Introduction
Osteoclasts are large multinucleated cells that are
derived from a monocyte-macrophage lineage and have been shown to
possess a number of essential physiological functions for
development, in particular, in the dynamic remodeling of bone
(1). Excessive bone resorption by
osteoclasts is observed in certain lytic bone diseases, including
osteoporosis, hypercalcemia and rheumatoid arthritis, as well as
tumor metastases in the bone, periodontitis and Paget’s disease
(2,3). Patients with lytic bone diseases are
at a higher risk of sustaining fractures. Therefore, lytic bone
diseases are an increasingly serious social and economic issue, due
to the high medical costs associated with hospitalization (4). Therefore, inhibiting osteoclast
formation may represent a treatment option for disease involving
excessive bone resorption (5).
Receptor activator nuclear factor-κB ligand (RANKL)
is the primary cytokine responsible for the differentiation of
osteoclast precursors into osteoclasts in vitro and in
vivo (6). The binding of RANKL
to its receptor, RANK, leads to activation of components of the
nuclear factor-κB (NF-κB) and mitogen-activated protein kinase
(MAPK) pathways, including c-Jun N-terminal kinase (JNK),
extracellular signal-regulated kinase 1/2 (ERK1/2) and p38
(7). There is accumulating
evidence that nuclear factor of activated T cells, cytoplasmic 1
(NFATc1), which acts as the principal regulator of
osteoclastogenesis, is upregulated by RANKL in osteoclast
precursors through mechanisms that depend on NF-κB and MAPK
(8). NFATc1 is known to directly
regulate a number of osteoclast-related marker genes, including
tartrate-resistant acid phosphatase (TRAP), matrix
metalloproteinase 9 (MMP9), cathepsin K (CTSK) and
osteoclast-associated receptor (OSCAR) (8–10).
Therefore, suppression of RANKL signaling may be beneficial in the
treatment of osteoclast-related diseases (5).
Amiloride is a small molecule diuretic, which was
discovered in 1965 and is used for the long-term treatment of
hypertension in combination with other diuretics (11,12).
This drug is known to interact with the epithelial sodium channel
(ENaC) and acid-sensing ion channel proteins (ASICs), as well as
Na+/H+ antiporters and
Na+/Ca2+ exchangers (13–15).
Previous studies have shown that isolated human monocytes express
ASIC1, 2, and 3, and that this expression persists following
induction of osteoclast differentiation (16). Additionally, ASIC1a is involved in
the induction of osteoclastic genes, known to be direct
transcriptional targets of NFATc1, during osteoclastogenesis
(17). The
Na+/H+ antiporter may also be involved in the
recovery of intracellular pH during osteoclast activation (18). Furthermore,
Na+/Ca2+ exchangers, which are expressed in
osteoclasts, are important in Ca2+ transportation and
regulation during bone resorption (19). Therefore, since amiloride inhibits
these channels, it was hypothesized that amiloride may also
suppress osteoclast differentiation. The present study examined the
effects of amiloride on RANKL-induced osteoclastogenesis in a
murine RAW264.7 macrophage cell line, which is a classic model of
osteoclast precursors that differentiates into TRAP-positive
multinuclear osteoclasts following treatment with RANKL (20).
Materials and methods
Cell culture
The RAW264.7 murine macrophage cell line was
obtained from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). Dulbecco’s modified Eagle’s medium
(DMEM; HyClone, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco Life Technologies, Carlsbad, CA, USA) and 1%
penicillin-streptomycin (Gibco Life Technologies) was used for the
routine subculture of RAW 264.7 cells. Cells were maintained in a
humidified incubator (Shanghai Laboratory Instrument Works Co.,
Ltd, Shanghai, China) with 95% air/5% CO2 at 37°C and
were subcultured every two days.
Proliferation assays
Cell viability was assessed using a Cell Counting
kit-8 (CCK-8, Boster Biological Technology Ltd., Wuhan, China).
RAW264.7 cells were seeded at 3,000 cells per well in 96-well
plates. Following incubation for 24 h, cells were treated with 0,
10, 50 or 100 μM amiloride (Sigma-Aldrich, St. Louis, MO, USA)
dissolved in 0.1% dimethyl sulfoxide (DMSO; Sigma-Aldrich). Medium
(DMEM supplemented with FBS and 1% penicillin-streptomycin) with
fresh amiloride was exchanged daily. After 24, 72 or 120 h, 100 μl
of medium from each well (containing 10% CCK-8) was added and
incubated in darkness at 37°C for 2 h. The plate was then read
using a spectrophotometer (ELx808 Absorbance Microplate Reader,
BioTek Instruments, Inc. Winooski, VT, USA) at 450 nm. The number
of surviving cells was quantified by measuring the absorbance at
this wavelength.
TRAP staining
RAW264.7 cells were treated with RANKL (50 ng/ml,
Peprotech, Rocky Hill, NJ, USA) and different concentrations of
amiloride, and media were refreshed daily over the course of five
days. Cells were washed twice with phosphate-buffered saline
(Boster Biological Technology, Ltd), fixed with 4% formaldehyde
(Boster Biological Technology, Ltd) for 15 min and then subjected
to TRAP staining (Sigma-Aldrich) for 60 min, according to the
manufacturer’s instructions. Cells were considered osteoclasts if
they were stained dark red and contained ≥3 nuclei when viewed
under a light microscope (magnification, ×40; Nikon TE2000-S, Nikon
Corporation, Tokyo, Japan). For each sample three fields of vision
were examined.
Pit-formation assays
The bone resorption function of osteoclasts derived
from RAW264.7 cells induced by RANKL was analyzed using Osteo Assay
plates (Corning Inc., Corning, NY, USA). Briefly, RAW264.7 cells
(6,000 cells/well) were plated on Osteo Assay plates and treated
with amiloride (0, 10, 50 or 100 μM) in the presence RANKL (50
ng/ml) for 7 days. Subsequently, the cells were removed completely.
Relative resorption area per well was observed under a light
microscope (magnification, ×40; Nikon TE2000-S, Nikon Corporation,
Tokyo, Japan) and measured by ImageJ 1.47 software (National
Institutes of Health, Bethesda, MD, USA). For each sample three
fields of vision were examined.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted from RAW264.7 cells using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The
concentration and integrity of the extracted RNA were analyzed by
measurement of the OD260/280 (Eppendorf 22331,
Eppendorf, Hamburg, Germany). The purified RNA was converted into
cDNA using a First Strand cDNA Synthesis kit (TransGen Biotech Co.,
Ltd., Beijing, China). The resulting cDNAs were subjected to qPCR.
The total volume of the reaction was 20 μl, which consisted of 10
μl Top Green qPCR SuperMix (TransGen Biotech Co., Ltd.), 8 μl
RNase-free water, 1 μl primer solution and 1 μl cDNA. qPCR was
performed on a Bio-Rad Q5 instrument (Bio-Rad Laboratories,
Hercules, CA, USA) under the following conditions: Denaturation at
94°C for 30 sec, followed by 40 cycles of denaturation at 94°C for
5 sec and annealing at 60°C for 30 sec. The primers used in the
amplification were as follows: Forward:5′-ATTTCTGAATGGCCCAGGT-3′
and reverse: 5′-CTGCCTCAACACCTCAACC-3′ for β-actin, forward:
5′-GATGCCAGCGACAAGAGGTT-3′ and reverse: 5′-CATACCAGGGGATGTTGCGAA-3′
for TRAP, forward: 5′-GAAGAAGACTCACCAGAAGCAG-3′ and reverse:
5′-TCCAGGTTATGGGCAGAGATT-3′ for CTSK, forward:
5′-CTGGACAGCCAGACACTAAAG-3′ and reverse: 5′-CTCGCGGCAAGTCTTCAGAG-3′
for MMP-9, forward: 5′-CAGGAGAGGCATTATGAGCA-3′ and reverse:
5′-GGTACTTTCCTGGTTCGCAT-3′ for RANK, forward:
5′-CTGCTGGTAACGGATCAGCTCCCCAGA-3′ and reverse:
5′-CCAAGGAGCCAGAACCTTCGAAACT-3′ for OSCAR and forward:
5′-GGTAACTCTGTCTTTCTAACCTTAAGCTC-3′ and reverse:
5′-GTGATGACCCCAGCATGCACCAGTCACAG-3′ for NFATc1. β-Actin was
included as housekeeping gene. The comparative 2−ΔΔCt
method was used to calculate the relative expression levels of each
gene.
Western blotting
Serum-starved RAW264.7 cells were pretreated with
100 μM amiloride for 12 h and then stimulated with RANKL (50 ng/ml)
for the appropriate times (0, 5, 15, 30 and 60 min). Subsequently,
cells were lysed in a radioimmunoprecipitation assay lysis buffer
containing inhibitors of protease and a phosphorylase (Protease
Inhibitor Cocktail and Phosphatase Inhibitor cocktail; Boster
Biological Technology Ltd.). The lysates were centrifuged at 14,000
× g for 20 min, and the supernatants were collected. Protein
concentrations of the supernatants were determined using a
bicinchoninic acid Protein Assay kit (Boster Biological Technology
Ltd.), standardized with bovine serum albumin (Gibco Life
Technologies). Cellular proteins (30 μg) were resolved by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and were transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Nonspecific
interactions were blocked with 5% bovine serum albumin for 2 h and
probed with the following primary antibodies: Rabbit polyclonal
anti-α-Tubulin (1:1,000; #2148), rabbit monoclonal anti-inhibitor
of κB (IκB; 1:1,000; #4812), rabbit monoclonal anti-JNK (1:1,000;
#9258), rabbit monoclonal anti-phosphorylated (p-)JNK (1:1,000;
#4671), rabbit monoclonal anti-ERK (1:1,000; #4348), rabbit
monoclonal anti-p-ERK (1:1,000; #4094), rabbit monoclonal anti-p38
(1:1,000; #14451) and rabbit monoclonal anti-p-p38 (1:1,000;
#4511), which were all purchased from Cell Signaling Technology
(Danvers, MA, USA). Membranes were incubated with the appropriate
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit IgG, 1:200, BA1003; Boster Biological Technology Ltd.)
and immunoreactivity was detected using enhanced chemiluminescence
reagents (Pierce Biotechnology, Inc., Rockford, IL, USA).
Statistical analyses
Statistically significant differences between groups
were determined by one-way analysis of variance using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation of ≥3 independent experiments. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of amiloride on cell
viability
In order to determine whether the effects of
amiloride on osteoclastogenesis were due to the potential toxicity
of this drug, the cytotoxicity of amiloride was examined using a
CCK-8 assay in RAW264.7 cells. Cytotoxicity was very low in cells
treated with all concentrations of amiloride (10, 50 or 100 μM) for
1, 3 or 5 days (Fig. 1),
suggesting that the effects of amiloride on osteoclast
differentiation are not mediated by cytotoxicity of this
compound.
Effects of amiloride on osteoclast
differentiation in RANKL-induced RAW264.7 cells
In order to examine the effects of amiloride on
osteoclast differentiation, the formation of osteoclast-like cells
induced by RANKL (50 ng/ml) stimulation in RAW264.7 cells was
examined in the presence of various concentrations of amiloride by
counting the number of TRAP-positive multinucleated cells. The
following doses of amiloride were selected for subsequent
experiments: 0, 10, 50 and 100 μM. As shown in Fig. 2, TRAP-positive osteoclasts with
multiple nuclei were formed within 5 days in response to RANKL
stimulation, and this response was inhibited by amiloride in a
concentration-dependent manner.
Effects of amiloride on bone resorption
in RANKL-induced RAW264.7 cells
Osteo Assay plates were used to determine whether
amiloride treatment affected the bone resorption function of
osteoclasts. RAW264.7 cells were plated onto bone slices and
stimulated with RANKL (50 ng/ml) for 7 days in the presence of
amiloride (0, 10, 50 or 100 μM). As shown in Fig. 3, numerous resorption pits were
formed on the bone slices. Amiloride decreased the area of the bone
resorption pits in a concentration-dependent manner.
Effects of amiloride on the expression of
osteoclast differentiation marker genes in RANKL-induced RAW264.7
cells
Changes in the expression of osteoclast
differentiation genes in response to amiloride treatment were
examined using RT-qPCR analysis of osteoclasts. The expression of
osteoclast differentiation markers, including TRAP, MMP9, CTSK and
OSCAR, have previously been shown to be detectable following the
formation of mature osteoclasts (8–10).
As shown in Fig. 4, the expression
of these genes decreased in response to amiloride treatment, in a
concentration-dependent manner. The expression of the NFATc1
gene, which acts as the master switch in osteoclastogenesis and
regulates the expression of osteoclastogenic genes (8), was downregulated by amiloride in a
concentration-dependent manner. A downregulation of 80% was
observed following treatment with 100 μM amiloride.
 | Figure 4Amiloride downregulated
osteoclast-specific gene expression in RANKL-induced RAW264.7
cells. RAW264.7 cells were cultured with RANKL in the presence or
absence of amiloride for five days. The relative mRNA expression
levels of TRAP, CTSK, MMP9, OSCAR, and
NFATc1 genes were determined by quantitative polymerase
chain reaction, following normalization to β-actin mRNA expression.
*P<0.05 vs. control, #P<0.05 vs. 10 μM
amiloride and ΔP<0.05 vs. 50 μM amiloride. RANKL,
receptor activator of nuclear factor-κB; Ami, amiloride; TRAP,
tartrate-resistant acid phosphatase; CTSK, cathepsin K; MMP-9,
matrix metalloproteinase-9; OSCAR, osteoclast-associated receptor;
NFATc1, nuclear factor of activated T cells cytoplasmic 1. |
Effects of amiloride on the
phosphorylation of NF-κB and MAPK family proteins in RANKL-induced
RAW264.7 cells
Activation of the NF-κB and MAPK is important in
osteoclastogenesis (21). In order
to evaluate the effects of 12 h amiloride treatment on these
signaling pathways, following RANKL stimulation in RAW264.7 cells,
the phosphorylation of p38, JNK and ERK, and the degradation of IκB
was evaluated using western blot analysis. The results demonstrated
that amiloride significantly inhibited the RANKL-induced activation
of JNK, ERK and p38, and the degradation of IκB (Fig. 5). These results indicate that
amiloride inhibits RANKL-induced activation of the NF-κB and MAPK
pathways in osteoclasts.
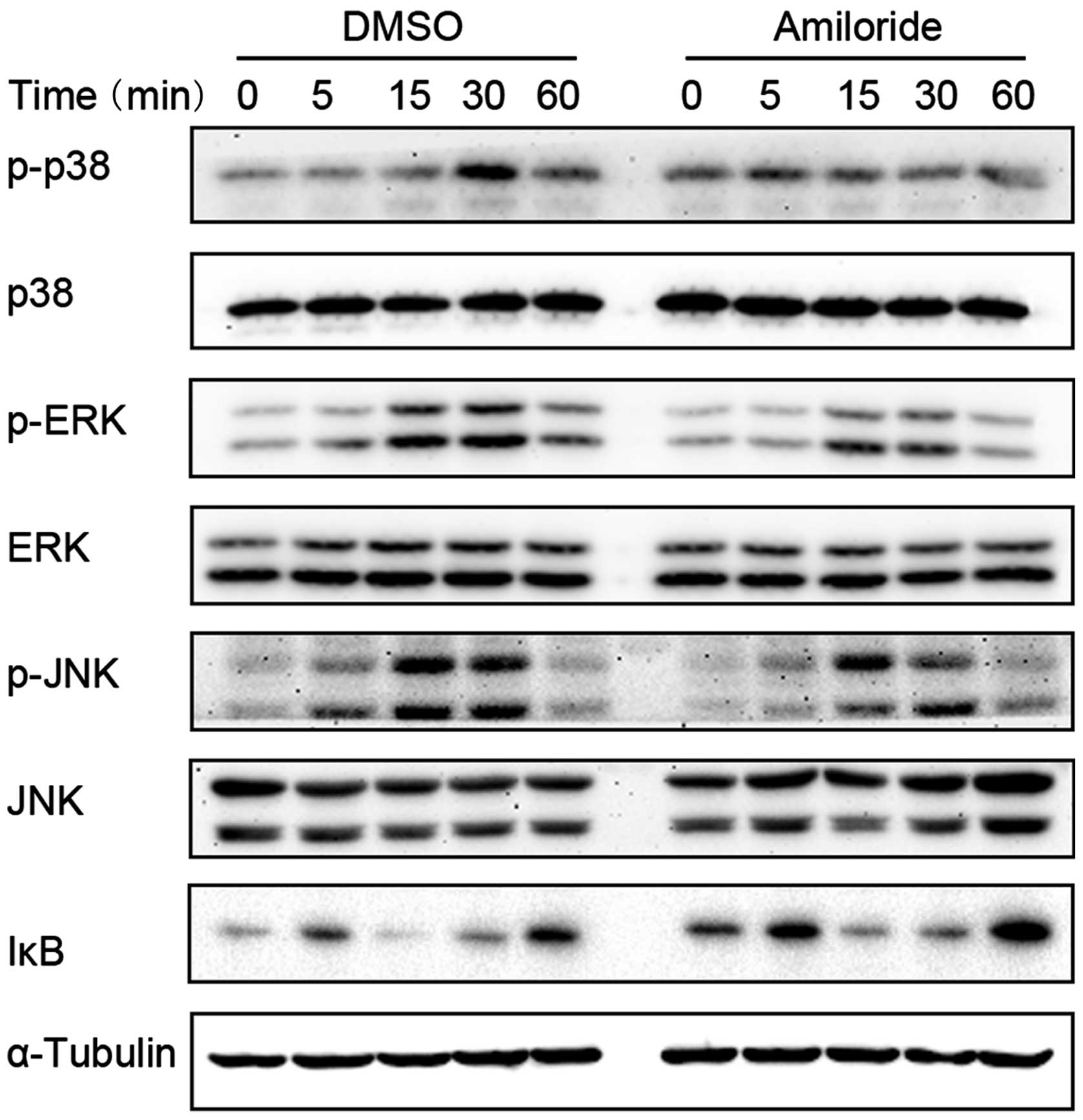 | Figure 5Amiloride downregulated RANKL-induced
IκB degradation and MAPK phosphorylation in RAW264.7 cells. Cells
were pretreated with amiloride (100 μM) or vehicle (DMSO, equal
final concentrations) for 12 h and then stimulated with RANKL (100
ng/ml) for the indicated times. Whole cell lysates were extracted
and subjected to western blotting analysis with antibodies
targeting p-p38, p38, p-ERK, ERK, p-JNK, JNK, and IκB. α-Tubulin
served as a reference protein. RANKL, receptor activator of nuclear
factor-κB; MAPK, mitogen-activated protein kinase; p-p38,
phosho-p38; ERK, extracellular signal-regulated kinase; p-ERK,
phospho-ERK; JNK, c-Jun N-terminal kinase; p-JNK, phospho-JNK;
DMSO, dimethyl sulfoxide. |
Discussion
In the present study, the effects of amiloride on
RANKL-induced osteoclast differentiation and its function in murine
RAW264.7 cells were investigated, and the signaling mechanisms
associated with this process were examined. The results
demonstrated that amiloride markedly suppressed RANKL-induced
osteoclast differentiation and resorption, and that these effects
were not due to the cytotoxicity of the drug. It was also shown
that amiloride inhibited the expression of NFATc1 and other
osteoclast-related genes through RANKL-induced inhibition of the
NF-κB and MAPK signaling pathways.
Activation of the NF-κB pathway is essential for
RANKL-induced osteoclast differentiation (22). NF-κB is localized in the cytoplasm,
in association with a number of inhibitory IκB proteins, including
IκBα, IκBβ and IκBɛ, of which IκBα is the most abundant (23). The results of the present indicated
that amiloride inhibits the degradation of IκBα, suggesting that
suppression of the NF-κB pathway is a mechanism underlying the
anti-osteoclastogenic effect of amiloride.
Three major MAPKs have been identified in mammalian
cells: JNK, ERK and p38. These proteins are activated by RANKL
stimulation and have been shown to be involved in
osteoclastogenesis (21,24). Moreover, inhibitors of JNK, ERK and
p38 have been shown to inhibit RANKL-induced osteoclastogenesis
(24–26). In the current study, it was shown
that amiloride markedly inhibited the phosphorylation of JNK, ERK
and p38, thereby contributing to the inhibition of RANKL-induced
osteoclast differentiation.
It was also demonstrated that amiloride
downregulates RANKL-induced expression of NFATc1 mRNA in
RAW264.7 cells. NFATc1 has been shown to be the predominant
regulator of osteoclastogenesis, and overexpression of the
NFATc1 gene is associated with the efficient induction of
mature osteoclasts. The expression of this gene is known to be
dependent on NF-κB and MAPKs (8,27).
NFATc1 also regulates the expression of a number of
osteoclast-specific genes, including TRAP, CTSK,
MMP9 and OSCAR, which, in the present study, were
shown to be inhibited by amiloride in a concentration-dependent
manner. Therefore, the inhibition of RANKL-induced NFATc1
expression by amiloride is likely to be involved in the inhibition
of osteoclastogenesis. Further investigation is required in order
to confirm the mechanisms involved in osteoclastogenesis
inhibition. In addition, the efficacy of amiloride in treating
excessive bone resorption should be evaluated in vivo.
In conclusion, the results of the present study
demonstrated that amiloride markedly inhibited osteoclastogenesis
in vitro without exerting cytotoxic effects. It also reduced
the RANKL-induced expression of osteoclastic marker genes and
suppressed the expression of NFATc1, the principal regulator
of osteoclastogenesis. In addition, amiloride attenuated
RANKL-induced JNK, ERK and p38 activation, and the degradation of
IκB. These findings indicated that amiloride may have a potential
indication in the treatment of bone loss-related diseases.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81272058).
References
|
1
|
Suda T, Takahashi N and Martin TJ:
Modulation of osteoclast differentiation. Endocr Rev. 13:66–80.
1992.PubMed/NCBI
|
|
2
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon SJ, Ahn IE, Jung H, et al: Temporal
differential effects of proinflammatory cytokines on
osteoclastogenesis. Int J Mol Med. 31:769–777. 2013.PubMed/NCBI
|
|
4
|
Lippuner K, von Overbeck J, Perrelet R,
Bosshard H and Jaeger P: Incidence and direct medical costs of
hospitalizations due to osteoporotic fractures in Switzerland.
Osteoporos Int. 7:414–425. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takayanagi H, Kim S, Koga T, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim N, Takami M, Rho J, Josien R and Choi
Y: A novel member of the leukocyte receptor complex regulates
osteoclast differentiation. J Exp Med. 195:201–209. 2002.PubMed/NCBI
|
|
10
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benos DJ: Amiloride: a molecular probe of
sodium transport in tissues and cells. Am J Physiol. 242:C131–C145.
1982.PubMed/NCBI
|
|
12
|
Cragoe EJ Jr, Woltersdorf OW Jr, Bicking
JB, Kwong SF and Jones JH: Pyrazine diuretics. II
N-amidino-3-amino-5-substituted 6-halopyrazinecarboxamides. J Med
Chem. 10:66–75. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Canessa CM, Schild L, Buell G, et al:
Amiloride-sensitive epithelial Na+ channel is made of
three homologous subunits. Nature. 367:463–467. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleyman TR and Cragoe EJ Jr: Amiloride and
its analogs as tools in the study of ion transport. J Membr Biol.
105:1–21. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ugawa S, Ueda T, Ishida Y, Nishigaki M,
Shibata Y and Shimada S: Amiloride-blockable acid-sensing ion
channels are leading acid sensors expressed in human nociceptors. J
Clin Invest. 110:1185–1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahr H, van Driel M, van Osch GJ, Weinans
H and van Leeuwen JP: Identification of acid-sensing ion channels
in bone. Biochem Biophys Res Commun. 337:349–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Xu RS, Jiang DL, et al: Acid-sensing
ion channel 1a is involved in acid-induced osteoclastogenesis by
regulating activation of the transcription factor NFATc1. FEBS
Lett. 587:3236–3242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rousselle AV and Heymann D: Osteoclastic
acidification pathways during bone resorption. Bone. 30:533–540.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li JP, Kajiya H, Okamoto F, Nakao A,
Iwamoto T and Okabe K: Three Na+/Ca2+
exchanger (NCX) variants are expressed in mouse osteoclasts and
mediate calcium transport during bone resorption. Endocrinology.
148:2116–2125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cuetara BL, Crotti TN, O’Donoghue AJ and
McHugh KP: Cloning and characterization of osteoclast precursors
from the RAW264.7 cell line. In Vitro Cell Dev Biol Anim.
42:182–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
22
|
Takayanagi H: Osteoimmunology: shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto M, Sudo T, Saito T, Osada H and
Tsujimoto M: Involvement of p38 mitogen-activated protein kinase
signaling pathway in osteoclastogenesis mediated by receptor
activator of NF-kappaB ligand (RANKL). J Biol Chem.
275:31155–31161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikeda F, Matsubara T, Tsurukai T, Hata K,
Nishimura R and Yoneda T: JNK/c-Jun signaling mediates an
anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res.
23:907–914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SE, Woo KM, Kim SY, et al: The
phosphatidylinositol 3-kinase, p38 and extracellular
signal-regulated kinase pathways are involved in osteoclast
differentiation. Bone. 30:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar
|



















