Introduction
Members of the tumor necrosis factor
receptor-associated factor (TRAF) family are overexpressed in
numerous types of human cancer and are involved in the activation
of multiple signal transduction pathways (1). TRAF4 has been demonstrated to promote
Smurf1-mediated ubiquitination-dependent cell migration,
transforming growth factor (TGF)-β receptor signaling and breast
cancer metastasis (2,3). It also serves as a critical molecule
in Akt activation in lung cancer (4). The Akt pathway has been indicated to
modulate TRAF4-mediated inhibition of Glut1 and HK2 expression,
leading to impairment of glucose metabolism (4). Although overexpression of TRAF4 has
been reported in several types of carcinoma (5), its biological function in
tumorigenesis remains to be elucidated.
Girders of actin filament (Girdin) is activated by
Akt-induced phosphorylation in response to epidermal growth factor
receptor (EGFR) signaling and insulin-like growth factor 1
stimulation (6,7); the Girdin-Gai molecular complex binds
to EGFR to determine whether cells migrate or proliferate (8). Girdin has been observed to facilitate
cell migration and metastasis in breast and colon cancer (9–11),
and its expression has been correlated with histological grade and
distant metastasis in breast cancer (12).
TRAF4 regulates a set of cancer-related genes that
are involved in biological processes such as migration, invasion,
metastasis and angiogenesis, in which Girdin is strongly implicated
(13). Therefore, it is considered
as the member of the TRAF family that may influence Girdin. TRAF4
and Girdin have been implicated in the phosphatidylinositol
3-kinase (PI3-K)/Akt signal transduction pathway, which is central
to cancer progression (14). TRAF4
was reported to activate Akt, a pivotal cell survival kinase,
through ubiquitination in lung cancer. In addition, TRAF4 was
identified to mediate activation of nuclear factor-κB (NF-κB)
through glucocorticoid-induced tumor necrosis factor receptor
(15). The p65 and p50 forms of
NF-κB interact physically with signal transducer and activator of
transcription-3 (STAT3), facilitating NF-κB recruitment to STAT3
promoters (16). Girdin was
previously demonstrated to be a direct target of STAT3 (17), suggesting that TRAF4 may influence
Girdin through NF-κB-mediated STAT3 activity.
Based on these observations, the present study
hypothesized that TRAF4 and Girdin cooperate in tumor invasion and
metastasis. To test this hypothesis, the expression status of
Girdin in breast cancer cells and its association with TRAF4 was
investigated. The results may aid in the development of novel
methods for the management of breast cancer treatment.
Materials and methods
Patients and tissue samples
Tissue samples were collected from 38 patients who
had been diagnosed with breast cancer between 1998 and 2005 and had
presented to the First Affiliated Hospital of China Medical
University (Shenyang, China). None of the patients had received
radiotherapy or chemotherapy prior to surgical resection, and all
the patients were treated with routine chemotherapy subsequent to
the operation. The median age of the patients was 58 years (range:
37–72 years). The tissue specimens were independently reviewed by
three pathologists and the diagnoses were confirmed according to
the World Health Organization criteria for the classification of
breast cancer. The study received ethical approval from and was
conducted according to the Regulations of the Institutional Review
Board of the China Medical University. Informed consent was
obtained from all the patients enrolled in the study, or their next
of kin.
Reagents and antibodies
HEPES-buffered Dulbecco’s modified Eagle’s medium
(H-DMEM), Leibovitz’s L-15 medium and fetal bovine serum (FBS) were
purchased from Gibco Life Technologies (Grand Island, NY, USA).
Bovine serum albumin (BSA) and Triton X-100 were purchased from
Beyotime Institute of Biotechnology (Haimen, China).
The following primary antibodies were used:
Anti-Girdin (human), anti-TRAF4 (rabbit), anti-β-actin (mouse) and
anti-lamin B1 (goat; Santa Cruz Biotechnology, Texas, TX, USA).
Anti-mouse and anti-rabbit IgG secondary antibodies conjugated with
horseradish peroxidase were purchased from Santa Cruz
Biotechnology. Biotinylated secondary antibodies
(anti-mouse/rabbit, Fuzhou Maixin Biotechnology Development Co.,
Ltd., Fuzhou, China) were used for immunohistochemistry and
fluorescein isothiocyanate (FITC)/tetramethylrhodamine
(TRITC)-conjugated secondary antibodies (anti-rabbit and
anti-mouse, respectively; Santa Cruz Biotechnology) were used for
immunofluorescence.
Cell lines and cell culture
The non-tumorigenic mammary epithelial cell line
MCF-10A, and the breast cancer cell lines MCF-7 and MDA-MB-231 were
obtained from the American Type Culture Collection (Manassas, VA,
USA).
MCF-7 cells and MCF-10A cells were grown in H-DMEM
medium and MDA-MB-231 cells were grown in Leibovitz’s L-15 medium.
All culture media contained 10% FBS supplemented with 100 IU/ml
penicillin and 100 μg/ml streptomycin (Invitrogen Life
Technologies, New York, NY, USA). The cells were cultured at 37°C
in a humidified atmosphere with 5% CO2.
Plasmids and transfection
The pcDNA3-TRAF4 plasmid (plasmid 16375) was
purchased from Addgene (Cambridge, MA, USA). MCF-7 cells were
transiently transfected using Attractene Transfection Reagent or
HiPerFect Transfection Reagent (Qiagen, Hilden, Germany) according
to the manufacturer’s instructions. Untransfected MCF-7 cells and
cells transfected with empty plasmid were used as negative
controls. Lamin B1 was used as a nuclear internal control and
β-actin was used as a cytoplasmic internal control.
Preparation of nuclear and cytoplasmic
extracts
To detect the cellular localization of Girdin,
nuclear and cytoplasmic fractions were isolated using the Pierce
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher
Scientific; Rockford, IL, USA) according to the manufacturer’s
instructions.
Immunohistochemistry
The anti-Girdin and anti-TRAF4 primary antibodies
(1:100) were incubated at 4°C overnight, followed by incubation
with biotinylated secondary antibodies at 37°C for 20 min. The
peroxidase reaction was developed with DAB (Fuzhou Maixin
Biotechnology Development Co., Ltd.). Hematoxylin was used as a
counterstain, and the sections were dehydrated in alcohol prior to
being mounted on slides. The primary antibodies were replaced with
phosphate-buffered saline (PBS) for the negative control.
Western blot analysis
Proteins were extracted using
radioimmunoprecipitation assay (RIPA) buffer containing a protease
and phosphatase inhibitor cocktail (Beyotime, Shanghai, China). The
protein concentrations were determined using a Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology). Equal
amounts of protein from the samples were separated by 8% SDS-PAGE
and transferred to polyvinylidene fluoride membranes (EMD
Millipore; Bedford, MA, USA). The membranes were blocked with 5%
non-fat milk in PBS, prior to incubation with the primary
antibodies against Girdin (1:200), TRAF4 (1:200), β-actin (1:1,000)
or lamin B1 (1:500). The membranes were then incubated with the
respective secondary antibody (1:2,000) and the protein bands were
imaged using Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific). The protein expression was quantified using ImageJ
(National Institute of Health, Bethesda, MD, USA).
Immunofluorescence
The cells were seeded in Nunc 24-well plates (Thermo
Fisher Scientific) at a density of 2×104 cells/well,
fixed with 4% paraformaldehyde (Invitrogen Life Technologies) and
permeabilized with 0.2% Triton X-100. Subsequent to blocking with
5% BSA in PBS, the cells were incubated with either Girdin (1:50)
or TRAF4 (1:50) primary antibody at 4°C overnight, followed by
incubation with a FITC/TRITC-conjugated secondary antibody (1:50;
Santa Cruz Biotechnology) for 2 h at room temperature. The nuclei
were stained with DAPI (Beyotime Institute of Biotechnology) and
the cells were examined under a Leica DM5500 B microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Pearson’s
χ2 test was used to determine correlations between
TRAF4/Girdin coexpression and clinicopathological factors.
Spearman’s correlation test was used to determine the association
between TRAF4 and Girdin expression (positive vs. negative).
Independent-samples t-test was used to compare data between two
groups. One-way analysis of variance and Bonferroni correction were
used to compare data between three or more groups. The data are
expressed as the means ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Overexpression of TRAF4 correlates with
Girdin expression in the cytoplasm of breast carcinoma tissues
Immunohistochemical analysis revealed that the TRAF4
and Girdin proteins were expressed in the cytoplasm and nuclei of
cells from breast cancer tissues. The staining patterns indicated
that 35/38 (92.11%) samples stained positive for TRAF4 protein and
26/38 (68.42%) samples stained positive for Girdin protein. In
addition, 14/38 (36.84%) samples presented coexpression of the
TRAF4 and Girdin proteins (Fig. 1;
Table I). However, more intense
staining patterns were observed in invasive ductal carcinoma (IDC)
tissue samples compared with ductal carcinoma in situ (DCIS)
tissue samples, and TRAF4/Girdin coexpression was significantly
correlated with lymph node metastasis in tissue samples from
patients.
 | Table ICorrelations between TRAF4 and Girdin
coexpression and clinicopathological features. |
Table I
Correlations between TRAF4 and Girdin
coexpression and clinicopathological features.
| | TRAF4/Girdin
coexpression | |
|---|
| |
| |
|---|
| Feature | N | Negative | Positive | χ2 |
|---|
| Age (years) |
| ≤50 | 17 | 12 | 5 | 0.73 |
| >50 | 21 | 12 | 9 | |
| Histology-type |
| DCIS | 8 | 8 | 0 | 5.911a |
| IDC | 30 | 16 | 14 | |
| Tumor size (cm) |
| ≤3.0 | 14 | 9 | 6 | 0.106 |
| >3.0 | 23 | 15 | 8 | |
| LNM |
| negative | 24 | 18 | 6 | 4.786a |
| positive | 13 | 5 | 8 | |
| ER |
| negative | 30 | 20 | 10 | 0.754 |
| positive | 8 | 4 | 4 | |
| PR |
| negative | 29 | 18 | 11 | 0.062 |
| positive | 9 | 6 | 3 | |
| CerbB-2 |
| negative | 15 | 5 | 10 | 9.474a |
| positive | 23 | 19 | 4 | |
| P53 |
| negative | 15 | 9 | 6 | 0.106 |
| positive | 23 | 15 | 8 | |
Further analyses indicated that the coexpression of
TRAF4 and Girdin proteins was significantly higher in
c-erbB2-negative cases as compared with c-erbB2-positive cases
(66.67 vs. 17.39%). By contrast, no significant differences in
coexpression rates were observed between samples that were positive
or negative for the estrogen receptor, progesterone receptor or p53
(Table I).
The association between the positive and negative
cytoplasmic expression rates of TRAF4 and Girdin in breast cancer
cells are summarized in Table II.
There were a total of 35 samples displaying positive cytoplasmic
expression of TRAF4 protein. Of these, 14 samples were
Girdin-negative while 21 were Girdin-positive. By contrast, 3
samples were TRAF4-negative, all of which were Girdin-negative.
These observations demonstrated that the expression of Girdin in
the cytoplasm of breast cancer tissues was positively correlated
with expression of TRAF4 (P<0.05).
 | Table IIAssociation between cytoplasmic TRAF4
and Girdin expression in breast cancer tissue. |
Table II
Association between cytoplasmic TRAF4
and Girdin expression in breast cancer tissue.
| Girdin
expression | |
|---|
|
| |
|---|
| negative | positive | χ2 |
|---|
| TRAF4
expression |
| negative | 3 | 0 | 4.024a |
| positive | 14 | 21 | |
Expression patterns of Girdin protein in
breast cancer cell lines
The levels of Girdin protein were compared in three
breast cell lines: MCF-10A, normal human breast epithelia; MCF-7,
ER-positive weakly invasive luminal A breast cancer cells (18); and MDA-MB-231, ER-negative highly
proliferative and invasive triple-negative breast cancer cells
(18). Western blot analysis and
relative quantification indicated that the Girdin protein was
highly expressed in MDA-MB-231 and MCF-7 breast cancer cell lines;
whereas only trace amounts were observed in normal MCF-10A breast
cells (Fig. 2A).
The cell extracts were separated into cytoplasmic
and nuclear fractions, and the relative abundance of Girdin in the
fractions were analyzed by Western blotting (Fig. 2B). In agreement with a previous
observation (7), Girdin was more
strongly expressed in c-erbB2-positive tumor tissues than
c-erbB2-negative tissues, and the western blotting results
demonstrated that Girdin was more highly expressed in the cytoplasm
of the weakly invasive MCF-7 cells than in the strongly invasive
MDA-MB-231 cells.
The expression and localization of Girdin protein in
MCF-7 and MDA-MB-231 breast cancer cells was evaluated by indirect
immunofluorescence (Fig. 2C). The
fluorescent staining patterns indicated that Girdin was mainly
localized in the cytoplasm in MCF-7 and MDA-MB-231 cells; however
it was also detectable in the nuclei of the MDA-MB-231 cells. These
observations are consistent with the immunohistochemical and
immunofluorescence results (Fig. 1
and Fig. 3, respectively), which
indicated that Girdin was mainly localized in the cytoplasm in
breast cancer tissues, with no significant staining in the
nuclei.
TRAF4 and Girdin colocalize in the
cytoplasm of breast cancer cells
Immunohistochemistry indicated that cytoplasmic
overexpression of TRAF4 and Girdin were positively correlated in
breast cancer cells. In order to verify these results,
immunofluorescence experiments were performed. These demonstrated
that TRAF4 and Girdin proteins colocalized in the cytoplasm of
MCF-7 and MDA-MB-231 breast cancer cells (Fig. 3).
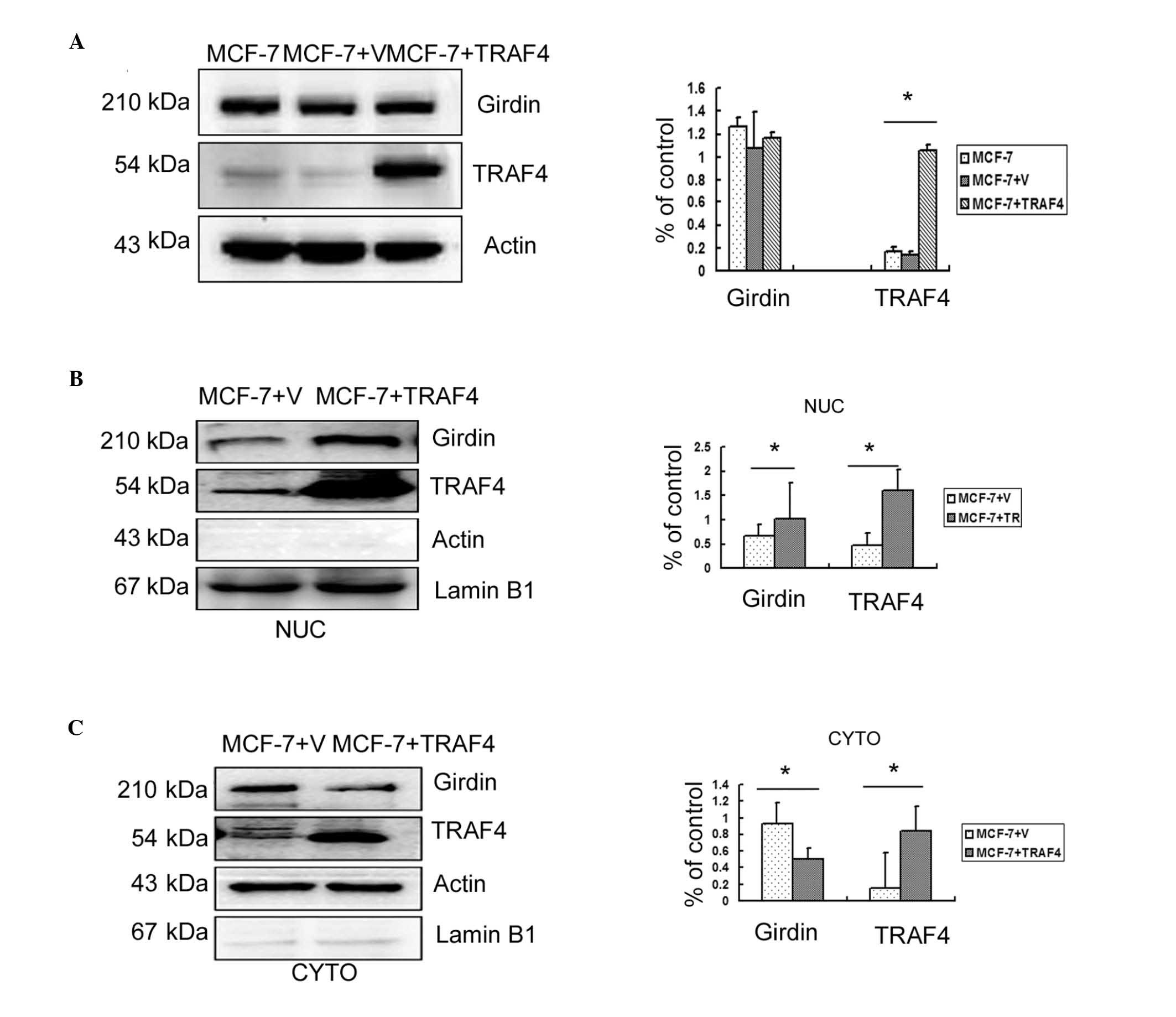
TRAF4 promotes the translocation of
Girdin from the cytoplasm to the nuclei in breast cancer cells
Previous studies had demonstrated that TRAF4 was
essential for the migration of normal mammary epithelial and breast
cancer cells (2,3); Girdin has also been implicated in
migration and metastasis (9–11).
In order to determine whether the molecular mechanisms of
TRAF4-induced migration involved the regulation of Girdin
expression, TRAF4 was overexpressed in the weakly invasive MCF-7
breast cancer cell line. However, no significant increase in the
overall Girdin protein levels were observed compared with
untransfected cells or empty vector controls (P>0.05; Fig. 4A). Therefore, Girdin protein levels
in the cytoplasm and nuclei of transfected MCF-7 cells were
analyzed individually by western blotting. The results revealed
that overexpression of TRAF4 significantly reduced cytoplasmic
expression of Girdin (P<0.05) but significantly increased its
nuclear expression (P<0.05; Fig. 4B
and C).
Discussion
Girdin is an actin-binding protein that is essential
in remodeling the actin cytoskeleton (19). It is also important for cell
motility and is implicated in tumor progression (13). TRAF4 is required for Akt Ub-Lys63
ubiquitination (4), which is
essential for Akt activity (20,21).
TRAF4 is also associated with NF-κB activation, and thereby STAT3
transcription. As Girdin is activated by Akt and has been
identified as a direct target of STAT3 (16), TRAF4 may potentially influence
Girdin via the Akt signaling pathway. However, the association
between TRAF4 and Girdin and the underlying mechanisms of action
remain to be established.
The current study demonstrated that the cytoplasmic
overexpression of TRAF4 in breast cancer tissues was positively
correlated with cytoplasmic expression of Girdin. In addition, it
was observed that TRAF4 was more highly expressed in the cytoplasm
of breast cancer cells than in normal breast cells; that TRAF4 and
Girdin were more highly expressed in IDC tissue samples than in
DCIS tissue samples; and that coexpression of TRAF4 and Girdin was
higher in tissue samples taken from patients with lymph node
metastases than those without metastasis.
A previous study reported that Girdin expression was
significantly correlated with c-erbB2 and Ki67 expression, by
demonstrating that Girdin expression was significantly higher in
c-erbB2- and Ki67-positive cases than in c-erbB2- and Ki67-negative
cases (12). In the current study,
it was observed that TRAF4 and Girdin coexpression was
significantly higher in tissues from c-erbB2-negative cases than in
tissues from c-erbB2-positive cases. However, further analysis is
required to verify this finding.
Although Girdin expression has previously been
identified in MDA-MB-231 breast cancer cells (9,17),
the expression levels were not compared with normal breast cell
lines or other breast cancer cell lines. In the current study,
Girdin expression levels were analyzed in normal MCF-10A breast
cells and ER-positive MCF-7 breast cancer cells, in addition to
ER-negative MDA-MB-231 cells. It was demonstrated that the
expression levels of Girdin were lower in normal breast cells
compared to breast cancer cells (P<0.01), and were higher in
ER-positive MCF-7 cells than in ER-negative MDA-MB-231 cells.
However the difference between the two breast cancer cell lines was
not statistically significant.
Consistent with a previous study that examined
immunohistochemical staining patterns of Girdin in breast cancer
tissues (7), the
immunofluorescence observations of the present study revealed that
Girdin was mainly localized in the cytoplasm of breast cancer
cells. To further explore the association between TRAF4 and Girdin
in breast cancer, TRAF4 was overexpressed in MCF-7 cells to
determine the effect on cytoplasmic and nuclear expression of
Girdin. These results demonstrated that the overexpression of TRAF4
promoted translocation of Girdin from the cytoplasm to the nuclei
in breast cancer cells. This finding remains to be verified in the
highly invasive MDA-MB-231 cell line.
In conclusion, the present study demonstrated that
cytoplasmic expression of TRAF4 was positively correlated with
cytoplasmic expression of Girdin in breast cancer cells, and that
the coexpression of TRAF4 and Girdin was highest in patients with
lymph node metastases. Furthermore, it was observed that Girdin was
predominantly expressed in the cytoplasm of breast cancer cells,
but that TRAF4 was able to promote its translocation from the
cytoplasm to the nucleus. Although the underlying mechanisms remain
to be established, the present findings suggest that cytoplasmic
expression of TRAF4 is a potential novel marker for migration in
breast cancer.
References
|
1
|
Xie P: TRAF molecules in cell signaling
and in human diseases. J Mol Signal. 8:72013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Zhou F, García de Vinuesa A, et
al: TRAF4 promotes TGF-β receptor signaling and drives breast
cancer metastasis. Mol Cell. 51:559–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Jin C, Tang Y, Tang LY and Zhang
YE: Ubiquitination of tumor necrosis factor receptor associated
factor 4 (TRAF4) by Smad ubiquitination regulatory factor 1
(Smurf1) regulates motility of breast epithelial and cancer cells.
J Biol Chem. 288:21784–21792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Peng C, Lee MH, et al: TRAF4 is a
critical molecule for Akt activation in lung cancer. Cancer Res.
73:6938–6950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camilleri-Broët S, Cremer I, Marmey B, et
al: TRAF4 overexpression is a common characteristic of human
carcinomas. Oncogene. 26:142–147. 2007. View Article : Google Scholar
|
|
6
|
Garcia-Marcos M, Jung BH, Ear J, Cabrera
B, Carethers JM and Ghosh P: Expression of GIV/Girdin, a
metastasis-related protein, predicts patient survival in colon
cancer. FASEB J. 25:590–599. 2011. View Article : Google Scholar :
|
|
7
|
Ling Y, Jiang P, Cui SP, et al: Clinical
implications for Girdin protein expression in breast cancer. Cancer
Invest. 29:405–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh P, Garcia-Marcos M and Farquhar MG:
GIV/Girdin is a rheostat that fine-tunes growth factor signals
during tumor progression. Cell Adh Migr. 5:237–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang P, Enomoto A, Jijiwa M, et al: An
actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jun BY, Kim SW, Jung CK, et al: Expression
of girdin in human colorectal cancer and its association with tumor
progression. Dis Colon Rectum. 56:51–57. 2013. View Article : Google Scholar
|
|
11
|
Ghosh P, Garcia-Marcos M, Bornheimer SJ
and Farquhar MG: Activation of Galphai3 triggers cell migration via
regulation of GIV. J Cell Biol. 182:381–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Zhang Y, Xu H, et al: Girdin
protein: a new potential distant metastasis predictor of breast
cancer. Med Oncol. 29:1554–1560. 2012. View Article : Google Scholar
|
|
13
|
Enomoto A, Ping J and Takahashi M: Girdin,
a novel actin-binding protein, and its family of proteins possess
versatile functions in the Akt and Wnt signaling pathways. Ann NY
Acad Sci. 1086:169–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weng L, Enomoto A, Ishida-Takagishi M,
Asai N and Takahashi M: Girding for migratory cues: roles of the
Akt substrate Girdin in cancer progression and angiogenesis. Cancer
Sci. 101:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esparza EM and Arch RH: TRAF4 functions as
an intermediate of GITR-induced NF-kappaB activation. Cell Mol Life
Sci. 61:3087–3092. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Liao X, Agarwal MK, Barnes L,
Auron PE and Stark GR: Unphosphorylated STAT3 accumulates in
response to IL-6 and activates transcription by binding to
NFkappaB. Genes Dev. 21:1396–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunkel Y, Ong A, Notani D, Mittal Y, Lam
M, Mi X and Ghosh P: STAT3 protein up-regulates Gα-interacting
vesicle-associated protein (GIV)/Girdin expression, and GIV
enhances STAT3 activation in a positive feedback loop during wound
healing and tumor invasion/metastasis. J Biol Chem.
287:41667–41683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lautenschlaeger T, Perry J, Peereboom D,
et al: In vitro study of combined cilengitide and radiation
treatment in breast cancer cell lines. Radiat Oncol. 8:2462013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enomoto A, Murakami H, Asai N, et al:
Akt/PKB regulates actin organization and cell motility via
Girdin/APE. Dev Cell. 9:389–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang WL, Wu CY, Wu J and Lin HK:
Regulation of Akt signaling activation by ubiquitination. Cell
Cycle. 9:487–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin K: The Akt DUBbed InAktive. Sci
Signal. 6:pe12013.PubMed/NCBI
|