Introduction
Hepatitis B virus (HBV) is a highly infectious virus
with an estimated two billion people infected worldwide (1). Approximately 240 million individuals
worldwide are chronically infected with HBV, leading to ~600,000
mortalities as a result of acute or chronic hepatitis B annually
(2). One of the major risk factors
of HBV is acute or chronic liver disease which may progress into
HBV-associated liver failure, leading to an increased risk of
mortality from liver cirrhosis or primary hepatocellular carcinoma
(3). Chronic HBV infection may
result in sustained hepatic injury and liver fibrosis; therefore,
an accurate method for the assessment of hepatic injury and liver
fibrosis is vital for patients with chronic HBV infections. At
present, a lack of accurate, reproducible and easily applied
assessments for hepatic injury and liver fibrosis in patients with
HBV is a major limitation of the clinical management of HBV.
Current clinical indicators, including ultrasonography, liver
function tests, markers of coagulation function and serum markers
for liver fibrosis may reflect disease progression in patients with
chronic hepatitis B (CHB) (4).
Liver biopsies provide an accurate reflection of the progression of
liver disease in patients with CHB (1). However, the clinical applications of
this test remain limited due to its invasive nature. Therefore, the
identification of a simple, non-invasive serum marker for
evaluating disease progression in patients with CHB is
required.
Golgi protein 73 (GP73), a novel transmembrane
protein, is expressed in human epithelial cells (5). In normal human liver tissue, GP73 is
primarily expressed in biliary epithelial cells and rarely
expressed in healthy hepatocytes (6,7).
Studies have revealed that hepatocellular GP73 mRNA levels and
protein expression are significantly upregulated in acute and
chronic hepatitis, regardless of the etiology, and accompanies the
advanced fibrogenesis stage (7.8). Furthermore, a significant
increase in GP73 protein expression has been reported in the liver
tissue of patients with CHB and GP73 was highly expressed in liver
tissue infected with HBV and adenovirus (9,10).
Multiple studies have demonstrated that increased serum GP73
protein concentrations were positively correlated with the
progression of chronic liver disease (8,11).
However, the correlation between serum GP73 protein levels and
liver pathological grading or fibrosis staging in patients with CHB
has remained unclear, and the association between serum GP73
protein levels and HBV replication are under dispute (12,13).
The current study aimed to evaluate the correlation between serum
GP73 protein levels and liver pathological grading and staging in
patients with CHB. Serum GP73 levels were also investigated in
order to determine the correlation between serum GP73 protein
levels and HBV replication.
Materials and methods
Subjects
A total of 253 patients with chronic HBV infections
were enrolled in the present study, including 183 patients with
CHB, 35 patients with acute-on-chronic liver failure (ACLF) and 35
patients with HBV-associated decompensated liver cirrhosis
(HBV-DLC). Patients with chronic HBV infections enrolled in this
study were admitted to the 180th Hospital of the People’s
Liberation Army (Quanzhou, China) during the period between January
2012 and September 2013. The demographic and clinical data of each
patient were recorded. Thirty healthy individuals were selected as
normal control subjects during the same period. The study was
approved by the Ethical Review Committee of the 180th Hospital of
People’s Liberation Army. All participants provided written
informed consent.
The diagnoses of CHB and HBV-DLC were made according
to the program of prevention and cure for viral hepatitis (14). Briefly, cases of CHB were
classified as mild, moderate and severe. A diagnostic
classification of mild CHB was defined as albumin (ALB) levels ≥35
g/l, alanine aminotransferase (ALT) levels ≤3 upper limits of
normal (ULN), total bilirubin (TBIL) levels ≤2 ULN and prothrombin
activity (PTA) >70%. A diagnostic classification of moderate CHB
required criterion of ALB 32–35 g/l, ALT >3 ULN, TBIL 2–5 ULN
and a PTA of 60–70%. The diagnostic criterion for severe CHB
required an ALB ≤32 g/l, ALT >3 ULN, TBIL >5 ULN and a PTA of
40–60%. HBV-DLC diagnostic criteria were advanced liver cirrhosis
and a Child-Pugh score (15) of B
or C class. The diagnosis of ACLF was made according to the
consensus on ACLF recommended by the Asian Pacific Association for
the study of the liver (16).
Patients with liver damage caused by hepatitis A, C, D and E or
other factors were excluded. The serum GP73 level, hepatitis B e
antigen (HBeAg), HBV DNA and liver function of each patient were
measured. Prior to the initiation of drug therapy and following six
months of treatment, serum samples were collected and stored at
−80°C for subsequent investigations.
Determination of serum GP73 level
Quantitative determination of GP73 in serum was
performed using a commercially available enzyme-linked
immunosorbent assay (ELISA) kit according to the manufacturer’s
instructions (Beijing Hotgen Biotech Co., Ltd, Beijing, China). All
reagents were provided in the kit. Briefly, a 20 μl serum blood
sample was added to each well of the ELISA microplate with 50 μl
dilution solution, and the microplate was sealed and incubated at
37°C for 60 min. Subsequently, 100 μl ELISA reagent was added to
each well and the microplate was sealed and incubated at 37°C for
30 min. All solutions were removed, and each well was washed with
phosphate-buffered saline (PBS)/Tween (Fuzhou Maixin Biotechnology
Development Co., Ltd, Fuzhou, China)five times. Chromogenic
substrates A and B (50 μl) were added to each well and the plate
was incubated in darkness at 37°C for 15 min. Subsequently, 50 μl
stop solution was added to each well, and the plate was read in a
Bio-Rad 860 microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Determination of serum liver fibrosis
indices
Serum liver fibrosis indices, including hyaluronic
acid (HA), type IV collagen (CIV), laminin (LN) and type III
procollagen (PIIINP), were determined using the up-converting
luminescence technique with an up-converting phosphor bioanalytical
system (Beijing Hotgen Biotech Co., Ltd.) according to the
manufacturer’s instructions.
Liver pathology and
immunohistochemistry
Liver pathology
Liver biopsies were obtained from 91 CHB patients
using 16 G disposable needles (C. R. Bard, Inc., Murray Hill, NJ,
USA). The liver biopsy specimens were considered reliable when the
liver specimen length was ≥1.5 cm or the portal tract number was
≥6. The liver biopsy specimens were fixed in 4% formaldehyde
(Shanghai Xi Hua Trade Co., Ltd, Shanghai, China), embedded in
paraffin (Maoming Huayue Group Co., Ltd, Maoming, China) and cut
into 4-μm sections, and conventionally stained with hematoxylin and
eosin (Shanghai Xi Hua Trade Co. Ltd), reticular fiber stain and
Masson’s trichrome (Beijing Sequoia Jinqiao Biological Technology
Co. Ltd, Beijing, China). Images were acquired using an Olympus
BX51 microscope (Tokyo, Japan). According to the Scheuer scoring
system (17), hepatic inflammation
activity grade (G) was divided into G0 (no hepatic
necroinflammation) and G1–G4, and liver fibrosis stage (S) was
divided into S0 (no fibrosis) and S1–S4. Liver specimens were
interpreted by an experienced liver pathologist.
Immunohistochemistry
Immunohistochemical (IHC) staining of liver sections
was performed using the EliVision™ Plus IHC kit (Fuzhou Maixin
Biotechnology Development Co., Ltd) according to the manufacturer’s
instructions. All reagents were provided in the kit. Briefly, the
tissue microarray blocks were cut into 3-μm sections,
deparaffinized with xylene and rehydrated through a graded alcohol
series. Antigen retrieval was performed using a high-temperature
and high-pressure antigen repairing method. Following rinsing in
Tris-buffered saline (pH 7.6), the sections were immersed in 3%
H2O2 to block endogenous peroxidase activity.
Samples were subsequently incubated with GP73 mouse monoclonal
antibody (Beijing Hotgen Biotech Co., Ltd.) overnight at 4°C.
Following washing with Tris-buffered saline, GP73 horseradish
peroxidase-labeled goat anti-mouse antibody (Fuzhou Maixin
Biotechnology Development Co., Ltd) was added to each section for
30 min at 37°C, and then visualized using 3,3′-diaminobenzidine
(Beijing Sequoia Jinqiao Biological Technology Co., Ltd.).
Counterstaining was performed using hematoxylin. PBS in place of
the primary antibody was used as a blank control. According to the
staining intensity, based on the semi-quantitative evaluation
(6) of GP73 expression in liver
tissue, GP37 staining was divided into five categories: negative
(no expression), weakly positive (fine brown particle), positive
(coarse brown particle), moderately positive (lumpy brown particle)
and highly positive (chunky dark brown particle).
Determination of serum HBV DNA
content
The serum HBV DNA was determined by the fluorescent
quantitative polymerase chain reaction (qPCR) technique according
to the manufacturer’s instructions (Shanghai Fosun Industrial
Limited by Share, Ltd, Shanghai, China). Primer sequences were
synthesized by Shanghai Shenyou Biotechnology Co., Ltd and
fluorescence was measured using the LightCycler 600 real-time
fluorescence quantitative PCR instrument manufactured by Roche
(Roche Diagnostics, Basel, Switzerland). The sensitivity of HBV DNA
for the determination was <5.0E+02 copies/ml.
Biochemical indices of liver function
analysis
The liver function-associated biochemical indices of
ALB, TBIL, ALT and aspartate aminotransferase (AST) were determined
by the chemical colorimetric method with a TBA-120 FR fully
automatic biochemical analyzer (Toshiba, Tokyo, Japan) according to
the manufacturer’s instructions (Beijing Condor-Teco Medical
Technology Co., Ltd, Beijing, China).
Changes in serum GP73 following
antiviral treatment
A total of 86 patients with CHB received antiviral
therapy [0.5 mg Entecavir (Zhengda Tianqing Pharmaceutical Group
Ltd, Jiangsu, China) every morning prior to eating ordrinking,
administered orally, once a day over one year; or 5×106
units recombinant human interferon α1b (Kexing Biological
Engineering Co., Ltd, Shenzhen, China), administered by
subcutaneous injection, once every other day over a course of six
months)]. Changes in condition were observed and the serum levels
of GP73 were detected prior to the initiation of drug therapy and
following six months of treatment. Prognoses were graded as: 1,
Clinical remission, where clinical symptoms had disappeared and
liver function had returned to normal or 2, clinical disease
progression, where clinical symptoms were not relieved and were
accompanied by recurrent or persistent abnormal liver function.
Statistical analysis
All values are expressed as the mean ± standard
deviation, and all statistical analyses were performed using
GraphPad Prism version 5.0 (Graphpad Software Inc., La Jolla, CA,
USA). The difference of the means of the data that satisfied the
homogeneity of variance were tested with analysis of variance
(ANOVA), and comparison of the data that did not satisfy the
homogeneity of variance was analyzed with ANOVA following rank
transformation. The correlation analyses among variables were
performed using Pearson’s correlation analysis and linear
regression analysis. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
General characteristics
Between January 2012 and September 2013, 253
patients with chronic HBV infections were enrolled in the present
study, including 213 males and 40 females, aged 20–65 years, with a
mean age of 39.75±14.69 years. The subjects included 183 patients
with CHB, including 53 with mild CHB, 80 with moderate CHB and 50
with severe CHB, as well as 35 patients with ACLF and 35 patients
with HBV-DLC. The serum GP73 levels, HBeAg, HBV DNA and liver
function of each patient were assessed (Table I).
 | Table ISerum GP73 levels associated with
chronic HBV infection, liver function, serum HBeAg and HBV DNA
contents. |
Table I
Serum GP73 levels associated with
chronic HBV infection, liver function, serum HBeAg and HBV DNA
contents.
| | Serum GP73 level
(ng/ml) |
|---|
| |
|
|---|
| Parameter | Patients (n) | Mean ± SD | 95% CI |
|---|
| Clinical
diagnosis |
253a | | |
| Mild CHB | 53 | 74.14±36.65 | 64.04–84.24 |
| Moderate CHB | 80 | 139.78±67.05 | 124.86–154.70 |
| Severe CHB | 50 | 243.31±74.39 | 222.17–264.45 |
| ACLF | 35 | 304.45±98.48 | 270.62–338.28 |
| HBV-DLC | 35 | 255.46±92.02 | 223.85–287.07 |
| ALB (g/l) |
183b | | |
| <35 | 24 | 236.66±80.49 | 202.67–270.65 |
| 35–40 | 61 | 158.92±92.16 | 135.31–182.52 |
| 40–45 | 78 | 104.45±57.18 | 91.55–117.34 |
| >45 | 20 | 91.59±49.88 | 68.25–114.93 |
| TBIL (μmol/l) |
183b | | |
| <34.2 | 139 | 111.14±64.83 | 100.27–122.02 |
| 34.2–85.5 | 20 | 192.07±82.18 | 153.61–230.54 |
| >85.5 | 24 | 252.58±85.13 | 216.63–288.53 |
| ALT (U/l) |
183b | | |
| <40 | 18 | 88.76±54.79 | 61.51–116.02 |
| 40–120 | 52 | 90.23±56.52 | 74.49–105.96 |
| 120–400 | 59 | 145.83±86.39 | 123.32–168.35 |
| >400 | 54 | 193.68±83.40 | 170.91–216.44 |
| AST (U/l) |
183b | | |
| <40 | 38 | 78.62±41.88 | 64.85–92.38 |
| 40–120 | 65 | 112.91±67.44 | 96.20–129.62 |
| 120–400 | 53 | 163.54±79.51 | 141.63–185.46 |
| >400 | 27 | 235.48±87.26 | 200.96–269.99 |
| HBeAg |
183b | | |
| Positive | 117 | 136.76±86.21 | 118.32–155.63 |
| Negative | 66 | 139.52±81.73 | 121.17–165.92 |
| HBV
DNA(copies/ml) |
183b | | |
| <1.0E+06 | 29 | 143.52±96.15 | 102.31–186.52 |
| 1.0E+06 –
1.0E+07 | 40 | 151.16±92.32 | 105.71–191.65 |
| 1.0E+07 –
1.0E+08 | 61 | 155.21±68.16 | 123.29–183.51 |
| >1.0E+08 | 53 | 136.96±81.95 | 98.93–179.67 |
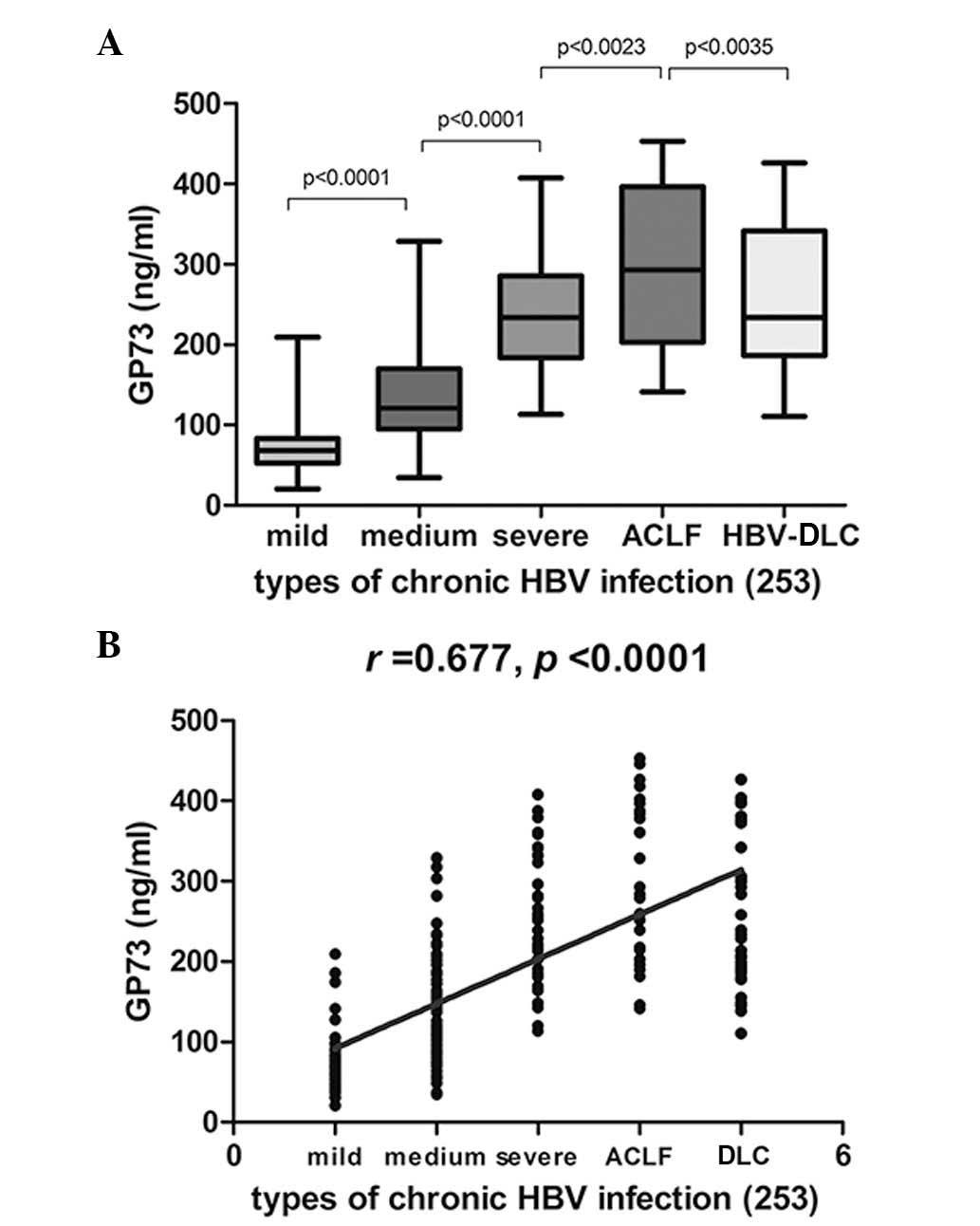
Serum GP73 levels are positively
correlated with HBV clinical disease progression
Elevation of serum GP73 was observed in patients
with mild, moderate and severe CHB, patients with HBV-DLC and
patients with hepatitis B-associated ACLF (Fig. 1A). Serum GP73 levels were
positively correlated with disease progression in patients with
chronic HBV infections (Fig. 1B;
r=0.677; P<0.0001).
Serum GP73 level is not correlated with
HBV replication
Serum HBeAg and HBV DNA are two major indices of HBV
replication. The association between serum GP73 levels and HBV
replication was investigated in the present study. The subjects
included 183 patients with CHB, including 117 (63.93%) with
HBeAg-positive CHB and 66 (36.07%) with HBeAg-negative CHB. Serum
levels of GP73 were not significantly different between patients
with HBeAg-positive CHB and those of patients with HBeAg-negative
CHB (t=0.212; P>0.05).
According to the serum HBV DNA content, 183 patients
with CHB were divided into four groups: <1.0E+06, 1.0E+06 –
1.0E+07, 1.0E+07 – 1.0E+08 and >1.0E+08 copies/ml. The
association between serum GP73 levels and HBV DNA contents are
exhibited in Table I. There was no
significant difference in serum GP73 levels between the four groups
(F=0.513; P>0.05).
Serum GP73 levels are correlated with
liver function-associated biochemical indices
Serum ALT and AST levels are the most commonly used
indicators of liver cell injury. In addition, serum TBIL levels are
associated with hepatic necrosis, whilst serum ALB levels reflect
hepatic synthetic function. These four serological biochemical
indices are frequently used to evaluate the degree of hepatic
injury and necrosis (18). The
correlation between GP73 and liver function-associated biochemical
indices was therefore evaluated. The serum ALB, TBIL, ALT and AST
levels of 183 patients with CHB were determined. The patients were
divided into four groups according to serum ALB levels (<35 g/l,
24 cases; 35–40 g/l, 61 cases; 40–45 g/l, 78 cases and >45 g/l,
20 cases) and three groups according to serum TBIL levels (≤34.2
μmol/l, 139 cases; 34.2–85.5 μmol/l, 20 cases and ≥85.5 μmol/l, 24
cases). Patients were also divided into four groups according to
serum ALT levels (<40 IU/l, 18 cases; 40–120 IU/l, 52 cases;
120–400 IU/l, 59 cases and >400 IU/l, 54 cases), and four groups
according to serum AST levels (<40 IU/l, 38 cases; 40–120 IU/l,
65 cases; 120–400 IU/l, 53 cases and >400 IU/l, 27 cases). The
associations between serum GP73 levels and the above indices are
exhibited in Table I and Fig. 2.
 | Figure 2Serum GP73 level closely correlates
with serum ALB, TBIL, ALT and AST levels. Serum GP73 levels in
patients with various (A) ALB, (B) TBIL, (C) ALT and (D) AST
levels. Correlations between serum GP73 level and (E) ALB, (F)
TBIL, (G) ALT and (H) AST levels. GP73, Golgi protein 73; ALB,
albumin; TBIL, total bilirubin; ALT, alanine aminotransferase; AST,
aspartate aminotransferase. |
Serum GP73 levels significantly increased
concurrently with a reduction in ALB levels, and there was a
significant difference between ALB expression level groups (all
P<0.001), except the group with an ALB level of >45 g/l
(Fig. 2A). A significant elevation
of serum GP73 level was observed with an increase in TBIL level,
and significant differences were identified between groups
(Fig. 2B; all P<0.05). The
serum GP73 level was significantly increased in correlation with an
elevation in ALT and AST levels, and there was a significant
difference between groups (Fig. 2C and
D, all P<0.01), except for the group with an ALT level of
<40 IU/l. Serum GP73 levels were negatively correlated with ALB
levels (Fig. 2E; r=-0.524;
P<0.0001), but positively correlated with TBIL levels (Fig. 2F; r=0.572; P<0.0001), ALT
levels (Fig. 2G; r=0.498;
P<0.0001) and the AST level (Fig.
2H; r=0.532; P<0.0001).
Serum GP73 levels are positively
correlated with serum liver fibrosis indices and liver tissue
pathology
Serum markers are indirect serological markers of
liver fibrosis, whereas liver biopsies represent the ‘gold
standard’ for the diagnosis of liver fibrosis (19,20).
In order to evaluate the correlation between serum GP73 levels and
disease progression in patients with chronic HBV infections, the
serum HA, CIV, LN and PIIINP levels of liver fibrosis indices were
determined in 183 patients with CHB, and liver biopsy was performed
on 91 patients with CHB (Table
II). Serum GP73 level was found to positively correlate with
serum HA (r=0.460), CIV (r=0.581), LN
(r=0.604) and PIIINP levels (r=0.592) (Table II, all P<0.0001).
 | Table IICorrelations between serum GP73
level, serum liver fibrosis indices and liver tissue pathology. |
Table II
Correlations between serum GP73
level, serum liver fibrosis indices and liver tissue pathology.
| Serum GP73 level
(ng/ml) |
|---|
|
|
|---|
| Parameter | Patients (n) | Mean ± SD | 95% CI |
|---|
| Serum liver
fibrosis index |
183a | | |
| HA | 183 | 180.76±185.69 | 152.03–209.48 |
| CIV | 183 | 214.00±210.46 | 181.45–246.55 |
| LN | 183 | 209.49±180.17 | 180.17–238.82 |
| PIIINP | 183 | 216.55±200.09 | 185.60–247.50 |
| GP73 | 183 | 168.37±95.93 | 153.53–183.20 |
| Pathological
grading and staging of liver tissues |
| Hepatic
inflammation grade | 91b | | |
| G1 | 28 | 70.55±29.63 | 59.06–82.04 |
| G2 | 31 | 112.08±57.26 | 91.08–133.09 |
| G3 | 17 | 190.27±73.19 | 152.64–227.90 |
| G4 | 15 | 250.28±85.76 | 202.79–297.77 |
| Liver fibrosis
stage | 91b | | |
| S1 | 31 | 75.31±35.76 | 62.19–88.43 |
| S2 | 24 | 101.92±47.67 | 81.79–122.04 |
| S3 | 14 | 192.60±93.47 | 138.63–246.57 |
| S4 | 22 | 221.38±83.05 | 184.99–257.76 |
The correlation between serum GP73 levels and liver
pathological grading and staging are illustrated in Fig. 3. Serum GP73 levels were
significantly increased with increased hepatic inflammation
activity grade (F=51.50; P<0.0001) and increased liver
fibrosis stage (F=28.85; P<0.0001). There was a
significant difference amongst all hepatic inflammation activity
grades (G1–G4) and amongst the liver fibrosis stages (S1–S3),
regardless of the serum GP73 levels (Fig. 3A and B; all P<0.05). Serum GP73
levels were positively correlated with hepatic inflammation
activity grade (Fig. 3C;
r=0.737; P<0.0001) and liver fibrosis stage (Fig. 3D; r=0.692; P<0.0001).
GP73 protein expression is correlated
with hepatic inflammation grade and stage in liver tissue
samples
GP73 protein expression was validated in liver
tissue specimens of patients with chronic HBV infections by
staining biopsy samples. Immunohistochemical analysis indicated
that the GP73 protein was expressed in a small number of
hepatocytes in normal liver tissue, whereas in patients with
chronic HBV infections, GP73 expression was identified scattered in
the cytoplasm of hepatocytes, but not in the infiltrating
inflammatory cells or within the fibrous septa (Fig. 4, G2 and S4). In patients with mild
CHB, the GP73 staining was brown and the granules were fine, and
scattered in the cytoplasm of liver cells, suggesting weakly
positive GP73 expression. In severe CHB, GP73 staining was brown
and the granules were lumpy, and diffusely distributed in the
cytoplasm of liver cells, suggesting moderately positive GP73
expression. In samples from patients with HBV-DLC, GP73 staining
was dark brown and the granules coarse blocks, widely distributed
in the parenchyma of the liver cytosol, suggesting strong positive
expression. These results indicated that the expression levels of
GP73 increased with disease progression from mild to severe CHB and
HBV-DLC. Compared with mild CHB tissue specimens, GP73 was
significantly expressed in severe CHB and HBV-DLC.
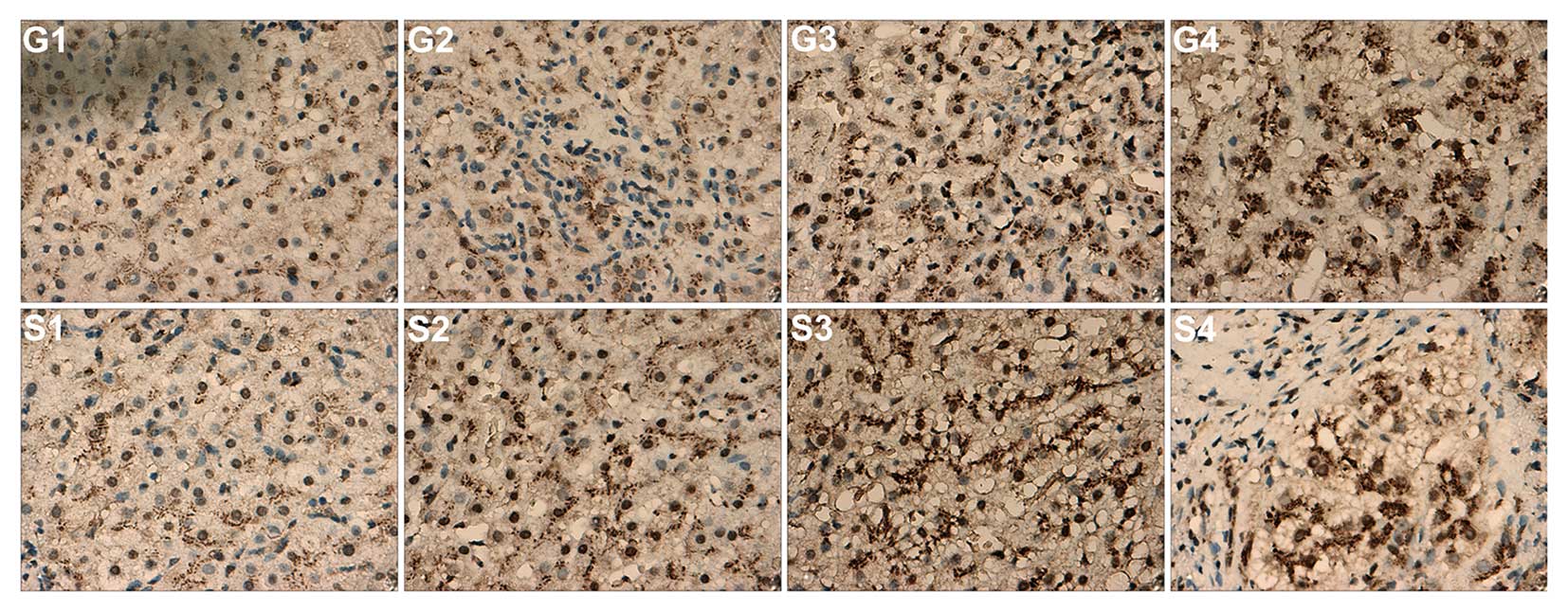 | Figure 4GP73 expression in pathological
grading and staging of liver tissue of 16 patients with chronic
hepatitis B (8 individual patients, representative of the total 16
evaluated). GP73 expression was scattered in the cytoplasm of
hepatocytes, but not in the infiltrating inflammatory cells (G2 and
S4) or within the fibrous septa (S4). G1 and S1, weakly positive
for GP73; G2 and S2, positive for GP73; G3 and S3, moderately
positive for GP73; G4 and S4, highly positive for GP73
(magnification, ×400). GP73, Golgi protein 73; G1–G4, hepatic
inflammation activity grades; S1–S4, liver fibrosis stages. |
To further validate GP73 expression in liver tissue
with regard to various hepatic inflammation grades (G0–G4) and
fibrosis stages (S0–S4), GP73 protein was stained in various biopsy
samples. Immunohistochemical analysis demonstrated that GP73
protein expression increased gradually with increased liver
histological inflammation activity grade and liver fibrosis stage.
The results of the immunohistochemical analyses are shown in
Fig. 4.
Serum GP73 levels are decreased following
clinical remission of CHB
Following six months of antiviral therapy, 62
(72.09%) patients with CHB reached clinical remission with
significantly decreased serum GP73 levels (Fig. 5A; t=6.699; P<0.0001).
However, 24 (27.91%) patients with CHB that had achieved clinical
progression, retained elevated levels of serum GP73 (Fig. 5B, t=-4.547, P=0.0001). These
alterations in serum GP73 are presented in Table III.
 | Table IIIChanges in serum golgi protein 73
expression prior to and following treatment. |
Table III
Changes in serum golgi protein 73
expression prior to and following treatment.
| Clinical
prognosis | Patients (n) | Mean ± SD | 95% CI |
|---|
| Clinical
remission | 62a | | |
| Prior to
treatment | 62 | 165.56±84.09 | 144.20–186.91 |
| Following
treatment | 62 | 96.13±43.99 | 84.96–107.30 |
| Clinical
progression | 24b | | |
| Prior to
treatment | 24 | 121.09±61.97 | 94.92–147.26 |
| Following
treatment | 24 | 172.75±61.20 | 146.90–198.59 |
Discussion
GP73 is also known as a type II Golgi transmembrane
protein (Golgi phosphoprotein 2, GOLPH2) and Golgi membrane protein
(Golgi membrane protein 1, GOLM1), and has an estimated molecular
weight of 73 kDa (5). The
GP73 gene is located on chromosome 9 and was originally
cloned from a library derived from liver tissue of a patient with
adult giant-cell hepatitis (5).
The function and regulatory mechanisms of GP73, however, have
remained elusive. Significant upregulation of GP73 has been
detected in the hepatocytes of patients with hepatocellular
carcinoma, and therefore GP73 may present a potential novel serum
marker of hepatocellular carcinoma (21–28).
The potential predictive value of GP73 in the early diagnosis of
hepatocellular carcinoma has received significant attention, and
elevated GP73 has been detected in patients with multiple benign
liver diseases (29). Studies have
demonstrated that GP73, expressed in the majority of hepatocytes,
was upregulated in acute or chronic hepatitis and serum GP73
concentration was positively correlated with the progression of
chronic liver diseases (8,11). Serum GP73 levels have previously
been associated with liver inflammation injury and fibrosis
(30,31), which is similar to the data
obtained in the present study.
The correlation between serum GP73 levels and
disease progression in patients with chronic HBV infections was
evaluated in the present study. Serum GP73 level was elevated in
patients with mild, moderate and severe CHB, patients with HBV-DLC
and patients with ACLF. Serum GP73 levels were positively
correlated with disease progression in patients with chronic HBV
infections. Consistent with the results of the present study, a
positive correlation between liver disease progression and GP73
protein expression has been previously reported, where a
significant increase in GP73 was observed with the progression of
liver disease (32). The
correlation between serum GP73 levels and certain biochemical
indices was also investigated. The results revealed that serum GP73
levels were negatively correlated with ALB levels, but positively
correlated with TBIL, ALT and AST levels indicating that serum GP73
levels were associated with liver function. The higher the serum
GP73 levels, the more severe the hepatic injury. This association
illustrated that GP73 levels may represent a serological marker of
hepatic injury. Subsequently, the correlation between serum GP73
levels and liver pathological grading and staging were further
investigated. The results indicated that serum GP73 levels
increased gradually with the elevation of hepatic inflammation
activity grade and liver fibrosis stage. These findings
demonstrated that serum GP73 levels represent an effective
serological indicator of the degree of hepatic inflammation injury
and the extent of liver fibrosis. The high expression of GP73
observed in acute and chronic liver diseases suggested that, whilst
serum GP73 protein levels alone may not aid the elucidation of the
underlying causes of liver disease, serum GP73 protein levels may
be used as a serum marker for the diagnosis of liver diseases and
the monitoring of liver disease progression (32).
Immunohistochemical analysis indicated that GP73
protein expression was mainly distributed in the cytoplasm of
hepatocytes, and expression levels increased gradually with hepatic
inflammation grade and liver fibrosis stage. The results revealed
that GP73 expression was associated with disease progression in
patients with chronic HBV infections, and this was consistent with
liver pathological grading and staging. However, due to the limited
number of immunohistochemical specimens analyzed in this study,
these results require validation in a larger cohort.
In the present study, serum GP73 protein levels were
positively correlated with disease progression in patients with
CHB. The regulatory mechanism underlying GP73 expression is yet to
be elucidated and the association between serum GP73 level and HBV
replication remains unclear. A previous study indicated that HBV
replication may significantly increase the expression of GP73
protein (12). A further study
revealed that GP73 expression was significantly increased in liver
disease due to viral causes (HBV, hepatitis C virus) compared to
non-viral causes of liver disease (7). The results of these studies indicated
that serum GP73 protein levels may be associated with viral
infection or replication level. However, several studies have
reported contrasting results. It was reported that GP73 protein
expression increased significantly in patients with various liver
diseases (viral and non-viral due to drug and alcohol abuse and
autoimmune hepatitis) (11), and
in cases of viral and non-viral liver disease, GP73 protein levels
were significantly upregulated (9,33).
In another study, an elevation of serum GP73 was not caused by HBV
infection (13). The results of
the current study demonstrated that the serum GP73 level was not
significantly associated with HBV replication, regardless of serum
HBeAg (positive or negative) or serum HBV DNA contents. These
results differ from those of certain studies in the literature and
may be associated with selected cases, and have occurred due to a
variety of factors (32). Patients
with chronic HBV infections frequently exhibit immune hepatic
injury due to active HBV replication (34). Hepatic injury may induce the
upregulation of GP73 expression in hepatocytes, or serum GP73
levels may potentially act as a novel virus-responding protein
(35) associated with clinical
disease progression and disease severity in patients with chronic
HBV infections.
These data indicated that GP73 upregulation may be a
feature of early-stage disease in patients with chronic HBV
infections. In the present study, alterations in serum GP73 levels
in patients with CHB were investigated following drug treatment for
six months. The levels of serum GP73 were gradually decreased
concurrently with the remission of CHB disease, however for cases
of clinical progression, serum GP73 levels remained elevated. These
results indicated that the upregulation of GP73 expression was
reversible, and that serum GP73 levels were closely associated with
the prognosis of patients with CHB. The present study, in
accordance with previous studies (7,11)
demonstrated that the upregulation of GP73 expression was
reversible. There is a possibility that GP73 may be an acute
reaction protein, the expression of which is triggered by
hepatocyte injury and in cases of clinical remission or
progression, the serum levels of GP73 are altered. These results
suggested that serum GP73 levels may represent an effective index
to indicate the prognosis of clinical disease.
In conclusion, serum GP73 levels are positively
correlated with disease progression in patients with chronic HBV
infections, and are closely correlated with liver pathological
grading and staging. Therefore, serum GP73 levels may be an
important indicator in the evaluation of clinical disease
progression and prognosis in patients with chronic HBV infections.
Whilst serum GP73 levels were not associated with HBV replication,
the upregulation of GP73 expression is associated with hepatocyte
injury following HBV infection. Further studies with larger sample
sizes and the use of evidence-based medicine are required in order
to validate the results of the current study.
Acknowledgements
The study was supported by grants from the Hospital
of Science and Technology plan projects (no. 11D3006). The authors
would like to acknowledge Dr Weirong Jin from Shanghai’s National
Human Genome Research Center (Shanghai, China) for his technical
assistance. The authors would also like to thank Hotgen Biotech Co.
(Beijing, China) for providing the antibody.
References
|
1
|
European Association for the Study of the
Liver. EASL clinical practice guidelines: Management of chronic
hepatitis B virus infection. J Hepatol. 57:167–185. 2012.PubMed/NCBI
|
|
2
|
World Health Organization. Hepatitis B
Factsheet 204. http://www.who.int/mediacentre/factsheets/fs204/en/index.html.
Accessed 2013
|
|
3
|
Zhou YH, Wu C and Zhuang H: Vaccination
against hepatitis B: the Chinese experience. Chin Med J (Engl).
122:98–102. 2009.
|
|
4
|
Rotman Y, Brown TA and Hoofnagle JH:
Evaluation of the patient with hepatitis B. Hepatology. 49(5
Suppl): S22–S27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kladney RD, Bulla GA, Guo L, Mason AL, et
al: GP73, a novel Golgi-localized protein upregulated by viral
infection. Gene. 249:53–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riener MO, Stenner F, Liewen H, Soll C, et
al: Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and
its value as a serum marker in hepatocellular carcinomas.
Hepatology. 49:1602–1609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kladney RD, Cui X, Bulla GA, Brunt EM, et
al: Expression of GP73, a resident Golgi membrane protein, in viral
and nonviral liver disease. Hepatology. 35:1431–1440. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Yang H, Mao Y, Xu H, et al:
Increased Golgi protein 73 expression in hepatocellular carcinoma
tissue correlates with tumor aggression but not survival. J
Gastroenterol Hepatol. 26:1207–1212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maitra A and Thuluvath PJ: GP73 and liver
disease: a (Golgi) complex enigma. Am J Gastroenterol.
99:1096–1098. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wright LM, Yong S, Picken MM, Rockey D, et
al: Decreased survival and hepato-renal pathology in mice with
C-terminally truncated GP73 (GOLPH2). Int J Clinical Exp Pathol.
2:34–47. 2009.
|
|
11
|
Iftikhar R, Kladney RD, Havlioglu N,
Schmitt-Graff A, et al: Disease- and cell-specific expression of
GP73 in human liver disease. Am J Gastroenterol. 99:1087–1095.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sterling RK, Jeffers L, Gordon F, Sherman
M, et al: Clinical utility of AFP-L3% measurement in North American
patients with HCV-related cirrhosis. Am J Gastroenterol.
102:2196–2205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WL, Peng T, Chen XP, et al: Clinical
significance of serum GP73 test in diagnosis of liver diseases.
Chin J Lab Med. 33:333–336. 2010.(In Chinese).
|
|
14
|
Chinese Society of Infectious Diseases and
Parasitology and Chinese Society of Hepatology of Chinese Medical
Association. The programme of prevention and cure for viral
hepatitis. Chin J Hepatol. 8:324–329. 2000.(In Chinese).
|
|
15
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC, et al: Transection of the oesophagus for bleeding
oesophageal varices. Br J Surg. 60:646–649. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarin SK, Kumar A, Almeida JA, Chawla YK,
et al: Acute-on-chronic liver failure: consensus recommendations of
the Asian Pacific Association for the study of the liver (APASL).
Hepatol Int. 3:269–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheuer PJ: Classification of chronic
viral hepatitis: a need for reassessment. J Hepatol. 13:372–374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chinese Society of Hepatology and Chinese
Society of Infectious Diseases, Chinese Medical Association. The
guideline of prevention and treatment for chronic hepatitis B (2010
version). Zhonghua Gan Zang Bing Za Zhi. 19:13–24. 2011.(In
Chinese).
|
|
19
|
Rockey DC, Caldwell SH, Goodman ZD, Nelson
RC, et al: Liver biopsy. Hepatology. 49:1017–1044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mani H and Kleiner DE: Liver biopsy
findings in chronic hepatitis B. Hepatology. 49(5 Suppl): S61–S71.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bachert C, Fimmel C and Linstedt AD:
Endosomal trafficking and proprotein convertase cleavage of cis
Golgi protein GP73 produces marker for hepatocellular carcinoma.
Traffic. 8:1415–1423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fimmel CJ and Wright L: Golgi protein 73
as a biomarker of hepatocellular cancer: development of a
quantitative serum assay and expression studies in hepatic and
extrahepatic malignancies. Hepatology. 49:1421–1423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao Y, Yang H, Xu H, Lu X, et al: Golgi
protein 73 (GOLPH2) is a valuable serum marker for hepatocellular
carcinoma. Gut. 59:1687–1693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marrero JA, Romano PR, Nikolaeva O, Steel
L, et al: GP73, a resident Golgi glycoprotein, is a novel serum
marker for hepatocellular carcinoma. J Hepatol. 43:1007–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Norton PA, Comunale MA, Krakover J,
Rodemich L, et al: N-linked glycosylation of the liver cancer
biomarker GP73. J Cellular Biochem. 104:136–149. 2008. View Article : Google Scholar
|
|
26
|
Schwegler EE, Cazares L, Steel LF, Adam
BL, et al: SELDI-TOF MS profiling of serum for detection of the
progression of chronic hepatitis C to hepatocellular carcinoma.
Hepatology. 41:634–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y, Chen J, Li L, Sun Z, et al: A study
of diagnostic value of golgi protein GP73 and its genetic assay in
primary hepatic carcinoma. Technol Can Res Treat. 10:287–294.
2011.
|
|
28
|
Yamamoto K, Imamura H, Matsuyama Y, Kume
Y, et al: AFP, AFP-L3, DCP, and GP73 as markers for monitoring
treatment response and recurrence and as surrogate markers of
clinicopathological variables of HCC. J Gastroenterol.
45:1272–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao Y, Chen J, Li X, Wei H, et al: Serum
gp73 is also a biomarker for diagnosing cirrhosis in population
with chronic HBV infection. Clin Biochem. 47:216–222. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian L, Wang Y, Xu D, Gui J, et al:
Serological AFP/Golgi protein 73 could be a new diagnostic
parameter of hepatic diseases. International journal of cancer. J
Int Du Cancer. 129:1923–1931. 2011. View Article : Google Scholar
|
|
31
|
Wright LM, Huster D, Lutsenko S, Wrba F,
et al: Hepatocyte GP73 expression in Wilson disease. J Hepatol.
51:557–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Wan X, Li Z, Lin C, et al: Golgi
protein 73 (GP73), a useful serum marker in liver diseases. Clin
Chem Lab Med. 49:1311–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao Q, Mak KM and Lieber CS: Leptin
represses matrix metalloproteinase-1 gene expression in LX2 human
hepatic stellate cells. J Hepatol. 46:124–133. 2007. View Article : Google Scholar
|
|
34
|
Hoofnagle JH: Reactivation of hepatitis B.
Hepatology. 49(5 Suppl): S156–S165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Block TM, Comunale MA, Lowman M, Steel LF,
et al: Use of targeted glycoproteomics to identify serum
glycoproteins that correlate with liver cancer in woodchucks and
humans. Proc Natl Acad Sci USA. 102:779–784. 2005. View Article : Google Scholar : PubMed/NCBI
|