Introduction
There are multiple conditions in which damage leads
to fibroblast activation and excessive collagen production, which
can result in fibrosis of various tissues (1). For example, fibroproliferative
disorders occurring following dermal trauma may lead to
hypertrophic scarring (HS). HS results from abnormal and excessive
deposition of extracellular matrix (ECM) during skin wound healing,
particularly collagen I and III, in different proportions depending
upon the type of tissue wounded and the age of the individual
(2). Collagen metabolism is
crucial to scar formation and determines its properties (3). In addition, HS is characterized by
fibrosis and inflammation, which is associated with several
inflammatory cytokines and growth factors affecting fibroblast
activity, including transforming growth factor β-1 (TGF-β1);
fibroblast growth factor; platelet-derived growth factor;
macrophage-derived growth factor; interleukin-1 and tumor necrosis
factor-α (4). By modulating levels
of these proteins, HS formation may be attenuated or prevented.
However, there are currently no effective therapeutic methods for
HS that prevent fibroblast activation.
Induced pluripotent stem cells (iPSCs) are novel
bioengineered embryonic-like stem cells (5) that were initially created from mouse
adult fibroblasts with four factors (Oct3/4;Sox2; Klf4; and
c-Myc) by optimizing retroviral transduction (5). A previous study demonstrated that
with either iPSC transplantation or iPSC-conditioned medium
(iPSC-CM) injection, interstitial and vascular fibrosis may be
significantly inhibited (6).
Additionally, iPSCs have previously been suggested to be effective
for the treatment of myocardial (6,7),
pulmonary (8) and renal (9) fibrosis.
These observations support the hypothesis that iPSCs
may suppress HS fibrosis by inhibiting fibroblast activation.
Although iPSCs have the ability to differentiate into cell types of
the three germ layers, it is difficult to manage the direction of
this differentiation. iPSCs cannot be maintained in an
undifferentiated state by a simple alteration in culture medium,
and previous studies have demonstrated that they may develop into
tumors, lose their self-renewal capacity or lose the potential to
differentiate into the cell type required for therapeutic
transplantation in vivo (8,10).
One study observed that the therapeutic effects of iPSC-CM are
similar to iPSCs in lung injury, and act via a similar signaling
pathway (11). Therefore, the
current study aimed to determine whether iPSC-CM is able to inhibit
fibroblast activation, by examining fibroblast-associated
properties, including activation, contraction and adhesion to human
acute monocytic leukemia (THP-1) cells in cultured human skin
fibroblasts.
Materials and methods
Cell culture and conditioned medium
iPSCs were generated from embryonic fibroblasts of
C57/B6 mice and were provided as a gift by Dr. Kazutoshi Takahashi
(Institute for Frontier Medical Sciences, Kyoto University, Kyoto,
Japan). The iPSCs were reprogrammed by the transduction of
retroviral vectors encoding four transcription factors, Oct-4,
Sox2, c-Myc and Klf4, and cultured in iPSC medium to
maintain an undifferentiated state, as previously described
(12). Human dermal fibroblasts
(HDFs) were isolated from normal human foreskin. All primary human
fibroblasts were obtained from each sample prior to tissue fixation
in 10% formalin (Nanchang Yulu Co., Jianxi, China) for routine
histological examination. The tissue sections were cut into 1–3-mm
cubes and incubated with 200 U/ml type I collagenase (Worthington
Biochemical Corporation, Lakewood, NJ, USA) for 4 h at 37°C. The
fibroblast cell cultures were maintained in Dulbecco’s modified
Eagle’s medium (DMEM; 11965-092) supplemented with 10% fetal bovine
serum (FBS), 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml
streptomycin (all from Gibco Life Technologies, Grand Island, NY,
USA). THP-1 cells (American Type Culture Collection, Manassas, VA,
USA) were maintained in RPMI 1640 medium (Gibco Life Technologies)
supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml
streptomycin and 0.5 mM/l β-mercaptoethanol (Gibco Life
Technologies). All cell lines were incubated at 37°C in a
humidified incubator with 5% CO2 and cells from passages
6–8 were used. Conditioned medium from iPSCs (2×105
cells/cm2) was diluted to 50, 30 and 0% by
HDF-conditioned medium (HDFs-CM).
A total of 15 foreskin samples were collected from
the Shanghai Jiaotong University Affiliated Sixth People’s Hospital
(Shanghai, China) following approval by the ethics committee for
human studies. The patients provided informed consent, and none had
a systemic disease or had been previously treated for scars.
Total protein synthesis assay
Following treatment with 0, 30, 50 or 100% iPSC-CM
for 24 h, 4×105 cells HDFs were harvested. The total
protein was determined by the microplate bicinchoninic acid method
using the BCA Protein Assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) in accordance with the manufacturer’s
instructions.
Cell adhesion assay
Cell adhesion assays were perfomed as described
previously (13). HDFs were seeded
at a density of 3×105 cells/well into 24-well plates
until confluence was reached, then were incubated with DMEM
supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and
100 mg/ml streptomycin. THP-1 cells were maintained in RPMI 1640
medium, supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml
streptomycin and 0.5 mM/L β-mercaptoethanol for 24 h and then were
labeled fluorescently using 2.5 mM Cellstain-calcein-AM-solution
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan). THP-1 and
HDF cells were co-cultured with the THP-1 suspension at a
concentration of 5×105 cells/ml and 0, 30, 50 or 100%
iPSC-CM was added into each well for 3 h. The plates were
centrifuged at 134 × g for 3 min and subsequently incubated at 37°C
for 60 min. The medium and the nonadherent THP-1 cells were removed
and each well was washed with phosphate-buffered saline three
times. Adherent THP-1 cells were then microscopically quantified at
a magnification of ×100 in four random visual fields for each well,
and were subsequently imaged using an Axiovert 200 inverted
fluorescence microscope (Zeiss, Oberkochen, Germany).
Three dimensional (3D) collagen gel
contraction assay
HDFs were seeded into 32-mm bacteriological plates
(density, 6×104 cells/ml; 2 ml/dish) in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin, sodium ascorbate (50 mg/ml; Gibco Life Technologies)
and 0.3 mg/ml acid-extracted collagen I from newborn calf skin
(IBFB Pharma GmbH, Leipzig, Germany), as previously described
(14), with 0, 30, 50 or 100%
iPSC-CM. Furthermore, the contraction efficiency of the iPSC-CM was
compared between quiescent and activated HDFs treated with TGF-β1
(Sigma-Aldrich, St. Louis, MO, USA). The cells were cultured at
37°C for 60 min to allow collagen polymerization to occur. The gels
were then released from the plates by tilting them slightly.
Gradual gel contraction was assessed by measuring the gel area at
four time points, including 6, 12, 18 and 24 h. The data are
presented as the mean ± standard error of three independent
experiments, each conducted in triplicate.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cultured HDFs using
TRIzol reagent (Invitrogen Life Technologies), and the integrity of
the RNA was determined by 2% UltraPure agarose gel (Gibco Life
Technologies) electrophoresis (15). For RT-qPCR, 2 μg total RNA was
reverse transcribed at 37°C for 1 h in a 25-μl reaction medium
containing 250 mM Tris-hydrochloric acid (HCl), 375 mM potassium
chloride (KCl), 15 mM magnesium chloride (MgCl2), 50 mM
dithiothreitol, 10 mM deoxynucleotide triphosphates (dNTPs), 0.5 μg
oligo (dT) 20 primer, 100 U reverse transcriptase (M-MLV) and 25 U
ribonuclease inhibitor (all from Takara Bio, Inc., Otsu, Japan) and
were subjected to PCR amplification with the primers described in
Table I. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was amplified as the internal
control. The RT products (0.5-l.0 μg) were amplified with 1 U Taq
DNA polymerase (Takara Bio, Inc.) and 1 mM of each primer in a 50
μl reaction mix containing 50 mM KCl, 10 mM Tris-HCl, 1.5 mM
MgCl2 and 0.02 mM each of four dNTPs as follows: Initial
denaturation for 3 min at 94°C, 30 cycles of amplification, 1 min
of denaturation at 94°C, annealing temperatures of 57°C and 53°C
for the collagen Iα1 and GAPDH primers, respectively, 1 min of
extension at 72°C and a final 5-min elongation period at 72°C.
Parallel PCR assays without reverse transcriptase were performed
for each sample to confirm that the PCR products resulted from cDNA
rather than from genomic DNA. The PCR products (10 μl) were
analyzed by 2% agarose gel electrophoresis (15). The relative abundance of mRNA was
calculated by densitometric analysis using Digital Science 1D Image
Analysis software, version 3.0 (Kodak, Rochester, NY, USA).
 | Table IPrimer design and product lengths for
RT-PCR products. |
Table I
Primer design and product lengths for
RT-PCR products.
| Gene | Primer | Length (bp) | Tm |
|---|
| COLIA1 |
5′-AAAGACGGGAGGGCGAGTG-3′ | | |
|
5′-GCCATAGGACATCTGGGAAGCAA-3′ | 242 | 62 |
| GAPDH |
5′-GTCGTGGAGTCTACTGGCGTCTT-3′ | | |
|
5′-CAGTCTTCTGAGTGGCAGTGATGG-3′ | 280 | 58 |
Western blotting
Cells were lysed with radio-immunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Jiangsu,
China) supplemented with 1 mM phenylmethylsulfonyl fluoride
(Adamas-Beta, Ltd., Shanghai, China). The cell lysates were subject
to western blot analysis, which was conducted as described in
previous studies (15). The
primary antibodies used were as follows: Polyclonal rabbit
anti-human α-SMA IgG (1:500; ab15263; Abcam, Cambridge, UK) and
monoclonal mouse anti-human collagen I IgG1 (1:200; sc-59772; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA).
Statistical analysis
Statistical analysis was performed using SPSS,
version 13.0 (SPSS, Inc., Chicago, IL, USA) and a paired samples
t-test was used to identify any differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxicity of iPSC-CM
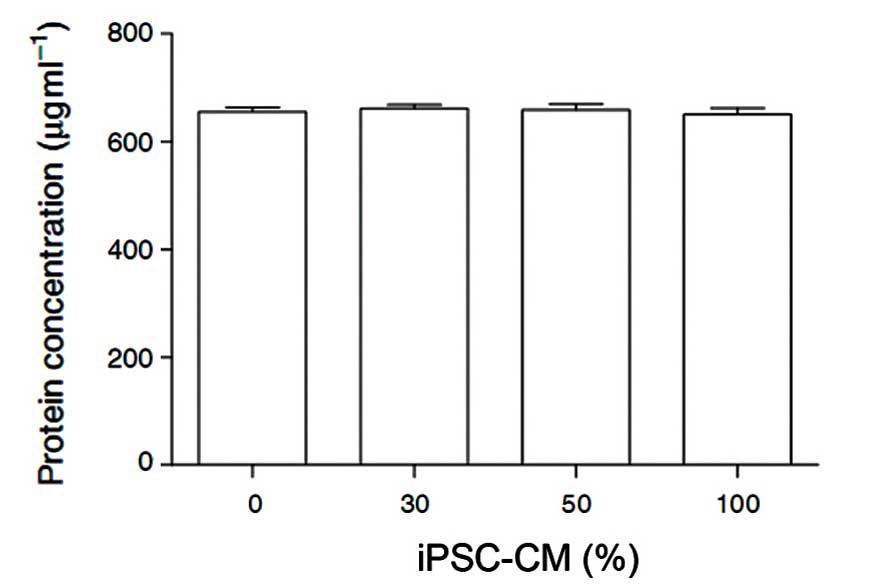
To evaluate the cytotoxicity of iPSC-CM on
fibroblasts, the total protein products following treatment of HDFs
with 0, 30, 50 or 100% iPSC-CM for 24 h were measured. No
significant differences were observed in the total protein among
the four groups, which suggests that total fibroblast activity is
not markedly affected by iPSC-CM (Fig.
1).
Suppressive effect of fibroblast
activation by iPSC-CM
HS is formed by the abnormal accumulation of ECM.
This predominantly consists of collagen, particularly type I
collagen, which is produced by fibroblasts and is vital to HS
formation. The proliferative stage of HS is marked by proliferation
and activation of fibroblasts, thus, the level of type I collagen
may be a marker of fibroblast activation. Myofibroblasts (activated
fibroblasts) are identified by α-SMA expression and stress fiber
formation (16), and their
recruitment, retention and differentiation are commonly triggered
by local stimuli of the microenvironment. Those stimuli include
TGF-β1, mechanical force and matrix stiffness (17).
To determine whether in vitro TGF-β1-induced
fibroblast activation may be suppressed by iPSC-CM in the present
study, the level of α-SMA expression was assayed using western blot
analysis. The results indicated that iPSC-CM significantly
suppressed TGF-β1-induced α-SMA expression in a dose-dependent
manner in cultured HDFs (P<0.01; Fig. 2A). Additionally, the alterations in
collagen type Iα1 expression and type I collagen protein levels
were confirmed by RT-qPCR and western blotting. The data
demonstrated that the expression levels of collagen type Iα1 mRNA
and collagen I protein were reduced, compared with control in 100%
iPSC-CM (P<0.05; Fig. 2B and
C). These observations suggest that fibroblast activation may
be effectively suppressed by iPSC-CM.
 | Figure 2iPSC-CM suppresses fibroblast
activation. (A) Protein levels of α-SMA following incubation with
iPSC-CM (0, 30, 50 and 100%) and TGF-β1 (2 ng/ml) for 12 h. (B)
Expression levels of collagen type Iα1 mRNA following incubation
with iPSC-CM (0, 30, 50 and 100%) for 24 h. (C) Protein levels of
collagen I following incubation with iPSC-CM (0, 30, 50 and 100%)
for 24 h. All values are presented as the mean ± standard error;
*P< 0.05, **P<0.01; n=3. iPSC-CM,
induced pluripotent stem cells-conditioned medium; α-SMA, α-smooth
muscle actin; TGF-β1, transforming growth factor-β1. |
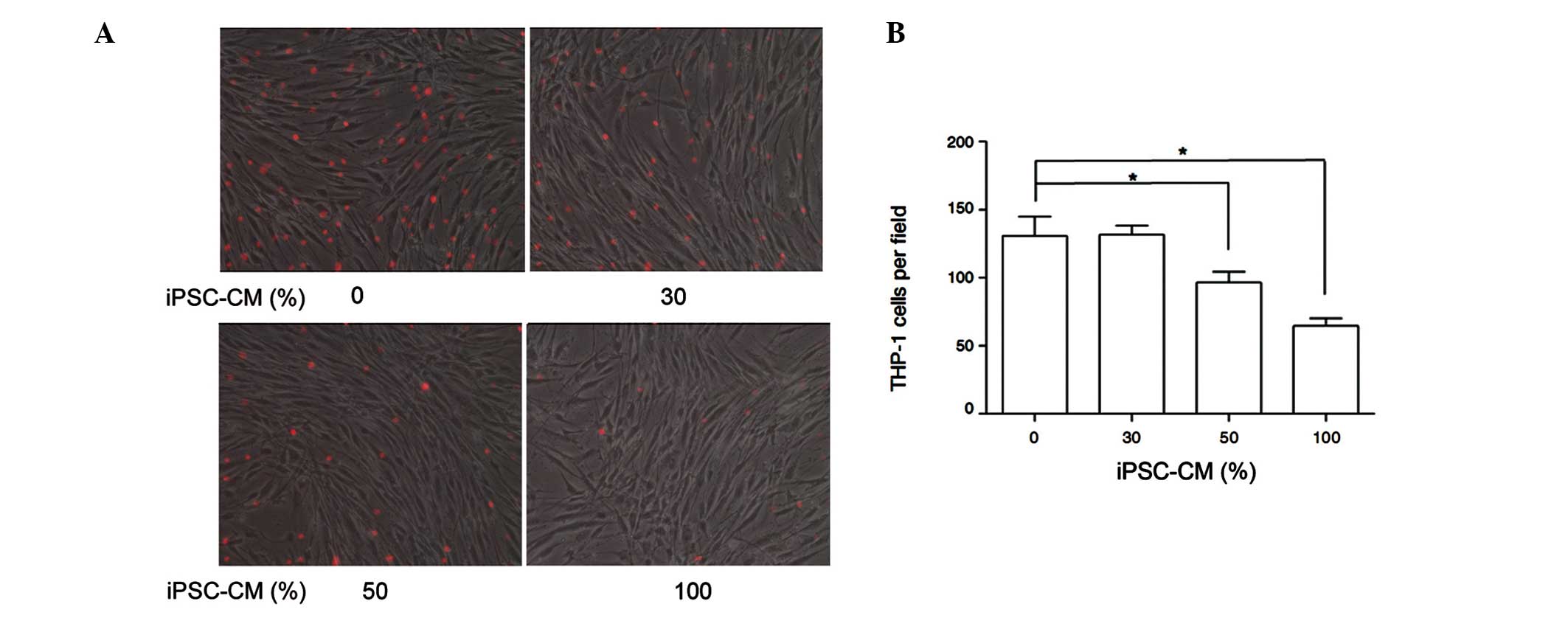
Inhibition of inflammatory cell adhesion
on HDFs by iPSC-CM
Monocytes and fibroblasts work together during
tissue repair, and fibroblasts regulate the responses of monocytes
to ECM-derived matrices (18).
Monocytes and lymphocytes are the main cells that induce chronic
inflammatory reactions in HS. In addition, THP-1 monocytes are
routinely used in monocyte assays (19). Therefore, the adhesion levels of
THP-1 and cultured HDFs were examined to identify the inhibition of
chronic inflammation by iPSC-CM.
In vitro adhesion assays of THP-1 and
cultured HDFs demonstrated that iPSC-CM significantly reduced the
level of adhesion in a dose-dependent manner (Fig. 3). These results suggest that
iPSC-CM may suppress fibroblast activation by inducing the
recruitment of inflammatory cells and blocking the direct
interaction of inflammatory cells and fibroblasts. However, to
confirm the attenuated effect of iPSC-CM on the inflammatory
response in HS, in vivo studies are required.
iPSC-CM reduces the contractile ability
of HDFs in 3D collagen gels
Tissue contraction is dynamic and is characterized
by intracellular and extracellular events (20). The contraction of HS is not
determined by fibroblast properties alone, but is also dependent on
the rate and extent of matrix contraction. To evaluate the effect
of iPSC-CM on the contractile ability of HDFs, a 3D collagen gel
fibroblast contraction assay was performed. Subsequent to treatment
with 0, 30, 50 or 100% iPSC-CM for 6, 12, 18 or 24 h, the
contraction of the collagen gels was monitored by measuring the gel
area. Significant dose-dependent inhibitory effects of iPSC-CM on
the contractile ability of HDFs were observed, and as time
progressed, the differences between 100% iPSC-CM and the other
three groups remained statistically significant (Fig. 4A). Significant time- and
dose-dependent inhibitory effects of iPSC-CM on the contractile
ability of HDFs were observed (Fig.
4A). Additionally, the contraction efficiency of iPSC-CM was
significantly lower in the quiescent HDFs compared with the
activated HDFs, at 18 and 24 h (P<0.05; Fig. 4B). This suggests that iPSC-CM may
more efficiently prevent alterations in the contractile ability of
activated HDFs treated with TGF-β, compared with quiescent HDFs.
These observations further support the hypothesis that iPSC-CM is
able to suppress fibroblast activation in vitro.
 | Figure 4iPSC-CM attenuates the contractile
ability of HDFs in 3D collagen gels. (A) Quantification of collagen
gel contraction following treatment with iPSC-CM (0, 30, 50 and
100%) for 6, 12, 18 and 24 h; n=3. (B) Contraction efficiency of
iPSC-CM on quiescent HDFs and activated HDFs treated with TGF-β1
for 6, 12, 18 and 24 h; n=3. All values are presented as the mean ±
standard error. *P<0.05, **P<0.01,
***P<0.001. iPSC-CM, induced pluripotent stem
cells-conditioned medium; HDFs, human dermal fibroblasts; TGF-β1,
transforming growth factor-β1. |
Discussion
HS is a complex and multifactorial fibrotic
abnormality that is associated with excessive fibroblast
proliferation and collagen synthesis. Previous studies have
reported that mutual regulation of chronic inflammation, mechanical
force and fibroblast activation leads to the formation of HS in
pathological scar formation (16,21,22).
The prolonged existence of chronic inflammation in
the active stage of pathological scarring has been investigated in
a number of studies (21–23). Histological observations suggest
that a large number of macrophages, lymphocytes and mast cells are
recruited to the focal site and the early immunological response is
important in HS formation (24).
Specific cytokines and inflammatory factors, such as TGF-β1 are
secreted from these cells and contribute to fibroblast activation
and the modulation of the fibroblast phenotype. The results of the
current study suggest that iPSC-CM is able to block cell-cell
adhesion of inflammatory cells and fibroblasts, while a previous
study indicated that fibroblasts can be activated by direct contact
with inflammatory cells, such as THP-1 cells (25). Thus, it was concluded that the
fibroblast activation suppression by iPSC-CM is partially due to
the inhibition of inflammatory cell adhesion.
During the active period of HS, myofibroblasts
rooted in irritated fibroblasts are transiently involved in wound
repair (26). Under normal
conditions, α-SMA (a myofibroblast marker) is not expressed by
dermal fibroblasts (27), however,
α-SMA expression is observed with mechanical force, which is key in
the activation of fibroblasts (28). Conversely, increased numbers of
myofibroblasts are responsible for the contractible collagen gels
and contraction of wounds during healing (?). In the present study,
iPSC-CM significantly reduced the expression of α-SMA in cultured,
activated HDFs and notably, produced a significantly greater
reduction in the contractile ability of activated HDFs than in
quiescent HDFs in 3D collagen gels. From these results, it can be
concluded that iPSC-CM may attenuate fibroblast activation via the
inhibition of the fibroblast phenotype switch.
Notably, mechanical force has been demonstrated to
induce a chronic-like inflammatory state and boost the recruitment
of inflammatory cells, including macrophages and lymphocytes
(21). Another study indicated
that physical force regulates fibrosis through the inflammatory
FAK-ERK-MCP-1 pathway (23). It is
thus clear that the various etiologies are not independent of each
other.
In fibrotic diseases, activated fibroblasts are the
main cells involved in the pathogenesis, with abnormal fibroblast
activation leading to excessive ECM deposition and increased
generation of myofibroblasts. However, the current treatments for
fibrotic disorders are unsatisfactory, with an urgent requirement
for an effective therapeutic strategy. iPSCs are embryonic-like
stem cells that have been demonstrated to have potential in
regenerative medicine, while iPSC-CM contains various components,
including cytokines and growth factors, which remain to be fully
elucidated. The identification of factors that may assist with scar
treatment is required and a therapeutic treatment able to prevent
abnormal fibroblast activation may be a novel and effective
treatment strategy for fibrosis.
In conclusion, the present study indicates that
iPSC-CM may be an effective compound in preventing processes
leading to HS formation, by attenuating fibroblast activation,
blocking inflammatory cell recruitment and adhesion and reducing
the contractibility of fibroblasts. However, additional research is
required to fully elucidate the biomolecular modulation of the
suppressive effect on fibroblast activation by iPSC-CM.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81000837 and
81370055).
References
|
1
|
Canady J, Arndt S, Karrer S and Bosserhoff
AK: Increased KGF expression promotes fibroblast activation in a
double paracrine manner resulting in cutaneous fibrosis. J Invest
Dermatol. 133:647–657. 2013. View Article : Google Scholar
|
|
2
|
Beanes SR, Dang C, Soo C and Ting K: Skin
repair and scar formation: the central role of TGF-beta. Expert Rev
Mol Med. 5:1–22. 2003. View Article : Google Scholar
|
|
3
|
Clark RA: The Molecular and Cellular
Biology of Wound Repair. 2nd edition. Springer; New York, NY: pp.
22–23. 1996
|
|
4
|
Kovacs EJ: Fibrogenic cytokines: the role
of immune mediators in the development of scar tissue. Immunol
Today. 12:17–23. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neel S and Singla DK: Induced pluripotent
stem (iPS) cells inhibit apoptosis and fibrosis in
streptozotocin-induced diabetic rats. Mol Pharm. 8:2350–2357. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singla DK, Long X, Glass C, Singla RD and
Yan B: Induced pluripotent stem (iPS) cells repair and regenerate
infarcted myocardium. Mol Pharm. 8:1573–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Q, Quan Y, Sun H, et al: A
site-specific genetic modification for induction of pluripotency
and subsequent isolation of derived lung alveolar epithelial type
II cells. Stem Cells. 32:402–413. 2014. View Article : Google Scholar :
|
|
9
|
Chou YH, Pan SY, Yang CH and Lin SL: Stem
cells and kidney regeneration. J Formos Med Assoc. 113:201–209.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colman A and Dreesen O: Induced
pluripotent stem cells and the stability of the differentiated
state. EMBO Rep. 10:714–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li LF, Liu YY, Yang CT, et al: Improvement
of ventilator-induced lung injury by IPS cell-derived conditioned
medium via inhibition of PI3K/Akt pathway and IP-10-dependent
paracrine regulation. Biomaterials. 34:78–91. 2013. View Article : Google Scholar
|
|
12
|
Chen SJ, Chang CM, Tsai SK, et al:
Functional improvement of focal cerebral ischemia injury by
subdural transplantation of induced pluripotent stem cells with
fibrin glue. Stem Cells Dev. 19:1757–1767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu MJ, Liu XJ, Zhao YL, et al:
Identification and characterization of an anti-fibrotic benzopyran
compound isolated from mangrove-derived Streptomyces xiamenensis.
Mar Drugs. 10:639–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ZG, Bothe I, Hirche F, et al:
Interactions of primary fibroblasts and keratinocytes with
extracellular matrix proteins: contribution of alpha2beta1
integrin. J Cell Sci. 119:1886–1895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mun JH, Kim YM, Kim BS, Kim JH, Kim MB and
Ko HC: Simvastatin inhibits transforming growth factor-β1-induced
expression of type I collagen, CTGF, and α-SMA in keloid
fibroblasts. Wound Repair Regen. 22:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu XJ, Xu MJ, Fan ST, et al: Xiamenmycin
attenuates hypertrophic scars by suppressing local inflammation and
the effects of mechanical stress. J Invest Dermatol. 133:1351–1360.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarrazy V, Billet F, Micallef L, Coulomb B
and Desmoulière A: Mechanisms of pathological scarring: role of
myofibroblasts and current developments. Wound Repair Regen.
19(Suppl 1): s10–s15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Riddle SR, Frid MG, et al: Emergence
of fibroblasts with a proinflammatory epigenetically altered
phenotype in severe hypoxic pulmonary hypertension. J Immunol.
187:2711–2722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung AS and Kao WJ: Fibroblasts regulate
monocyte response to ECM-derived matrix: the effects on monocyte
adhesion and the production of inflammatory, matrix remodeling, and
growth factor proteins. J Biomed Mater Res A. 89:841–853. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ngo P, Ramalingam P, Phillips JA and
Furuta GT: Collagen gel contraction assay. Methods Mol Biol.
341:103–109. 2006.PubMed/NCBI
|
|
21
|
Wong VW, Paterno J, Sorkin M, et al:
Mechanical force prolongs acute inflammation via T-cell-dependent
pathways during scar formation. FASEB J. 25:4498–4510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaker SA, Ayuob NN and Hajrah NH: Cell
talk: a phenomenon observed in the keloid scar by
immunohistochemical study. Appl Immunohistochem Mol Morphol.
19:153–159. 2011. View Article : Google Scholar
|
|
23
|
Wong VW, Rustad KC, Akaishi S, et al:
Focal adhesion kinase links mechanical force to skin fibrosis via
inflammatory signaling. Nat Med. 18:148–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van der Veer WM, Bloemen MC, Ulrich MM, et
al: Potential cellular and molecular causes of hypertrophic scar
formation. Burns. 35:15–29. 2009. View Article : Google Scholar
|
|
25
|
Clayton A, Evans RA, Pettit E, Hallett M,
Williams JD and Steadman R: Cellular activation through the
ligation of intercellular adhesion molecule-1. J Cell Sci.
111:443–453. 1998.PubMed/NCBI
|
|
26
|
Agarwal C, Britton ZT, Alaseirlis DA, Li Y
and Wang JH: Healing and normal fibroblasts exhibit differential
proliferation, collagen production, alpha-SMA expression, and
contraction. Ann Biomed Eng. 34:653–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darby I, Skalli O and Gabbiani G:
Alpha-smooth muscle actin is transiently expressed by
myofibroblasts during experimental wound healing. Lab Invest.
63:21–29. 1990.PubMed/NCBI
|
|
28
|
Wang J, Chen H, Seth A and McCulloch CA:
Mechanical force regulation of myofibroblast differentiation in
cardiac fibroblasts. Am J Physiol Heart Circ Physiol.
285:H1871–H1881. 2003.PubMed/NCBI
|