Introduction
Intracerebral hemorrhage (ICH), a common and
devastating disorder, accounts for 10–20% of all strokes (1) and causes brain edema and neuronal
cell death. The rapid release of blood into the parenchyma results
in limited hematoma, which causes blood-brain barrier (BBB)
disruption, brain edema and inflammation. Secondary injury occurs
resulting from the toxic effects of blood components, including
thrombin (2) and erythrocyte
rupture (3).
Thrombin, a serine protease, is an important
component in the process of blood coagulation. The quantity of
thrombin in the blood significantly exceeds the requirement of
coagulation (4). The excess
thrombin released from a hematoma or blood clot affects the
microenvironment surrounding astrocytes and microglia (5) via activation of protease activated
receptor-1 (PAR-1) (6). Matrix
metalloproteinases (MMPs) are a family of zinc-dependent
endopeptidase enzymes, which may cause degradation of extracellular
matrix proteins and lead to an increase in BBB permeability and
brain edema. Among the MMPs, MMP-9 is a major contributor to the
disruption of the major components of the basal lamina surrounding
cerebral blood vessels (7).
Aquaporin 4 (AQP4), a member of the aquaporin family of
water-selective transporting proteins, is important in cerebral
water balance (8). Studies have
demonstrated that increases in MMP-9 and AQP4 expression are
associated with thrombin-induced disruption of the BBB and brain
edema (9,10).
Hypothermia has been established to exert
neuroprotective properties in the acute phase following ICH
(9,10). It may effectively relieve brain
edema (11) and the disruption of
the BBB (12) caused by thrombin
and it reduces the accumulation of excitatory amino acids and free
radicals (13,14). Therefore, it is expected that
reducing brain temperature may limit cell death, thereby improving
recovery. However, this mechanism has not at present been clearly
demonstrated.
To gain an improved understanding of the effects of
hypothermia on thrombin-induced edema and the expression of PAR-1,
MMP-9 and AQP4, a time course was established for their comparison.
The current study aimed to elucidate whether hypothermic treatment
is practical and effective in intracerebral hemorrhage in rats.
Materials and methods
Animal preparation and experimental
groups
Adult male Sprague-Dawley rats (n=360; weight,
250–300 g) were used throughout the study. Animals were treated in
accordance with the guidelines set by The Chinese Council for the
Care and Use of Laboratory Animals and were approved by the
Institutional Animal Care and Use Committee at the Harbin Medical
University (Harbin, China). Rats were allowed free access to food
and water and were housed with a 12 h/12 h light/dark cycle. No
mortality or signs of illness observed in the experimental
animals.
The rats were anesthetized with chloral hydrate (0.3
g/kg body weight, intraperitoneally; Sigma-Aldrich, St. Louis, MO,
USA) prior to aseptic surgery as previously described (15). Briefly, animals were placed in a
stereotaxic frame (Bilaney Consultants GmbH, Düsseldorf, Germany).
A midline scalp incision was made and a hole was drilled in the
left side of the skull (0.2 mm anterior, 3.0 mm lateral and 5.0 mm
ventral, with respect to bregma); a total of 50 μl thrombin (10
U/ml; Sigma-Aldrich) was injected into the right caudate nucleus
(rats in the sham group received an injection of 50 μl saline
only). The syringe was subsequently removed slowly. The hole in the
skull was sealed with bone wax (Shanghai Sanyou Medical Instrument
Co., Ltd., Shanghai, China) and the scalp was sutured. Body
temperature was maintained at 37°C during surgery with the use of a
feedback-controlled heating pad. Rats were randomly assigned to
three groups: The normothermia (NT) group, the hypothermia (HT)
group and the sham group. Rats in the NT and HT groups were
euthanized using an overdose (0.6 g/kg) of chloral hydrate at 6,
24, 48 h or 3, 5 or 7 days after surgery (n=6 for each time point).
Rats in the sham group (n=6) were euthanized at 24 h after the
infusion of saline.
Focal mild hypothermia
The rats in the HT group were cooled immediately
following surgery with a hypothermia instrument (Patent no.
ZL98236936.0; Harbin Institute of Technology, Harbin, China)
following the thrombin injection as previously described (16). Briefly, following the injection of
thrombin, the heads of the rats were fixed on the metallic plate of
the hypothermia instrument, which reduced the cranial temperature
to 33±0.5°C; hypothermia was applied for 4 h from when the target
temperature was reached. During the treatment of hypothermia, the
temperature probes of the SL-4 temperature sensor (Tongji Medical
University, Wuhan, China) were inserted into the ipsilateral and
contralateral basal ganglia and rectum to monitor cranial and core
temperatures. A heating pad was also used to maintain the core
temperature at ~37°C. Rats rewarmed spontaneously following
hypothermia and were allowed free access to food and water. Rats in
the NT group were subject to the same conditions as the HT group
with the exception of the hypothermia treatment.
Western blot analysis
The brains were removed and dissected rapidly and
the striatal tissues (3×3×3 mm, ~100 mg) were collected. Western
blot analysis was performed as previously described (17). Briefly, brain tissues were ground
in liquid nitrogen and proteins were extracted using a Tissue
Protein Extraction Reagent (Boster Biological Technology, Inc.,
Wuhan, China). The protein concentration was detected using a
bicinchoninic acid protein assay kit (Santa Cruz Biotechnology,
Inc., Lake Placid, NY, USA). Subsequently, proteins were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene fluoride membrane (Santa Cruz
Biotechnology, Inc.). Following blocking with 5% bovine serum
albumin for 2 h, membranes were subsequently incubated with mouse
monoclonal thrombin R (ATAP2; sc-13503; 1:1,000; Santa Cruz
Biotechnology, Inc.), mouse monoclonal anti-AQP4 (C-19; sc-9888;
1:1,000; Santa Cruz Biotechnology, Inc.) and goat polyclonal
anti-MMP-9 (C-20; sc-6840; 1:1,000; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. The following day, the membrane was washed with
Tris-buffered saline with Tween® (TBS-T; 3×10 min) and
subsequently incubated with horseradish peroxidase-conjugated goat
anti-mouse (BA1051) or rabbit anti-goat (BA1060) secondary
antibodies (1:500; Boster Biological Technology, Inc.) at room
temperature for 2 h. The membrane was washed again with TBS-T (3×10
min) and the results were visualized using electrochemiluminescence
and luminol reagent (sc-2048; 1:1 ratio of solutions A and B; Santa
Cruz Biotechnology, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to determine mRNA expression levels
of PAR-1, MMP-9 and AQP4 in the brain. Total RNA was extracted from
the frozen tissue samples with TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) (17) at each time point following thrombin
injection. PCR procedures were performed as follows: Each reaction
volume was 25 μl. PCR was performed at 95°C for 2 min, 94°C for 1
min, 58°C for 45 sec, followed by 25 cycles of 74°C for 10 min. The
primer sequences were as follows: Forward:
5′-TGCGGTCCTTTGCTGTCTTC-3′ and reverse: 5′-GTCTTCTGTCTCCACTTGGCT-3′
for PAR-1; forward: 5′-TCCTCTACCTGGTCACACCC-3′ and reverse:
5′-GTCTTCTGTCTCCACTTGGCT-3′ for AQP4; forward:
5′-ACCTCCAACCTCACGGACA-3′ and reverse: 5′-GTCTTCTGTCTCCACTTGGCT-3′
for MMP-9; and forward: 5′-TGTGATGGTGGGTATGGG-3′ and reverse:
5′-TAGAAGCATTTGCGGTGC-3′ for β-actin.
Determination of BBB permeability
BBB permeability was evaluated using the Evans Blue
dye method (EB) enabling the identification of extravasation
(18). Briefly, EB (2%, 4 ml/kg)
was injected intravenously and allowed to circulate for 2 h. The
brain was subsequently transcardially perfused with 200 ml saline
through the left ventricle until colorless liquid was obtained from
the right atrium. Following decapitation, the brain was removed and
100 mg of tissue surrounding the brain injury was dissected. For
quantitative measurements, the brain samples were placed in 5 ml
formamide solution and incubated for 72 h at 37°C. The optical
density of the EB formamide solution was determined by
spectrophotometry (Multiskan MK3; Thermo Fisher Scientific,
Waltham, MA, USA) at 620 nm and the absorbance of the supernatant
solution was measured according to the EB/formamide standard
samples. The BBB permeability was expressed as EB/g of brain
tissue.
Determination of brain water content
Brain water content was determined using the dry-wet
weight method (19). Following
decapitation, rat brain samples were immediately weighed on an
electronic analytical balance (JA1003A Electronic Precision
Balance; Changzhou Keyuan Balance Instrument Co., Ltd., Changzhou,
China) to obtain the wet weight. The tissues were subsequently
dried in an oven at 100°C for 24 h to obtain the dry weight. Brain
water content was calculated as: Wet weight-dry weight)/wet weight
× 100.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The analyses for multiple groups were performed using an analysis
of variance followed by Tukey’s honest significant difference post
hoc test; independent two-sample t-tests were used to compare the
means of the two groups at each time point. All data were analyzed
with SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Brain water content and BBB
permeability
Following the injection of thrombin, the brain water
content in the basal ganglia began to increase within 6 h in the NT
group (n=6) and reached a maximum at 24 h. Furthermore, increased
levels were maintained at 72 h. The HT group exhibited a lower
brain water content in the basal ganglia compared with the NT group
(P<0.05). The brain water content began to decline at 48 h after
thrombin injection (Fig. 1A).
The BBB permeability to EB, which is a
protein-binding dye that binds to albumin and a marker of BBB
extravasation, demonstrated a trend similar to the brain water
content. A previous study identified that hypothermia did not alter
the BBB permeability in normal tissue (11). The extent of EB extravasation
observed in the damaged brain tissue increased rapidly within 6 h
and reached a maximum at 24 h. The level of EB extravasation was
attenuated at 48 h. Hypothermia significantly reduced the EB
extravasation (Fig. 1B).
Effects of focal hypothermia on
thrombin-induced AQP4 expression
To determine whether hypothermia affected AQP4,
levels of AQP4 were measured at 6, 24, 48 and 72 h and 3, 5 and 7
days by RT-qPCR and western blot analysis (Fig. 2). AQP4 expression reached a peak at
48 h. Compared with the NT group, in the HT group there was a
significant decrease in AQP4 expression at each time point.
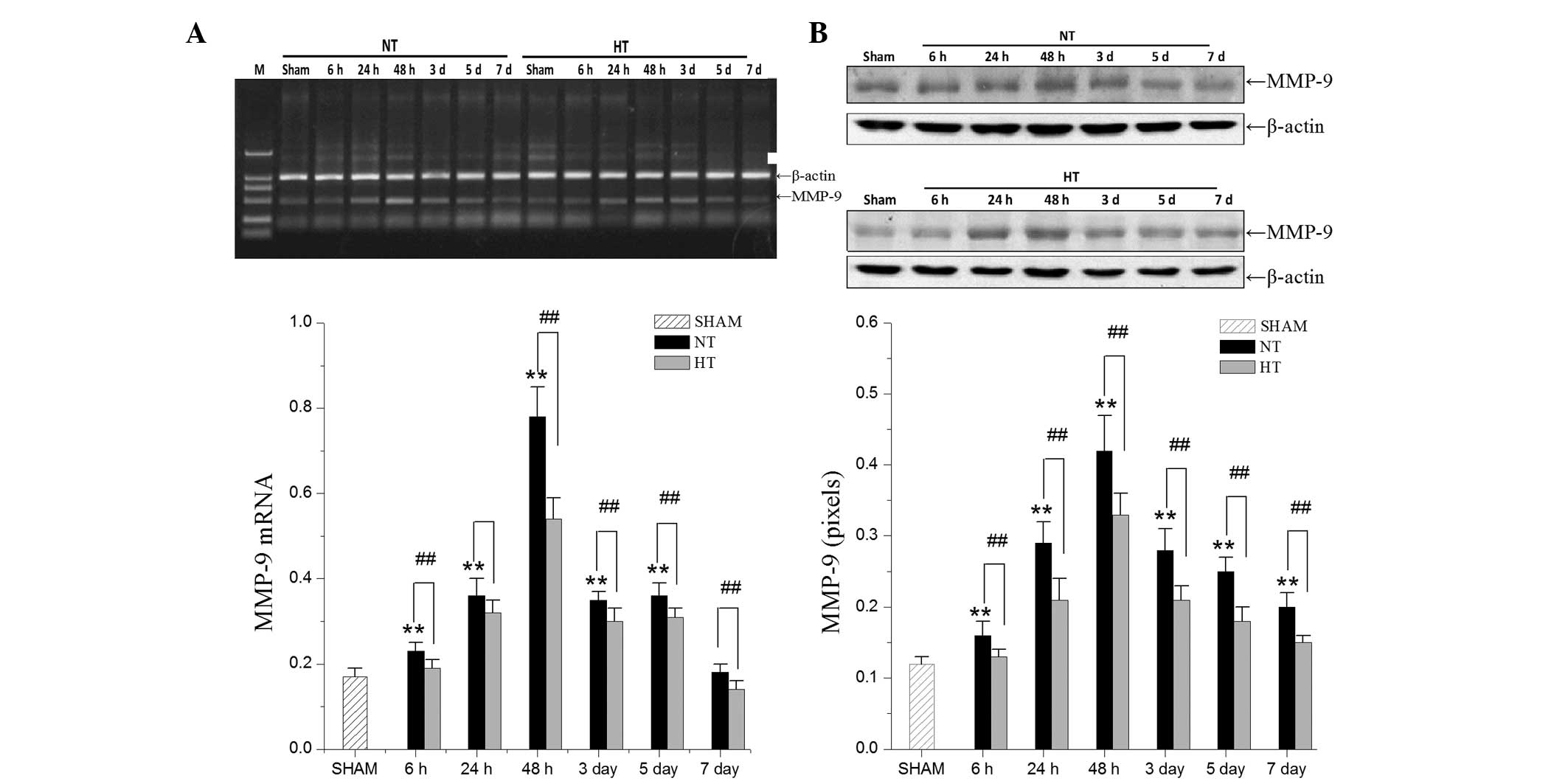
Effects of focal hypothermia on
thrombin-induced MMP-9 expression
Using RT-qPCR and western blot analysis, the
expression levels of MMP-9 in the HT group and the NT group were
compared. MMP-9 mRNA and protein expression (Fig. 3) significantly increased at 24 h
and peak levels were observed at 48 h. Hypothermia led to a
decreased expression of MMP-9.
Effects of focal hypothermia on
thrombin-induced PAR-1 expression
To investigate whether hypothermia decreased PAR-1
expression, RT-qPCR and western blot analysis was used to analyze
the differences between the HT group and the NT group (Fig. 4). The data revealed that
hypothermia in the HT group attenuated PAR-1 mRNA and protein
expression compared with the NT group.
Discussion
Hypothermia is a neuroprotective strategy in ICH
(20) and has been demonstrated to
moderate perihematomal edema (11,21).
Edema formation, following ICH has a close association with
thrombin (2); however, little is
known of the time course of effects of mild focal hypothermia on
thrombin-induced edema formation in rats. The principal aims of the
present study were to examine the mechanisms underlying the
neuroprotective effects of focal mild hypothermia following
ICH.
The results indicated that edema in the HT group
significantly decreased within 24 and 72 h compared with the NT
group following thrombin infusion. However, the mechanisms
responsible for the edema induced by thrombin remain to be
elucidated. Previous evidence has indicated that thrombin-induced
vasogenic brain edema formation responds to the activation of PAR-1
and the disruption of the BBB (22,23).
In the present study, PAR-1 within the NT group increased within 6
h and reached a maximum level at 48 h after thrombin infusion.
Treatment with focal mild hypothermia downregulated this level
markedly, which indicated that focal mild hypothermia effected
thrombin-PAR-1 signaling to reduce BBB permeability.
The present study identified that MMP-9 expression
was correlated with the breakdown of BBB integrity (24). Furthermore, upregulation of MMP-9
expression in the perihematomal region following ICH has been
reported in clinical patients (25) and rats (26). The present study demonstrated that
MMP-9 expression in the NT group reached a maximal level at 48 h
and remained increased at 72 h after thrombin infusion, which is
consistent with previous findings (19,27).
Compared with the NT group, the HT group had downregulated the
maximal level of MMP-9 at 48 h. According to the present data and a
previous study (16), the
reduction of MMP-9 expression caused by mild focal hypothermia was
responsible for moderating BBB disruption.
The effect of thrombin on AQP4 expression is
inconclusive. A study has suggested that AQP4 expression is
upregulated following ICH (28),
whereas other evidence has demonstrated that thrombin inhibits AQP4
expression (29). The present
study revealed that AQP4 expression in the NT group increased
within 6 h, reached a maximum level at 48 h and increased levels
were maintained for 7 days. Therapy with focal mild hypothermia
induced a robust reduction of AQP4 levels at 48 h and returned to
approximately normal levels at 7 days in the HT group. These
findings demonstrate that focal mild hypothermia was associated
with a downregulation in AQP4 expression.
In conclusion, the present study has demonstrated
that focal mild hypothermia may be neuroprotective and ameliorates
the edema induced by thrombin, which disrupts the BBB through the
activation of the PAR-1 pathway. In addition, focal mild
hypothermia downregulates the expression of MMP-9 and AQP4, which
are closely associated with brain edema. These findings provide
evidence for using hypothermia in the treatment of ICH.
Acknowledgements
This study was supported by The Natural Sciences
Foundation of Heilongjiang Province, China (grant no.
QC2009C77).
References
|
1
|
Sacco S, Marini C, Toni D, Olivieri L and
Carolei A: Incidence and 10-year survival of intracerebral
hemorrhage in a population-based registry. Stroke. 40:394–399.
2009. View Article : Google Scholar
|
|
2
|
Lee KR, Colon GP, Betz AL, Keep RF, Kim S
and Hoff JT: Edema from intracerebral hemorrhage: the role of
thrombin. J Neurosurg. 84:91–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xi G, Keep RF and Hoff JT: Erythrocytes
and delayed brain edema formation following intracerebral
hemorrhage in rats. J Neurosurg. 89:991–996. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arand AG and Sawaya R: Intraoperative
chemical hemostasis in neurosurgery. Neurosurgery. 18:223–233.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noorbakhsh F, Vergnolle N, Hollenberg MD
and Power C: Proteinase-activated receptors in the nervous system.
Nat Rev Neurosci. 4:981–990. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue M, Hollenberg MD, Demchuk A and Yong
VW: Relative importance of proteinase-activated receptor-1 versus
matrix metalloproteinases in intracerebral hemorrhage-mediated
neurotoxicity in mice. Stroke. 40:2199–2204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenberg GA: Matrix metalloproteinases in
neuroinflammation. Glia. 39:279–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zador Z, Stiver S, Wang V and Manley GT:
Role of aquaporin-4 in cerebral edema and stroke. Handb Exp
Pharmacol. 190:159–170. 2009. View Article : Google Scholar
|
|
9
|
Dietrich WD, Atkins CM and Bramlett HM:
Protection in animal models of brain and spinal cord injury with
mild to moderate hypothermia. J Neurotrauma. 26:301–312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
MacLellan CL, Clark DL, Silasi G and
Colbourne F: Use of prolonged hypothermia to treat ischemic and
hemorrhagic stroke. J Neurotrauma. 26:313–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawai N, Kawanishi M, Okauchi M and Nagao
S: Effects of hypothermia on thrombin-induced brain edema
formation. Brain Res. 895:50–58. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawanishi M, Kawai N, Nakamura T, Luo C,
Tamiya T and Nagao S: Effect of delayed mild brain hypothermia on
edema formation after intracerebral hemorrhage in rats. J Stroke
Cerebrovasc Dis. 17:187–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Busto R, Globus MY, Dietrich WD, et al:
Effect of mild hypothermia on ischemia-induced release of
neurotransmitters and free fatty acids in rat brain. Stroke.
20:904–910. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Globus MY, Alonso O, Dietrich WD, Busto R
and Ginsberg MD: Glutamate release and free radical production
following brain injury: effects of posttraumatic hypothermia. J
Neurochem. 65:1704–1711. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fingas M, Clark DL and Colbourne F: The
effects of selective brain hypothermia on intracerebral hemorrhage
in rats. Exp Neurol. 208:277–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao JK, Guan FL, Duan SR, et al: Effect
of focal mild hypothermia on expression of MMP-9, TIMP-1, Tau-1 and
β-APP in rats with cerebral ischaemia/reperfusion injury. Brain
Inj. 27:1190–1198. 2013. View Article : Google Scholar
|
|
17
|
Wu H, Zhang Z, Li Y, et al: Time course of
upregulation of inflammatory mediators in the hemorrhagic brain in
rats: correlation with brain edema. Neurochem Int. 57:248–253.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arican N, Kaya M, Kalayci R, Kucuk M,
Cimen V and Elmas I: Effects of acute cold exposure on blood-brain
barrier permeability in acute and chronic hyperglycemic rats.
Forensic Sci Int. 125:137–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song EC, Chu K, Jeong SW, et al:
Hyperglycemia exacerbates brain edema and perihematomal cell death
after intracerebral hemorrhage. Stroke. 34:2215–2220. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang J, Yu M and Zhu C: Effect of
long-term mild hypothermia therapy in patients with severe
traumatic brain injury: 1-year follow-up review of 87 cases. J
Neurosurg. 93:546–549. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kollmar R, Staykov D, Dörfler A,
Schellinger PD, Schwab S and Bardutzky J: Hypothermia reduces
perihemorrhagic edema after intracerebral hemorrhage. Stroke.
41:1684–1689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue M, Fan Y, Liu S, Zygun DA, Demchuk A
and Yong VW: Contributions of multiple proteases to neurotoxicity
in a mouse model of intracerebral haemorrhage. Brain. 132:26–36.
2009. View Article : Google Scholar
|
|
23
|
Xi G, Keep RF and Hoff JT: Mechanisms of
brain injury after intracerebral haemorrhage. Lancet Neurol.
5:53–63. 2006. View Article : Google Scholar
|
|
24
|
Valable S, Montaner J, Bellail A, et al:
VEGF-induced BBB permeability is associated with an MMP-9 activity
increase in cerebral ischemia: both effects decreased by Ang-1. J
Cereb Blood Flow Metab. 25:1491–1504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mun-Bryce S, Wilkerson A, Pacheco B, et
al: Depressed cortical excitability and elevated matrix
metalloproteinases in remote brain regions following intracerebral
hemorrhage. Brain Res. 1026:227–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J and Tsirka SE: Neuroprotection by
inhibition of matrix metalloproteinases in a mouse model of
intracerebral haemorrhage. Brain. 128:1622–1633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heo JH, Lucero J, Abumiya T, Koziol JA,
Copeland BR and del Zoppo GJ: Matrix metalloproteinases increase
very early during experimental focal cerebral ischemia. J Cereb
Blood Flow Metab. 19:624–633. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qing WG, Dong YQ, Ping TQ, et al: Brain
edema after intracerebral hemorrhage in rats: the role of iron
overload and aquaporin 4: Laboratory investigation. J Neurosurg.
110:462–468. 2009. View Article : Google Scholar
|
|
29
|
Tang Y, Cai D and Chen Y: Thrombin
inhibits aquaporin 4 expression through protein kinase C-dependent
pathway in cultured astrocytes. J Mol Neurosci. 31:83–93. 2007.
View Article : Google Scholar : PubMed/NCBI
|