Introduction
Cerebral hemorrhage, also termed intracerebral
hemorrhage (ICH), is a subtype of intracranial hemorrhage occurring
within the brain tissue, which can be caused by brain trauma or can
occur spontaneously in hemorrhagic stroke (1). ICH is a common fatal subtype of
stroke and can cause mortality or severe disability (1,2). ICH
causes mortality in >30,000 individuals annually in the United
States (3). Following ICH, the
resulting hematoma within the brain parenchyma triggers a series of
adverse events, which lead to secondary insults and severe
neurological deficits (4).
Epidemiological studies have identified differences in the
incidence of cerebral hemorrhage between different ethnicities,
with African American and Japanese individuals having an incidence
rate twice and four times that of Caucasians, respectively
(5). Although hypertensive
arteriolosclerosis and cerebral amyloid angiopathy are risk factors
that contribute to the majority of ICH cases, several genetic risk
factors have been identified (6).
Characterization of these genetic variations may enable
improvements in the prognosis and prevention of ICH.
Reactive oxygen species (ROS), including superoxide,
hydrogen peroxide and hydroxyl radicals, are generated by the
partial reduction of oxygen (7).
ROS in the vasculature are produced by several enzymatic systems,
including xanthine oxidase, nitric oxide synthases and nicotinamide
adenine dinucleotide phosphate [NAD(P)H] oxidases. Previous studies
have indicated that NAD(P)H oxidases are major factors involved in
the aberrant production of ROS in the vasculature (7,8).
Cumulative evidence has demonstrated that ROS are essential in the
pathogenesis of various types of cardiovascular disease, including
stroke and atherosclerotic lesions (9). ROS-mediated oxidative modification of
phospholipids can damage endothelial cells and induce the
expression of adhesion molecules, which leads to attachment of
monocytes, T lymphocytes and macrophages to the endothelial cells
(10). It is hypothesized that the
diversity of novel oxidative modification to lipids and proteins
mediated by ROS is associated with foam cell formation, the
development of atherosclerotic lesions and ultimately a fibrous
plaque that protrudes into the arterial lumen (11). The formation and release of thrombi
may occlude vessels, which causes cardiovascular diseases,
including hypertension, congestive heart failure, ICH and stoke
(12).
There is accumulating evidence that the -A930G
polymorphism of the p22phox subunit of NAD(P)H oxidases is
associated with ICH (13).
Membrane-associated NAD(P)H oxidases are the main enzymes
responsible for ROS generation in neutrophil granulocytes and the
vasculature, which may be important in the pathogenesis of ischemic
injury and atherosclerosis (14).
The NAD(P)H oxidase system has been well characterized, comprising
of three cytosolic elements, p40phox, p47phox and p67phox, two
membrane-associated cytochromes, p22phox and gp91phox and a low
molecular weight G-protein (15).
Polymorphism of the p22phox gene has been investigated the most of
those in the NAD(P)H oxidase system. The gene encoding the p22phox
subunit, CYBA, is located on the long arm of chromosome 16 (16q24),
spans a length of 8.5 kb and contains six exons and five introns
(16). The -A930G polymorphism is
located at the promoter region and the G allele has increased
promoter activity and a higher level of ROS, leading to increased
vascular smooth muscle cell proliferation, endothelial dysfunction
and the formation of lesions, which can contribute to
cerebrovascular disease (13). It
has been observed that the -A930G polymorphism is associated with
hypertension. Hypertensive patients with a GG genotype exhibit
increased p22phox mRNA and protein levels and enhanced NAD(P)H
oxidase activity compared with those with an AA or AG genotype,
while no difference is observed in these parameters in healthy
subjects (13).
However, it remains unclear whether the -A930G
polymorphism of the p22phox gene is associated with ICH. The
purpose of the present case-control study was to determine whether
the -A930G polymorphism of the NAD(P)H oxidase p22phox gene is
important in Chinese Han patients with ICH, using the method of
polymerase chain reaction (PCR), restriction fragment length
polymorphism (RFLP) and gene sequencing.
Subjects and methods
Subjects
Recruitment of individuals for the present
case-control study was performed in the Department of Internal
Neurology from Shanghai Songjiang Central Hospital (Shanghai,
China). All individuals were from the Chinese Han population of
Shanghai. The ICH group included 118 patients, confirmed using
computed tomography scan and/or magnetic resonance imaging and
healthy controls, which included 147 individuals of matched age and
gender. Individuals with cardiocerebral injuries, tumors, surgery,
external injuries or severe heart, lung, kidney diseases were
excluded from the investigation and individuals with a history of
stroke or related diseases were also excluded from the control
group. The present study complied with the Helsinki declaration and
was approved by the Ethics Committee of Shanghai Songjiang Central
Hospital. All of the individuals provided informed consent. Blood
glucose and blood lipid examinations were performed on all
subjects. Previous diagnoses of hypertension and diabetes were also
recorded. Hypertension was diagnosed at a blood pressure level of
≥140/90 mmHg. Diabetes was diagnosed at a fasting glucose level of
>7 mmol/l. Individuals with levels of blood cholesterol ≥5.2
mmol/l, triglyceride ≥2.3 mmol/l, high density lipoprotein (HDL)
≤1.8 mmol/l, low density lipoprotein (LDL) ≥4.9 mmol/l,
apolipoprotein (Apo)A ≥2.25 g/l or ApoB ≥1.33 g/l, was diagnosed as
hyperlipidemia.
DNA extraction
Venous blood (2 ml) was collected into tubes
following treatment with ethylenediaminetetraacetic acid and stored
at −80°C. Genomic DNA was extracted using a DNA extraction kit (DNA
purification kit; Omega Bio-Tek, Norcross, GA, USA) according to
the manufacturer’s instructions. DNA quantity and purity were
measured using a spectrophotometer (BD 177742; BD Biosciences,
Franklin Lakes, NJ, USA).
Genotyping
The p22phox gene was amplified by PCR using the
following primers: Forward: 5′-GTGTGGCTGGAA TGGTGGCAGGAG-3′ and
reverse: 5′-GAGGGTCCCACC TGAGCCAATGTG-3′ designed using primer 5
software (Premier Biosoft, Palo Alto, CA, USA). The primers were
synthesized at Shanghai Shenggong Biological Engineering Co., Ltd.
(Shanghai, China). PCR was performed using 25 μl 2-fold PCR
mixture, 1 μl of each primer, 15 μl template DNA in a 50 μl final
volume. The PCR amplification program was as follows: Initial
denaturation at 95°C for 3 min, denaturation at 95°C for 30 sec,
annealing at 65.5°C for 30 sec, extension at 72°C for 45 sec (35
cycles) and a final extension step at 72°C for 10 min.
RFLP
The PCR products (10 μl) were digested with 2 μl
10-fold BseXI buffer, 1 μl BseXI and 7 μl
ddH2O, and the mixture was incubated for 12 h at 65°C.
Following digestion, 4 μl 6-fold DNA loading dye (Sigma-Aldrich,
St. Louis, MO, USA) was added to the mixture. All products were
separated by electrophoresis on a 2% agarose gel containing 0.1%
ethidium bromide and were visualized under ultraviolet light.
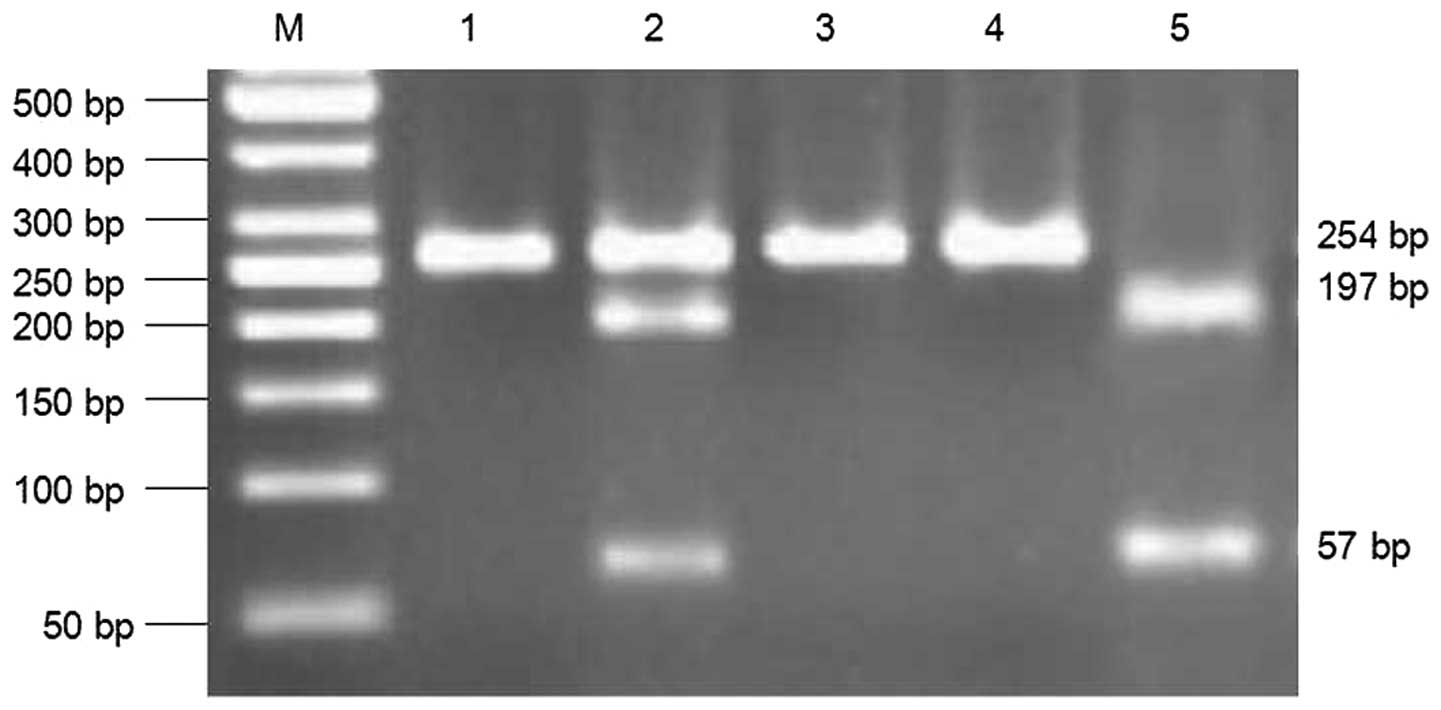
Digestion of the PCR products yielded bands of 254 bp for the
AA-homozygotes, 57 and 197 bp for the GG-homozygotes and three
fragments in the AG-heterozygotes (Fig. 1). DNA purification was performed
with a commercially available kit (QIAamp DNA kit; Qiagen,
Duesseldorf, Germany) according to the manufacture’s instruction.
The PCR products of the three genotypes were randomly selected for
DNA sequencing in an ABI 3130XL automated sequencer (Applied
Biosystems, Foster City, CA, USA), according to the manufacturer’s
instructions, and were then sent for gene sequencing by Invitrogen
Life Technologies (Carlsbad, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). All quantitative data are
expressed as the mean ± standard error of the mean. Student’s
t-test was used to compare between two groups. The χ2
test was used to compare categorical variables. Deviation of the
genotype and allele distribution from the Hardy-Weinberg
equilibrium was measured using the χ2 test. P<0.05
was considered to indicate a statistically significant difference.
Binary logistic regression analysis was performed to estimate the
independent risk factors for ICH, which were identified when
β>0, odds ratio (OR)>1 and P<0.05.
Results
Subject characteristics
No significant differences were observed in gender,
age or body mass index between the ICH and control groups
(P>0.05), suggesting that the ICH group was comparable with the
control group (Table I). The
prevalence of conventional risk factors for ICH were significantly
higher in the ICH group (P<0.05), which included systolic blood
pressure (SBP), diastolic blood pressure (DBP), cholesterol, LDL,
blood glucose, triglyceride, history of hypertension, smoking and
alcohol consumption. No significant difference was observed in HDL,
ApoA or ApoB between the patients and the controls (P>0.05).
 | Table ICharacteristics of intracerebral
hemorrhage patients and healthy controls. |
Table I
Characteristics of intracerebral
hemorrhage patients and healthy controls.
| Characteristic | Controls (n=147) | Patients (n=118) | F-value/χ2
value | P-value |
|---|
| Male, n (%) | 88 (59.9) | 70 (59.3) | 0.008 | 0.929 |
| Age (years) | 65.22±9.69 | 66.28±14.53 | 22.922 | 0.481 |
| BMI
(kg/m2) | 23.78±2.28 | 23.64±2.62 | 1.989 | 0.639 |
| SBP (mmHg) | 135.29±19.67 | 163.09±27.68 | 19.269 | 0.000 |
| DBP (mmHg) | 81.14±10.17 | 92.67±16.08 | 27.079 | 0.000 |
| Blood glucose
(mmol/l) | 6.01±2.14 | 6.80±3.02 | 8.494 | 0.015 |
| Triglycerides
(mmol/l) | 1.33±0.75 | 1.73±1.07 | 6.680 | 0.000 |
| Cholesterol
(mmol/l) | 4.59±0.98 | 4.48±0.94 | 0.019 | 0.037 |
| HDL (mmol/l) | 1.34±0.34 | 1.35±0.42 | 5.181 | 0.886 |
| LDL (mmol/l) | 3.00±0.88 | 2.82±0.79 | 0.676 | 0.036 |
| ApoA (g/l) | 1.39±0.27 | 1.34±0.33 | 5.693 | 0.204 |
| ApoB (g/l) | 0.94±0.25 | 0.88±0.26 | 0.710 | 0.090 |
| Smoker, n (%) | 19 (12.9) | 29 (24.6) | 5.991 | 0.014 |
| Alcohol drinker, n
(%) | 23 (15.6) | 35 (29.7) | 7.520 | 0.006 |
| History of
hypertension, n (%) | 44 (29.9) | 81 (68.6) | 39.364 | 0.000 |
Distribution of the -A930G
polymorphism
The distribution of the -A930G polymorphism of the
p22phox gene was accordance with the Hardy-Weinberg equilibrium in
the ICH and control individuals. The PCR products were digested by
BseXI at the 5′-GCAGCN8-3′ site. The DNA of the AA genotype
was not digested by the enzyme, yielding a 254 bp DNA fragment. The
DNA of GG genotype was digested into two fragments of 57 and 197
bp. As only one chain of the AG gene was digested, the AG genotype
DNA was cleaved into three fragments of 57, 197 and 254 bp
(Fig. 1). DNA sequencing was
performed with five PCR products of the AA, AG and GG genotypes
according to the results of the restriction digestion (Fig. 2). The arrows indicate mutation
sites, which were in accordance with the electrophoresis results.
The distribution of p22phox -A930G genotypes demonstrated
significant differences between the two groups (Table II, P=0.003), with frequencies of
the AA, AG, GG genotypes of 61.9, 29.3 and 8.8% in the controls,
and 40.7, 45.8 and 13.6% in the ICH patients, respectively. The G
allele frequency in the ICH patients was significantly higher
compared with the control group, 36.4 and 23.5%, respectively
(P<0.05). By contrast, the frequency of the A allele was
significantly lower in the ICH group compared with the controls,
63.6 and 76.5%, respectively (P<0.05).
 | Table IIGenotype and allele distributions for
the patients with intracerebral hemorrhage and the healthy control
group. |
Table II
Genotype and allele distributions for
the patients with intracerebral hemorrhage and the healthy control
group.
| Genotype/allele | Controls (n=147) | Patients (n=118) | χ2
value | P-value |
|---|
| −A930G genotype, n
(%) | | | 12.828 | 0.003 |
| AA | 91 (61.9) | 48 (40.7) | | |
| AG | 43 (29.3) | 54 (45.8) | | |
| GG | 13 (8.8) | 16 (13.6) | | |
| −A930G allele, n
(%) | | | 10.645 | 0.001 |
| A | 225 (76.5) | 150 (63.6) | | |
| G | 69 (23.5) | 86 (36.4) | | |
Binary logistic regression analysis of
risk factors for ICH
Differences between the patients and controls were
analyzed using binary logistic regression analysis, which revealed
that the -A930G polymorphism of the p22phox gene was an independent
risk factor for ICH (P=0.009; OR, 2.196; 95% CI, 1.003–3.586).
However, SBP, DBP, blood glucose and triglyceride levels, and a
history of hypertension and smoking may be associated with the risk
of ICH (Table III).
 | Table IIIIntracerebral hemorrhage risk factors
in binary logistic regression analysis. |
Table III
Intracerebral hemorrhage risk factors
in binary logistic regression analysis.
| Risk factor | β-value | SEM | P-value | OR-value | 95% CI |
|---|
| Gender | −0.696 | 0.347 | 0.095 | 0.499 | 0.253–0.984 |
| SBP | 0.815 | 0.202 | 0.010 | 2.260 | 1.521–3.358 |
| DBP | 0.418 | 0.228 | 0.067 | 1.519 | 0.972–2.375 |
| Smoking | 1.078 | 0.444 | 0.015 | 2.938 | 1.229–7.021 |
| History of
hypertension | 0.893 | 0.330 | 0.014 | 2.442 | 1.279–4.664 |
| Triglyceride
level | 1.039 | 0.497 | 0.037 | 2.826 | 1.066–7.489 |
| Blood glucose | 1.172 | 0.425 | 0.006 | 3.228 | 1.403–7.426 |
| −A930G | 0.640 | 0.325 | 0.009 | 2.196 | 1.003–3.586 |
Comparison of clinical data in different
-A930G genotypes
Following comparison of the clinical data from the
three -A930G genotypes, significant differences were observed in
the level of SBP among the AA, AG and GG genotypes (P<0.05),
while no significant differences were observed with the other
parameters (P>0.05). These data suggested that genetic mutation
of -A930G increased the level of SBP (Table IV).
 | Table IVComparison between the clinical
characteristics of different genotypes of the −A930G p22phox
polymorphism. |
Table IV
Comparison between the clinical
characteristics of different genotypes of the −A930G p22phox
polymorphism.
| Clinical
characteristic | AA (141) | AG (96) | GG (28) | F-value | P-value |
|---|
| BMI
(kg/m2) | 23.88±2.27 | 23.48±2.69 | 23.77±2.37 | 0.770 | 0.464 |
| SBP (mmHg) | 143.76±25.62 | 151.60±27.56 | 153.89±32.17 | 3.225 | 0.041 |
| DBP (mmHg) | 84.95±14.39 | 88.41±14.00 | 85.64±14.56 | 1.706 | 0.184 |
| Blood glucose
(mmol/l) | 6.30±2.45 | 6.66±3.07 | 5.76±1.08 | 1.438 | 0.239 |
| Triglycerides
(mmol/l) | 1.50±0.94 | 1.52±0.86 | 1.55±1.11 | 0.045 | 0.956 |
| Cholesterol
(mmol/l) | 4.56±0.96 | 4.58±0.99 | 4.36±0.90 | 0.613 | 0.542 |
| HDL (mmol/l) | 1.32±0.36 | 1.36±0.39 | 1.39±0.43 | 0.707 | 0.494 |
| LDL (mmol/l) | 2.93±0.83 | 2.95±0.89 | 2.71±0.79 | 0.971 | 0.380 |
| ApoA (g/l) | 1.36±0.28 | 1.37±0.30 | 1.35±0.34 | 0.076 | 0.927 |
| ApoB (g/l) | 0.92±0.27 | 0.92±0.25 | 0.86±0.21 | 0.606 | 0.546 |
Discussion
ROS are positively associated with cerebrovascular
diseases. Enhanced ROS production at sites of injury regulates all
the cellular responses to injury, including the induction of
inflammatory genes, expression of adhesion molecules, monocyte
adhesion and platelet aggregation (17). Alteration of the balance between
endothelial proliferation and apoptosis by ROS causes excessive
angiogenesis or loss of endothelial cells (18). ROS promote the activation,
aggregation and adhesion of platelets on the endothelium at sites
of thrombi and can cause mitochondrial dysfunction, leading to a
reduction in the production of adenosine triphosphate and marked
problems with energy metabolism (19). Evidence has revealed that p22phax
is an essential component of the NAD(P)H oxidase system and is
important in regulating the activity of NAD(P)H oxidases when
producing ROS (20,21).
The -A930G polymorphism of NAD(P)H p22phox is
significantly associated with NAD(P)H oxidase activation and the
production of ROS. San José et al (22) reported that hypertensive patients
with the GG genotype have increased mRNA and protein levels of
p22phox and higher NAD(P)H oxidase activity compared with those
with AA/AG genotypes, while no differences were observed between
the GG and AA/AG genotypes in the healthy control group.
Transfection experiments on vascular smooth muscle cells revealed
that the A to G substitution increases the expression of the
reporter gene in hypertensive cells and hypertensive subjects
carrying the GG genotype of the p22phox -A930G polymorphism are
highly exposed to NAD(P)H oxidase-mediated oxidative stress
(22). Golliasch et al
(23) suggested that there is a
protective association between the -A930G promoter polymorphisms in
the NAD(P)H p22phox gene and the development of myocardial
infarction in individuals ≤40 years of age. Zalba et al
(24) demonstrated the -A930G
polymorphism as a novel marker in determining the genetic
susceptibility of hypertensive patients to oxidative stress.
To the best of our knowledge, the present study
demonstrated for the first time that the -A930G polymorphism of the
p22phox gene affects the susceptibility of the Chinese Han
population to ICH. A significant difference was observed in the
frequencies of the AA, AG, GG genotypes and A and G alleles between
the ICH and control subjects. Logistic regression analysis
indicated that genetic mutations of the p22phox -A930G gene were
independent risk factors of ICH (P=0.009; OR, 2.196; 95% CI,
1.003–3.586). The -A930G polymorphism of the p22phox gene was
associated with the risk of developing ICH when the clinical data
of different genotypes were included. One explanation for -A930G
polymorphism as a potential risk factor of ICH is that the G allele
was associated with higher blood pressure in the brachial artery
and in the aorta (25). For this
reason, the -A930G polymorphism may have an indirect association
with ICH by increasing blood pressure (26). Another possibility is that the
-A930G polymorphism is located at the promoter region of the
p22phox gene and a 30% increase in promoter activity and increased
oxidative stress was observed in the G allele compared with the A
allele (27), leading to
endothelial dysfunction, thickening of the vessel wall and ICH.
In conclusion, the present study demonstrated that
the -A930G polymorphism of the NAD(P)H p22phox may be associated
with ICH in the Chinese Han population and screening this site may
aid the diagnosis of ICH patients in the future.
Acknowledgements
This study was supported by the Shanghai Songjiang
Health Bureau, a leading cooperation class A foundation (grant no.
2011LX03).
References
|
1
|
Hua Y, Keep RF, Gu Y and Xi G: Thrombin
and brain recovery after intracerebral hemorrhage. Stroke.
40:S88–S89. 2009. View Article : Google Scholar :
|
|
2
|
Dong XQ, Du Q, Yu WH, et al: Plasma
resistin, associated with single nucleotide polymorphism -420, is
correlated with C-reactive protein in Chinese Han patients with
spontaneous basal ganglia hemorrhage. Genet Mol Res. 11:1841–1850.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khatri R, Tariq N, Vazquez G, et al:
Outcomes after nontraumatic subarachnoid hemorrhage at hospitals
offering angioplasty for cerebral vasospasm: a national level
analysis in the United States. Neurocrit Care. 15:34–41. 2011.
View Article : Google Scholar
|
|
4
|
Aronowski J and Zhao X: Molecular
pathophysiology of cerebral hemorrhage: secondary brain injury.
Stroke. 42:1781–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qureshi AI, Mendelow AD and Hanley DF:
Intracerebral haemorrhage. Lancet. 373:1632–1644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim H, Crago E, Kim M, et al: Cerebral
vasospasm after sub-arachnoid hemorrhage as a clinical predictor
and phenotype for genetic association study. Int J Stroke.
8:620–625. 2013. View Article : Google Scholar
|
|
7
|
Taniyama Y and Griendling KK: Reactive
oxygen species in the vasculature: molecular and cellular
mechanisms. Hypertension. 42:1075–1081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Zhou K, Meng X, et al: Increased ROS
generation and SOD activity in heteroplasmic tissues of
transmitochondrial mice with A3243G mitochondrial DNA mutation.
Genet Mol Res. 7:1054–1062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng JC, Cheng HP, Tsai IC and Jiang MJ:
ROS-mediated downregulation of MYPT1 in smooth muscle cells: a
potential mechanism for the aberrant contractility in
atherosclerosis. Lab Invest. 93:422–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ng CK, Deshpande SS, Irani K and
Alevriadou BR: Adhesion of flowing monocytes to
hypoxia-reoxygenation-exposed endothelial cells: role of Rac1, ROS,
and VCAM-1. Am J Physiol Cell Physiol. 283:C93–C102. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y and Tabas I: Emerging roles of
mitochondria ROS in atherosclerotic lesions: causation or
association? J Atheroscler Thromb. 21:381–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel RP, Moellering D, Murphy-Ullrich J,
Jo H, Beckman JS and Darley-Usmar VM: Cell signaling by reactive
nitrogen and oxygen species in atherosclerosis. Free Radic Biol
Med. 28:1780–1794. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin YW, Peng J, Liang BY, et al: The A930G
polymorphism of P22phox (CYBA) gene but not C242T variation is
associated with hypertension: a meta-analysis. PLoS One.
8:e824652013. View Article : Google Scholar :
|
|
14
|
Muller G and Morawietz H: Nitric oxide,
NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal.
11:1711–1731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akasaki T, Ohya Y, Kuroda J, et al:
Increased expression of gp91phox homologues of NAD(P)H oxidase in
the aortic media during chronic hypertension: involvement of the
renin-angiotensin system. Hypertens Res. 29:813–820. 2006.
View Article : Google Scholar
|
|
16
|
Ushio-Fukai M, Zafari AM, Fukui T,
Ishizaka N and Griendling KK: p22phox is a critical component of
the superoxide-generating NADH/NADPH oxidase system and regulates
angiotensin II-induced hypertrophy in vascular smooth muscle cells.
J Biol Chem. 271:23317–23321. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fraser PA: The role of free radical
generation in increasing cerebrovascular permeability. Free Radic
Biol Med. 51:967–977. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papaharalambus CA and Griendling KK: Basic
mechanisms of oxidative stress and reactive oxygen species in
cardiovascular injury. Trends Cardiovasc Med. 17:48–54. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seligmann C, Schimmer M, Leitsch T, et al:
A thrombocyte-induced myocardial dysfunction in the ischemic and
reperfused guinea pig heart is mediated by reactive oxygen species.
Free Radic Biol Med. 29:1244–1251. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Collins-Underwood JR, Zhao W, Sharpe JG
and Robbins ME: NADPH oxidase mediates radiation-induced oxidative
stress in rat brain microvascular endothelial cells. Free Radic
Biol Med. 45:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding H, Aljofan M and Triggle CR:
Oxidative stress and increased eNOS and NADPH oxidase expression in
mouse microvessel endothelial cells. J Cell Physiol. 212:682–689.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
San José G, Moreno MU, Oliván S, et al:
Functional effect of the p22phox -930A/G polymorphism on p22phox
expression and NADPH oxidase activity in hypertension.
Hypertension. 44:163–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goliasch G, Wiesbauer F, Grafl A, et al:
The effect of p22-PHOX (CYBA) polymorphisms on premature coronary
artery disease (≤ 40 years of age). Thromb Haemost. 105:529–534.
2011. View Article : Google Scholar
|
|
24
|
Zalba G, San José G, Moreno MU, Fortuño A
and Díez J: Nadph oxidase-mediated oxidative stress: genetic
studies of the p22(phox) gene in hypertension. Antioxid Redox
Signal. 7:1327–1336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xaplanteris P, Vlachopoulos C, Baou K, et
al: The effect of p22(phox) -930A/G, A640G and C242T polymorphisms
of NADPH oxidase on peripheral and central pressures in healthy,
normotensive individuals. Hypertens Res. 33:814–818. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moreno MU, San José G, Fortuño A, Beloqui
O, Díez J and Zalba G: The C242T CYBA polymorphism of NADPH oxidase
is associated with essential hypertension. J Hypertens.
24:1299–1306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan M, Raitakari OT, Kähönen M, et al: The
association between cigarette smoking and carotid intima-media
thickness is influenced by the -930A/G CYBA gene polymorphism: the
Cardiovascular Risk in Young Finns Study. Am J Hypertens.
22:281–287. 2009. View Article : Google Scholar : PubMed/NCBI
|