Introduction
Upper respiratory tract infection (URTI) are
disorders caused by acute infections, usually affecting the nose,
sinuses, pharynx, or larynx. The various URTIs include the common
cold, sinusitis, laryngitis and pharyngitis, amongst others
(1). URTIs are usually caused by
viruses, such as rhinoviruses, coronaviruses, parainfluenza virus
and adenoviruses (2). However,
numerous URTIs are also caused by bacteria, including
Streptococcus pyogenes, Streptococcus pneumoneae, Heamophilus
influenzae, Corynebacterium diptheriae, Bordetella pertussis
and Bacillus anthracis. Bronchitis, which is an inflammation
of the mucous membranes of the bronchi, can also be caused by
certain types of bacteria. Approximately 10% of bronchitis cases
are caused by bacteria, such as Mycoplasma pneumoniae,
Chlamydophila pneumoniae, B. pertussis and S. pneumoniae
(3,4).
Essential oils are composed of an odoriferous
mixture of monoterpenes, sesquiterpenes and aromatic compounds, and
form an important part of naturopathic therapy, where they are
well-known for their antimicrobial properties. Essential oils were
among the first topical and gastrointestinal antimicrobial agents
used by mankind. Due to the recent, extensive use of conventional
antibiotics and synthetic antimicrobial drugs, there has been an
increase in the widespread development of drug resistant
microorganisms, including methicillin-resistant Staphylococccus
aureus (MRSA), and multidrug resistant strains of Klebsiella
pneumoniae and Pseudomonas aeruginosa. These
drug-resistant microbes pose a challenge for scientists to identify
alternative ways to treat microbial infections. Essential oils are
antimicrobial agents with multiple target sites. The essential oils
and their components target the bacterial cell wall and cytoplasmic
membrane, resulting in permeabilization, which is followed by the
loss of ions, reduction of the membrane potential, collapse of the
proton pump and depletion of the ATP pool (5–7). Due
to their multifunctionality, essential oils have a potentially
large application in medicine and aromatherapy. Essential oils have
been shown to exhibit potent antimicrobial actions against a wide
range of both Gram-positive and Gram-negative bacteria (8).
Essential oils have also been used traditionally for
treating respiratory tract infections, and are currently used as
alternative medicines for the treatment of colds (9–11).
Inhalation therapy using essential oils, has previously been used
to treat acute sinusitis, and acute and chronic bronchitis. It has
been reported that inhalation therapy using volatile essential oil
vapors, is capable of enhancing the respiratory tract fluid output
(12), maintaining the ventilation
and drainage of the sinuses, and reducing asthma and inflammation
of the trachea (13–15).
The present study aimed to determine the chemical
composition of the essential oil of Artemisia capillaris as
well as to evaluate the antibacterial effects of Artemisia
capillaris and its primary components against common clinically
relevant respiratory bacterial pathogens. Gas chromatography mass
spectrometry was used to study the chemical composition of the oil.
In addition, Agar well diffusion assays and micro-well dilution
methods were used to study the antibacterial susceptibility of the
microbes towards the essential oil. Furthermore, Scanning Electron
Microscopy (SEM) was used to study the morphological changes which
occurred following oil exposure.
Materials and methods
Materials
The major essential oil components: α-pinene,
β-pinene, limonene, 1,8- cineole, piperitone, β-caryophyllene and
capillin were all purchased from Sigma-Aldrich (St Louis, MO,
USA).
Plant material
The aerial portions of the Artemisia
capillaris plant were collected from a local region in Jianguo,
China, between June and July 2013. The plant was identified by an
experienced taxonomist.
Essential oil isolation
The extraction of the essential oil from the aerial
parts of A. capillaris, was carried out by hydrodistillation
for 3 h using Clevenger-type apparatus (Lianyungang Hightborn
Technology Co., Ltd, Jiangsu, China), as recommended in the
European Pharmacopoeia (16).
Three samples of the dried aerial parts (200 g) were subjected to
hydrodistillation at separate times. The essential oil was
collected, dehydrated with Na2SO4
(Sigma-Aldrich), and stored at 4°C until further use.
Essential oil analysis
The essential oil was analyzed by a combination of
gas chromatography-flame ionization detection (GC-FID) and gas
chromatography-mass spectrometry (GC-MS) analytical techniques.
GC-FID analysis
GC-FID was carried out using a Perkin Elmer
AutoSystem XL Gas Chromatograph 8500 series (Perkin Elmer, Waltham,
MA, USA), with a flame ionization detector and head space analyzer,
using a fused silica capillary column HP-5 (30 m × 0.25 mm × 0.25
μm) coated with dimethyl polysiloxane. The oven temperature was
programmed from 50–260°C at 2°C/min, with an injector temperature
of 250°C and a detector temperature of 260°C. The injection volume
was 0.8 μl, and nitrogen was used as the carrier gas (1.2
ml/min).
GC-MS analysis
GC-MS analysis was conducted using a Varian Gas
Chromatograph series 3800 (Varian Medical Systems, Palo Alto, CA,
USA) fitted with a VF-5 MS fused silica capillary column (60 m ×
0.25 mm, × 0.25 μm), using split/splitless injection, and coupled
with a 4000 series mass detector. The GC-MS was conducted under the
following conditions: injection volume 0.8 μl with a split ratio of
1:80, helium was used as the carrier gas (1.5 ml/min constant flow
mode), an injector temperature of 250°C, and the oven temperature
was programmed from 50–260°C at 2°C/min. Mass spectra was produced
with an electron impact (EI+) mode 70 ev, and ion source
temperature 260°C. The mass spectra were recorded at a range
between 50–500 a.m.u.
Identification of components
Identification of the essential oil constituents was
based on the Retention Index, which was determined with respect to
a homologous series of n-alkanes (C5–C28; Polyscience, Niles, IL,
USA), which underwent the same experimental conditions;
co-injection with standards (Sigma Aldrich and standard isolates);
an MS Library search (NIST 05 and Wiley); and by comparing the MS
data of the present study with the previous MS literature data
(17).
Antibacterial testing
Bacterial strains and culture
media
S pyogenes [American Type Culture Collection
(ATCC) 12344], MRSA (ATCC 43300), MRSA (clinical strain),
Methicillin-gentamicin resistant S. aureus (MGRSA; ATCC
33592), S. pneumoniae (ATCC 2730), K. pneumoniae
(ATCC 27853), H. influenzae (ATCC 33391), E. coli
(clinical strain) were used in the present study. All of these
bacterial strains were obtained from the State Key Laboratory of
Microbial Resources, the Institute of Microbiology, Chinese Academy
of Sciences (Beijing, China). The bacterial strains were grown on
nutrient agar plates, at 37°C, and maintained on nutrient agar
slants. Cell suspensions of the micro-organisms in 0.5% NaCl, were
adjusted at 0.5 Mcfarland to obtain ~106 cfu/ml.
Agar well diffusion assay
The antibacterial susceptibility test was conducted
using the agar well diffusion assay. The overnight bacterial
cultures were added to 30 ml of liquid nutrient agar. The contents
of the tubes were then transferred to petri plates. Following 20
min solidification of the agar petri plates, at 25°C, the wells of
the plates were filled with 20 μl neat A. capillaris
essential oil and the major chemical constituents. The plates were
then incubated for 24 h at 37°C. Following the incubation, the
antimicrobial efficacy of the essential oil and its individual
constituents was determined by calculating the width of the
inhibition zone. The inhibition zones were expressed in mm. All of
the experiments were repeated in triplicate. Ampicillin and
vancomycin, (10 μg/disc) were used as positive controls.
Determination of minimum inhibitory
concentration (MIC) and minimum bactericidal concentration (MBC)
values
The MIC of the essential oil was determined using
the micro-well dilution method, as recommended by the National
Committee for Clinical Laboratory Standards, as reported by
previous methods (18). The
essential oil and its chemical constituents were dissolved in
dimethylsulfoxide (2 mg/ml) and diluted to prepare concentrations
in the range of 0.10–392.8 μg/ml (0.1, 0.2, 0.4, 0.8, 1.6, 3.2,
6.4, 12.8, 24.6, 49.2, 98.4, 196 and 392.8 μg/ml). The inoculum
suspensions, with a final concentration of 0.5×106
cfu/ml, were added to 96-well microplates. A total of 150 μl
Mueller Hinton (MH) broth was added to the wells of the 10th
column, which was reserved for the bacterial growth control. The
wells of the 11th column were reserved for the control of broth
sterility. The wells of the final column were used as a negative
control, containing 150 μl of nutrient broth and 5 μl of inoculum.
Following a 24 h incubation period at 37°C, the plates were
screened visually for broth turbidity. The MIC was defined as the
lowest concentration of the essential oil and the components at
which the bacteria did not exhibit any visible growth, following
the 24 h incubation at 37°C. The MBC was defined as the lowest
concentration of the essential oil and the components at which
99.9% of the bacterial population were killed, following the 24 h
incubation at 37°C.
SEM
The essential oil-treated bacterial cells were
obtained for SEM. Briefly, an overnight culture of S. aureus
(clinical strain), grown on MH agar at 37°C, was added to a saline
solution containing 0.1% Tween®-80 (Shanghai Sungo
Technology and Trade Co., Ltd, Shanghai, China). Four different
concentrations of the A. capillaris essential oil (20, 40,
60 and 80 μg/ml) were prepared and added to the suspension, which
was then incubated at room temperature. Following a 24 h
incubation, the bacterial cells were centrifuged at 8000 × g for 15
min. The bacterial cells were then washed with 0.1 mol/l
Tris-acetate buffer (pH 7.1), fixed in Tris-acetate buffer
containing 1.5% glutaraldehyde, and freeze-dried. Each of the
bacterial cultures was observed using a SEM (Shenzhen Jinliyang
Technology Co., Ltd., Guangdong, China), at magnification 10,000x.
A bacterial cell suspension in saline, with no essential oil
treatment, was used as a positive control.
Determination of potassium and
phosphate ion efflux
Overnight bacterial cultures of S. aureus
were harvested and washed three times in deionized water. The
bacterial cells were treated with 50 μl A. Capillaris
essential oil and the concentration of extracellular potassium and
phosphate ions was estimated, using an ion selective electrode and
a phosphorous inorganic kit 670-A (Sigma-Aldrich), respectively.
The numerical values were compared with standard calibration curves
of HPO4 and KCl for determination of phosphate and
potassium ion efflux, respectively. To observe the dead and viable
cells both prior to resuspension in water, and during the
incubation period at 37°C in the absence of an antimicrobial agent,
70 μl of the bacterial cultures were transferred to 250 μl MH
broth, and variations in optical density were recorded using a
Bioscreen (Shanghai Vizai Trade and Development Co., Ltd, Shanghai,
China).
Results
Chemical composition of the essential oil
of A. capillaris
The yield of the essential oil obtained from the dry
aerial parts of A. capillaris was ~1.6% w/v. The chemical
components of the essential oil were identified using GC and GC-MS
techniques (Table I and Fig. 1). A total of 25 compounds were
identified in the A. capillaris essential oil, accounting
for 90.1% of the total oil composition. The major components of the
essential oil were: α-pinene (4.3%), β-pinene (12.1%), limonene
(4.5%), 1,8-cineole (6.2%), piperitone (4.2%), β-caryophyllene
(5.2%), capillin (24.2%), and germacrene D (3.9%) (Fig 2). The essential oil of A.
capillaris was dominated by the presence of monoterpene
hydrocarbons (29.3%), oxygenated monoterpenes (18%), sesquiterpene
hydrocarbons (12.4%), amongst others (24.2%). Previous studies have
also demonstrated that various Artemisia essential oils
contain α-pinene, β-pinene, limonene, 1,8-cineole and capillin as
major constituents (5,18,19).
 | Table IChemical components identified in the
essential oil of Artemisia capillaris. |
Table I
Chemical components identified in the
essential oil of Artemisia capillaris.
| Compound | RI | Relative peak area
(%) |
|---|
| Tricyclene | 919 | 2.1 |
| α-Thujene | 924 | 1.2 |
| α-Pinene | 931 | 4.3 |
| Camphene | 943 | 1.6 |
| β-Pinene | 981 | 12.1 |
| Limonene | 1021 | 4.5 |
| 1,8-Cineole | 1038 | 0.9 |
| (z)-Ocimene | 1038 | 0.9 |
| Artemesia
Ketone | 1045 | 3.5 |
| γ-Terpinene | 1057 | 2.6 |
| 4-Terpineol | 1179 | 0.7 |
| Citronellol | 1213 | 1.6 |
| Piperitone | 1220 | 4.2 |
| Pulegone | 1224 | 1.8 |
| Eugenol | 1356 | 1.7 |
| β-Cubebene | 1382 | 0.7 |
|
β-Caryophyllene | 1420 | 5.2 |
| β-Farnesene | 1438 | 0.9 |
| Capillin | 1457 | 24.2 |
| Germacrene D | 1479 | 3.9 |
| δ-Cadinene | 1520 | 1.7 |
| Nerolidol | 1564 | 2.0 |
| Spathulenol | 1578 | 2.6 |
| Globulol | 1587 | 0.3 |
| α-Cadinol | 1652 | 1.3 |
| Monoterpene
Hydrocarbons | | 29.3 |
| Oxygenated
Monoterpenes | | 18 |
| Sesquiterpene
Hydrocarbons | | 12.4 |
| Oxygenated
Sesquiterpenes | | 6.2 |
| Others | | 24.2 |
| Total (%) | | 90.1 |
Antibacterial activity
The antibacterial activities of the A.
capillaris essential oil, and its major constituents (α-pinene,
β-pinene, limonene, 1,8-cineole, piperitone, β-caryophyllene and
capillin) were evaluated against various clinically significant,
and respiratory infection causing, bacterial strains. These
included: S. pyogenes, MRSA, MRSA (clinical strain), MGRSA,
S. pneumoniae, K. pneumoniae, H. influenzae
and E. coli. The potency of the essential oil, and its
chemical constituents, was assessed by measuring inhibition zones,
and MIC and MBC values. The essential oil of A. capillaris
and its major constituents exhibited a broad spectrum and variable
degree of antibacterial activity against the various tested
bacterial strains. The essential oil exhibited potent growth
inhibition against S. pyogenes (MIC = 52 and MBC <52),
MRSA clinical strain (MIC= 56, MBC= 98), S. pneumoniae (MIC
= 32, MBC= 56), K. pneumoniae (MIC = 32, MBC = 56), H.
influenzae (MIC = 26, MBC = 72), and E. coli (MIC = 24,
MBC = 64) μg/ml (Table II). The
two bacterial strains MRSA (ATCC 43300) and MGRSA (ATCC 33592) were
less susceptible to the effects of the essential oil, and exhibited
higher values of MIC and MBC. This reduction in susceptibility may
arise from the drug-resistant nature of these bacterial
strains.
 | Table IIAntibacterial activity of the
Artemisia capillaris essential oil, minimum inhibitory
concentration (MIC) and minimum bacteriocidal concentration (MBC)
values. |
Table II
Antibacterial activity of the
Artemisia capillaris essential oil, minimum inhibitory
concentration (MIC) and minimum bacteriocidal concentration (MBC)
values.
| Essential oil
(μg/ml) | Ampicillin
(μg/ml) | Vancomycin
(μg/ml) |
|---|
|
|
|
|
|---|
| Bacterial
strain | MIC | MBC | MIC | MBC | MIC | MBC |
|---|
| Streptococcus
pyogenes (ATCC 12344) | 52 | >52 | 0.7 | >1.5 | 0.5 | 1 |
| MRSA (ATCC
43300) | 72 | 112 | 0.9 | 2 | 0.5 | 1.5 |
| MRSA (Clinical
strain) | 56 | 98 | 0.9 | 2 | 2 | 4 |
| MGRSA (ATCC
33592) | 98 | >98 | 0.5 | 1.2 | 1 | 2 |
| Streptococcus
pneumoniae (ATCC 2730) | 32 | 56 | 2 | 4 | 1 | 2 |
| Klebsiella
pneumoniae (ATCC 27853) | 32 | 56 | 2 | 4 | 2 | 4 |
| Haemophilus
influenzae (ATCC 33391) | 26 | 72 | 1 | 2 | 2 | |
| Escherichia
coli (Clinical strain) | 24 | 64 | 1 | 2 | 1 | 2 |
To identify which of the chemical compounds present
in the essential oil of A. capillaris, were active against
the respiratory bacteria, a further in vitro experiment was
conducted to evaluate the antibacterial effects of the major
constituents: α-pinene, β-pinene, limonene, 1,8-cineole,
piperitone, β-caryophyllene and capillin (Table III). Almost all of the tested
essential oil constituents exhibited moderate to potent
antibacterial effects against the various bacterial strains.
However, the essential oil remained much more potent, as compared
with the individual chemical constituents. These results indicate
there is a possible interplay between the various chemical
constituents of the essential oil. Among the chemical compounds,
piperitone and capillin exhibited more potent growth inhibition of
the bacterial strains. K. pneumoniae, H. influenzae
and E. coli were the most susceptible bacterial strains
towards the effects of piperitone and capillin. Piperitone
treatment resulted in MIC/MBC values of 86/>86, 72/>72 and
72/>72 μg/ml against K. pneumoniae, H. influenzae and
E. coli respectively; whereas capillin treatment resulted in
MIC/MBC values of 72/>72, 64/>64 and 64/>64 respectively
against the above bacterial strains. Among all of the chemical
constituents, capillin had the most potent effects against all of
the bacterial strains. Capillin was capable of inhibiting the
growth of drug resistant bacterial strains, including MRSA and
MGRSA, resulting in MIC/MBC values of 112/>112 and 156/>156,
respectively, against these bacteria.
 | Table IIIAntibacterial activity of the major
chemical constituents of Artemisia capillaris. |
Table III
Antibacterial activity of the major
chemical constituents of Artemisia capillaris.
| α-Pinene | β-Pinene | Limonene | 1,8-Cineole | Piperitone |
β-Caryophyllene | Capillin |
|---|
|
|
|---|
| Bacterial
strain | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
|---|
| S.
pyogenes | 132 | >132 | 144 | >144 | 156 | >156 | 112 | >112 | 102 | >102 | 126 | >126 | 98 | >98 |
| MRSA | 210 | >210 | 212 | >212 | 332 | >332 | 244 | >244 | 112 | >112 | 330 | >330 | 156 | > 156 |
| MRSA(Clinical) | 172 | >172 | 170 | >170 | 150 | >150 | 152 | >150 | 122 | >122 | 144 | >144 | 112 | >112 |
| MGRSA | 256 | >256 | 256 | >256 | 330 | >330 | 256 | >256 | 156 | >156 | 332 | >332 | 156 | >156 |
| S.
pneumoniae | 172 | >172 | 170 | >170 | 198 | >198 | 132 | >132 | 112 | >112 | 122 | >122 | 64 | >64 |
| K.
pneumoniae | 178 | >178 | 170 | >170 | 156 | >156 | 102 | >102 | 86 | >86 | 64 | >64 | 72 | >72 |
| H.
influenzae | 126 | >126 | 132 | >132 | 128 | >128 | 98 | >98 | 72 | >72 | 64 | >64 | 64 | >64 |
| E. coli
(Clinical strain) | 98 | >98 | 102 | >102 | 112 | >112 | 102 | >102 | 72 | >72 | 92 | >92 | 64 | >64 |
SEM results
The treatment of S. aureus with A.
capillaris essential oil, induced marked morphological changes
in the bacteria, as determined by SEM. The S. aureus
bacterial cells were bloated, 12 h following A. capillaris
essential oil treatment (20, 40, 60 and 80 μg/ml). The bacterial
cells were crushed and collected 48 h following the treatment with
the essential oil of A. capillaris. The essential oil
induced cell morphological changes in the S. aureus
bacterial cells, in a dose-dependent manner, whereas the untreated
control cells did not show any changes in cell morphology (Fig. 3). Treatment of S. aureus
with 80 μg/ml of the essential oil, for 12 h, killed >90%
of the bacterial cells. These results indicate that the A.
capillaris essential oil produces bactericidal effects against
S. aureus microbes.
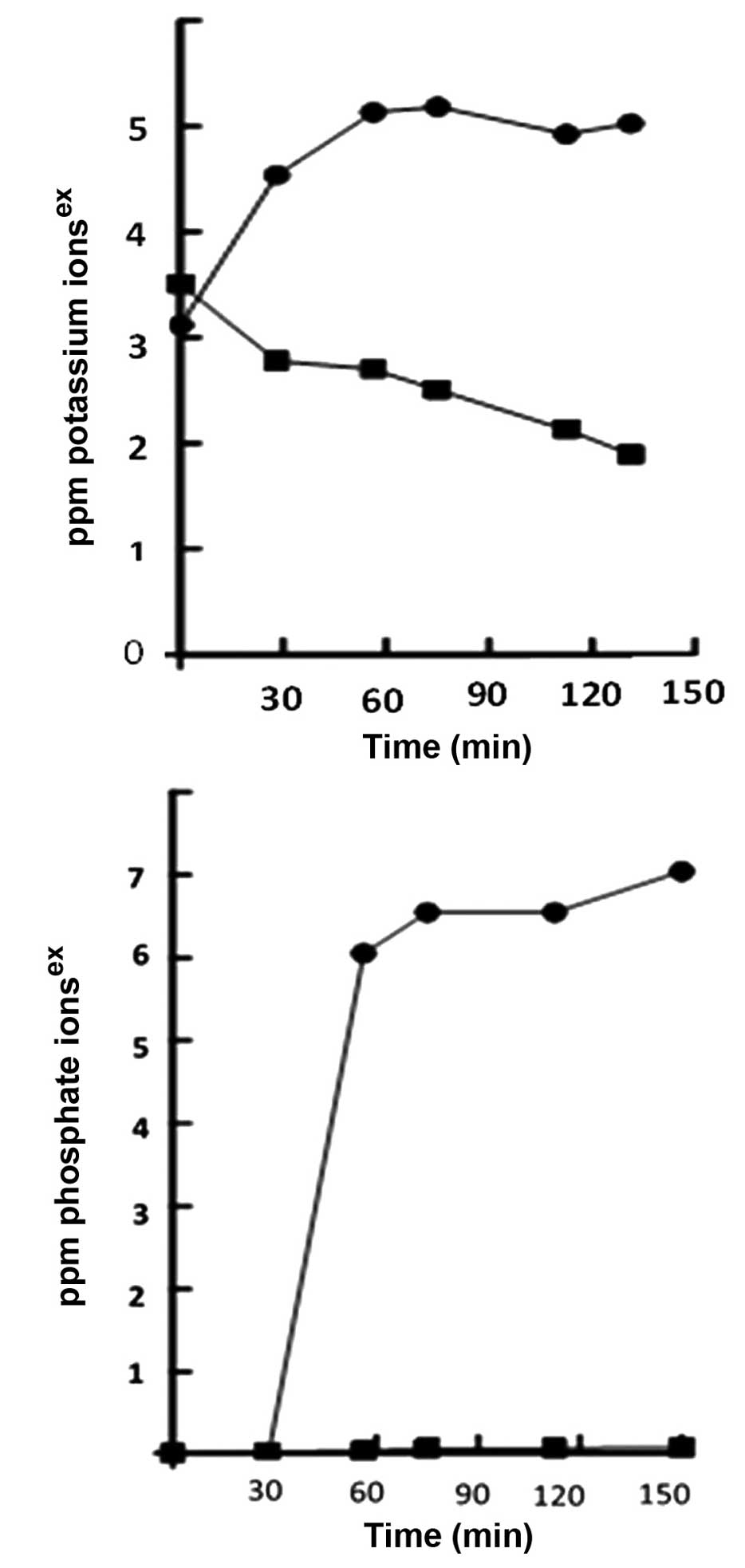
Leakage of phosphate and potassium
ions
A 50 μg/ml concentration of A. capillaris
essential oil was administered to a culture of ~1×106
cfu/ml S. aureus. The treated bacterial cultures exhibited
significant potassium and phosphate ion leakage following essential
oil administration, as compared with the untreated controls
(Fig. 4). These results indicate
that the antimicrobial actions of the essential oil are mediated
through the leakage of potassium and phosphate ions. It has
previously been reported that essential oils may induce the loss of
ions which are integral for the sustenance of bacterial strains,
and this is known to be one of the predominant mechanisms by which
essential oils exhibit their bactericidal effects (20).
Discussion
Essential oils have been used in traditional
medicine for the treatment of bacterial, viral and fungal
infections worldwide for centuries (19). Currently, essential oils are used
mainly in alternative and holistic medicine, for similar purposes,
and may be administered orally, topically or by aromatherapy. An
increasing number of studies have recently focussed on elucidating
the specific mechanisms of action of essential oils and their
components. Emerging evidence has shown that many essential oils
have both non-specific and specific mechanisms of action, which
vary based on the relative abundance and chemical composition of
the components. Elucidation of the mechanism of action of these
compounds may enable identification of new antibiotic targets, and
exploitation of novel biochemical pathways which are not currently
targeted by existing antibiotics. Furthermore, a combination of
existing drugs with essential oils and/or their components, may
provide an alternative approach to combat emerging drug resistance.
Antibiotic resistance is currently outpacing the research and
development required to identify new drugs; therefore, scientists
are facing a return to the ‘pre-antibiotic era’. Essential oils are
currently used in the food and beverage industries, and in perfumes
and cosmetics. In addition to this, essential oils have been shown
to exhibit a broad spectrum of biological activity, which has led
to increased interest among scientists. There has been extensive
recent research conducted to discover and determine the
antimicrobial activity of essential oils (21). The mechanism of action remains
unclear, but some studies have suggested that essential oil
compounds may penetrate the cell, where they interfere with
cellular metabolism (22). Other
studies have shown that phenols, such as carvacrol and eugenol,
disrupt the cellular membrane and react with the active sites of
enzymes. Essential oils and their components may interact with the
cell membrane and accumulate in the lipid bilayer of bacteria,
occupying a space between the chains of fatty acids (23, 24).
It has previously been demonstrated that various
Artemisia essential oils, as well as the major components
found in A. capillaris essential oil, may possess
antimicrobial activities. For example, A. indica (6), A. absinthium (22), A. biennis (22), A. cana (22), A. dracunculus (22), A. frigida (22), A. longifolia (22), A. ludoviciana (22) A. chamaemelifolia (23), A. turcomanica (23), A. annua (24) and A. fragrans (25), which are used in herbal medicines,
have been reported to possess antimicrobial properties.
Furthermore, the most abundant compounds present in the A.
capillaris essential oil: Germacrene D, α-pinene, β-pinene,
1,8-cineole, limonene, have been reported to exhibit antimicrobial
activity. (6,22,24).
The present study is, to the best of our knowledge, the first to
report on the antimicrobial activities of the essential oil of
A. capillaris. These results further support the importance
of the Artemisia species containing biologically active
metabolites for drug development.
In conclusion, the present study has demonstrated
that the essential oil of A. capillaris produces potent
antibacterial effects against various respiratory tract
infection-causing microbes. The bactericidal effects of the
essential oil are mediated by the induction of significant
morphological changes in bacterial cells, as well as promoting the
leakage of potassium and phosphate ions from the bacterial cells.
The results of the present study are significant, since numerous
bacterial strains used have attained drug resistance, and many
conventional drugs are not effective against them.
References
|
1
|
Eccles MP, Grimshaw JM, Johnston M, et al:
Applying psychological theories to evidence-based clinical
practice: identifying factors predictive of managing upper
respiratory tract infections without antibiotics. Implement Sci.
2:262007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mäkelä MJ, Puhakka T, Ruuskanen O, et al:
Viruses and bacteria in the etiology of the common cold. J Clin
Microbiol. 36:539–542. 1998.PubMed/NCBI
|
|
3
|
Albert RH: Diagnosis and treatment of
acute bronchitis. Am Fam Physicianl. 82:1345–1350. 2010.
|
|
4
|
Cohen J and Powderly W: Chapter 33:
Bronchitis, Bronchiectasis, and Cystic Fibrosis. Infectious
Diseases. 2nd edition. Day J: Mosby (Elsevier); Oxford: 2004
|
|
5
|
Rather MA, Dar BA, Dar MY, et al: Chemical
composition, antioxidant and antibacterial activities of the leaf
essential oil of Juglans regia L. and its constituents.
Phytomedicine. 19:1185–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rashid S, Rather MA, Shah WA and Bhat BA:
Chemical composition, antimicrobial, cytotoxic and antioxidant
activities of the essential oil of Artemisia indica Willd. Food
Chem. 138:693–700. 2013. View Article : Google Scholar
|
|
7
|
Bakkali F, Averbeck S, Averbeck D and
Idaomar M: Biological effects of essential oils - a review. Food
Chem Toxicol. 46:446–475. 2008. View Article : Google Scholar
|
|
8
|
Edris AE: Pharmaceutical and therapeutic
potentials of essential oils and their individual volatile
constituents: a review. Phytother Res. 21:308–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rantzsch U, Vacca G, Dück R and Gillissen
A: Anti-inflammatory effects of Myrtol standardized and other
essential oils on alveolar macrophages from patients with chronic
obstructive pulmonary disease. Eur J Med Res. 14:205–9. 2009.
View Article : Google Scholar
|
|
10
|
Federspil P, Wulkow R and Zimmermann T:
Effects of standardized Myrtol in therapy of acute sinusitis -
results of a double-blind, randomized multicenter study compared
with placebos. Laryngorhinootologie. 76:23–27. 1997.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schindl R: Inhalational effect of volatile
oils. Wien Med Wochenschr. 122:591–593. 1972.(In German).
PubMed/NCBI
|
|
12
|
Boyd EM and Sheppard P: Nutmeg oil and
camphene as inhaled expectorants. Arch Otolaryngol. 92:372–378.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burrow A, Eccles R and Jones AS: The
effects of camphor, eucalyptus and menthol vapours on nasal
resistance to airflow and nasal sensation. Acta Otolaryngol.
96:157–161. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shubina LP, Siurin SA and Savchenko VM:
Inhalations of essential oils in the combined treatment of patients
with chronic bronchitis. Vrach Delo. 5:66–67. 1990.(In
Russian).
|
|
15
|
Fröhlich E: Lavender oil, review of
clinical, pharmacological and bacteriological studies. Contribution
to classification of the mechanism of action. Wien Med Wochenschr.
118:345–350. 1968.(In German).
|
|
16
|
Europarådet, European Pharmacopoeia
Commission. European Pharmacopoeia. 3. Maisonneuve SA:
Sainte-Ruffine; pp. 68–71. 1975
|
|
17
|
Adams RP: Identification of essential oil
components by gas chromatography/mass spectrometry. Stream C:
Allured Publishing Corporation; Illinois, USA: 2007
|
|
18
|
Ashour ML, El-Readi M, Youns M, et al:
Chemical composition and biological activity of the essential oil
obtained from Bupleurum marginatum (Apiaceae). J Pharm Pharmacol.
61:1079–1087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ríos JL and Recio MC: Medicinal plants and
antimicrobial activity. J Ethnopharmacol. 22:80–84. 2005.
View Article : Google Scholar
|
|
20
|
Cox SD, Gustafson JE, Mann CM, Markham JL,
Liew YC, Hartland RP, Bell HC, Warmington JR and Wyllie SG: Tea
tree oil causes K+ leakage and inhibits re spirationin Escherichia
coli. Lett Appl Microbiol. 26:355–358. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saad Y, Muller D and Lobstein A: Major
bioactivities and mechanism of action of essential oils and their
components. Flavour Fragrance J. 28:269–279. 2013. View Article : Google Scholar
|
|
22
|
Kalemba D and Kunicka A: Antibacterial and
antifungal properties of essential oils. Curr Med Chem. 10:813–829.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dorman HJ and Deans SG: Antimicrobial
agents from plants: antibacterial activity of plant volatile oils.
J Appl Microbiol. 88:308–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adorjan B and Buchbauer G: Biological
properties of essential oils: an updated review. Flavour Fragr J.
25:407–426. 2010. View
Article : Google Scholar
|
|
25
|
Lopes-Lutz D, Alviano DS, Alviano CS and
Kolodziejczyk PP: Screening of chemical composition, antimicrobial
and antioxidant activities of Artemisia essential oils.
Phytochemistry. 69:1732–1738. 2008. View Article : Google Scholar : PubMed/NCBI
|