growth factor receptor
function. Biochem Biophys Res Commun. 106:711–728. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rebbaa A, Hurh J, Yamamoto K, Kersey D and
Bremer EG: Ganglioside GM3 inhibition of EGF receptor mediated
signal transduction. Glycobiology. 6:399–406. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meuillet E, Cremel G, Dreyfus H and Hicks
D: Differential modulation of basic fibroblast and epidermal growth
factor receptor activation by ganglioside GM3 in cultured retinal
Muller glia. Glia. 17:206–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yates AJ, Saqr HE and Van Brocklyn J:
Ganglioside modulation of PDGF receptor. A model for ganglioside
functions. J Neurooncology. 24:65–73. 1995. View Article : Google Scholar
|
|
24
|
Mukherjee P, Faber AC, Shelton LM, et al:
Thematic review series: sphingolipids. Ganglioside GM3 suppresses
the proangiogenic effects of vascular endothelial growth factor and
ganglioside GD1a. J Lipid Res. 49:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung TW, Kim SJ, Choi HJ, et al:
Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: direct
interaction of GM3 with VEGFR-2. Glycobiology. 19:229–239. 2009.
View Article : Google Scholar
|
|
26
|
Kawakami Y, Kawakami K, Steelant WF, et
al: Tetraspanin CD9 is a “proteolipid,” and its interaction with
alpha 3 integrin in microdomain is promoted by GM3 ganglioside,
leading to inhibition of laminin-5-dependent cell motility. J Biol
Chem. 277:34349–34358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ono M, Handa K, Sonnino S, et al: GM3
ganglioside inhibits CD9-facilitated haptotactic cell motility:
coexpression of GM3 and CD9 is essential in the downregulation of
tumor cell motility and malignancy. Biochemistry. 40:6414–6421.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Todeschini AR, Dos Santos JN, Handa K and
Hakomori SI: Ganglioside GM2/GM3 complex affixed on silica
nanospheres strongly inhibits cell motility through
CD82/cMet-mediated pathway. Proc Natl Acad Sci USA. 105:1925–1930.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suárez Pestana E, Greiser U, Sánchez B, et
al: Growth inhibition of human lung adenocarcinoma cells by
antibodies against epidermal growth factor receptor and by
ganglioside GM3: involvement of receptor-directed protein tyrosine
phosphatase(s). Br J Cancer. 75:213–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suárez Pestana E, Tenev T, Gross S, et al:
The transmembrane protein tyrosine phosphatase RPTP sigma modulates
signaling of the epidermal growth factor receptor in A431 cells.
Oncogene. 18:4069–4079. 1999. View Article : Google Scholar
|
|
31
|
Bassi R, Viani P, Giussani P, Riboni L and
Tettamanti G: GM3ganglioside inhibits endothelin-1-mediated signal
transduction in C6 glioma cells. FEBS Lett. 507:101–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|