Introduction
The solanum-steroid-alkaloids found in plants of the
Solanum species are of interest as a starting material for
the synthesis of steroid hormones and exhibit notable
pharmaceutical and toxicological properties (1,2).
α-tomatine and tomatidine (Fig.
1), occur naturally in tomatoes (Lycopersicon
esculentum), belonging to the group of
solanum-steroid-alkaloids. α-tomatine is a glycoalkaloid consisting
of an aglycone moiety (tomatidine) and a tetrasaccharide moiety
(β-lycotetraose), which is composed of two molecules of glucose,
one galactose and one xylose; the four monosaccharides form a
branched structure, which is attached at the C-3 position of the
aglycone (Fig. 1). Although it was
known that an enzyme, termed tomatinase, catalyzes the hydrolysis
of tomatine into tomatidine and β-lycotetraose (3,4), the
biosynthesis and metabolism of tomatine and tomatidine remain to be
elucidated. Unripe green tomatoes may contain up to 500 mg/kg
tomatine fresh fruit weight. The compound is partly degraded as the
tomato ripens until at maturity, levels in red tomatoes are ~5
mg/kg fresh fruit weight (5).
α-tomatine has also been found at high concentrations in leaves,
stems and roots, suggesting that it may be important in resistance
to potential pathogens (3,4).
α-tomatine has been reported to exert toxicity
against certain types of microorganisms (6–8).
Previous studies have also demonstrated that α-tomatine has
cytotoxic effects on insect and rat cells (9–11).
In previous years, the anticancer effect of α-tomatine has been
investigated. α-tomatine inhibits the growth of human cancer cells,
including the HT-29 colon cancer, HepG2 liver cancer, A549 lung
cancer, PC-3 prostate cancer and MCF-7 breast cancer cell lines
(12–15). α-tomatine also inhibits the growth
of lymphoma and leukemia cells (16,17).
Although α-tomatine has been revealed to have anticancer activities
in different cancer cells, the mechanisms of action and
particularly the primary target(s) remain to be elucidated. In the
present study, the effects and mechanisms of α-tomatine were
examined in HL-60 human myeloid leukemia cells, which are widely
used as a model system to investigate the effect of different
anticancer agents (18,19). The present study found that
α-tomatine markedly inhibited growth and induced apoptosis in HL-60
cells. α-tomatine also inhibited the in vivo growth of HL-60
cells in a mouse xenograft model.
Materials and methods
Cells and reagents
HL-60 cells were obtained from the American Type
Culture Collection (Rockville, MD, USA). Tomatidine, α-tomatine and
cholesterol were purchased from Sigma-Aldrich (St. Louis, MO, USA).
RPMI-1640, penicillin-streptomycin, L-glutamine and fetal bovine
serum (FBS) were purchased from Gibco-BRL (Grand Island, NY, USA).
HL-60 cells were maintained in RPMI-1640 culture medium containing
10% FBS that was supplemented with penicillin (100 U/ml),
streptomycin (100 μg/ml) and L-glutamine (300 μg/ml)
(Gibco-BRL). Cultured cells were grown at 37°C in a humidified
atmosphere of 5% CO2 and were passaged twice a week.
Determination of viable cells
Cell viability was determined by the trypan blue
exclusion assay, as described previously (20), which was performed by mixing 80
μl of cell suspension and 20 μl of 0.4% trypan blue
solution for 2 min. Blue cells were counted as dead cells and the
cells that did not absorb dye were counted as live cells.
Morphological assessment of apoptotic
cells
Apoptosis was determined by morphological assessment
in cells stained with propidium iodide (21). Briefly, cytospin slides were
prepared following each experiment and cells were fixed with
acetone/methanol (1:1) for 10 min at room temperature, followed by
10 min with propidium iodide staining (1 μg/ml in
phosphate-buffered saline; Gibco-BRL) and analyzed using a
fluorescence microscope (Nikon Eclipse TE200; Nikon, Tokyo, Japan).
Apoptotic cells were identified by classical morphological
features, including nuclear condensation, cell shrinkage and
formation of apoptotic bodies (21).
Nuclear extract preparation and
electrophoretic mobility shift assay (EMSA)
Mini-extracts prepared from cells (1×107
cells/ml) were collected by centrifugation (13000 × g for 10 min),
resuspended in hypotonic buffer and incubated on ice to obtain the
nuclear pellet as described in our previous study (22). Oligonucleotides were synthesized by
the DNA Synthesis and Sequencing Laboratory at the Cancer Institute
of New Jersey (New Brunswick, NJ, USA). Double-stranded
oligonucleotides were labeled by incubation with the Klenow enzyme
fragment of DNA polymerase in the presence of 32P-dCTP,
32P-dGTP, dATP and dTTP deoxynucleoside triphosphates.
Radiolabeled oligonucleotides (at least 1×108
cpm/μg) were incubated with 8 μg of nuclear protein
and 3 μg of poly(dI-dC) in a total volume of 16 μl.
DNA-protein complexes were analyzed by electrophoresis on 6%
acrylamide gels run in 1X Tris-borate buffer (Sigma-Aldrich)
(22).
HL-60 xengrafts in immunodeficient
mice
Female severe combined immunodeficient (SCID) mice
(6–7 weeks old) were obtained from Taconic Farms Inc. (Germantown,
NY, USA). The animals were housed in sterile filter-capped
microisolator cages and provided with sterilized food and water.
HL-60 cells (1.0×106 cells/0.1 ml/mouse) suspended in
50% Matrigel (Collaborative Research, Bedford, MA, USA) in
RPMI-1640 medium were injected subcutaneously into the right flank
of the mice. After 3 weeks, mice with subcutaneous tumors were
divided into two groups. One group of animals received
intraperitoneal (IP) injection of vehicle, which consisted of
propylene glycol, polysorbate 80, benzyl alcohol, ethanol and water
(40: 0.5: 1: 10: 48.5). The other group of animals received IP
injection of α-tomatine (5 mg/kg; 5 μl vehicle/g). The mice
received treatment three times a week for 3 weeks. Tumor size
(length × width) and body weight were measured. The animal
experiment was performed under an Institutional Animal Care and Use
Committee-approved protocol (#02-001; Rutgers University,
Piscataway, NJ, USA).
Statistical analysis
The analysis of variance method with the
Tukey-Kramer test was used for the comparison of growth inhibition
and apoptosis. Student’s t-test was used to assess the differences
of tumor size and body weight between the control group and the
treatment group.
Results
Effects of α-tomatine on the growth of
HL-60 cells
HL-60 cells were treated with different
concentrations of α-tomatine for 72 h. The number of viable cells
was determined at 24, 48 and 72 h using the trypan blue exclusion
assay (Fig. 2). Treatment of HL-60
cells with α-tomatine resulted in a time- and
concentration-dependent growth inhibition. α-tomatine at 2 and 5
μM markedly inhibited the growth of HL-60 cells. Treatment
with α-tomatine (5 μM) resulted in ~98% decrease in the
number of viable cells (Fig. 2A).
Cytosine arabinoside (Ara-C), a commonly used chemotherapeutic drug
for the treatment of myeloid leukemia, was included in the
experiment. Treatment of HL-60 cells with Arc-C also resulted in
growth inhibition in a time- and concentration-dependent manner
(Fig. 2B). α-tomatine at 5
μM exhibited a more significant growth inhibitory effect
than Ara-C. By contrast to the marked growth inhibitory effect of
α-tomatine, tomatidine (the aglycone of tomatine, see Fig. 1) had little or no effect on the
growth of HL-60 cells (Fig.
2C).
Effect of α-tomatine on the apoptosis of
HL-60 cells
The effects of α-tomatine and tomatidine on
stimulation of apoptosis in HL-60 cells were determined using
morphological assessment of apoptotic cells. Apoptotic cells were
identified by classical morphological features, including nuclear
condensation, cell shrinkage and formation of apoptotic bodies.
Morphologically distinct apoptotic cells from representative
samples are shown in Fig. 3B.
Treatment of HL-60 cells with α-tomatine stimulated apoptosis in a
concentration-dependent manner (Fig.
3C). A low concentration of α-tomatine (1 μM) had little
stimulatory effect on HL-60 cell apoptosis. Treatment with 2
μM α-tomatine resulted in ~30% apoptotic cells and treatment
with a higher concentration of α-tomatine (5 μM) resulted in
~60% apoptotic cells. By contrast, tomatidine had little or no
stimulatory effect on the apoptosis of HL-60 cells (Fig. 3C).
Effects of cholesterol on
α-tomatine-induced growth inhibition and apoptosis
It has been established that α-tomatine and
tomatidine can form complexes with cholesterol (23,24).
It was further investigated whether binding of α-tomatine with cell
membrane cholesterol is required for the effect of this compound on
growth inhibition and apoptosis in HL-60 cells. In these
experiments, HL-60 cells were treated with α-tomatine in the
presence or absence of cholesterol and cell growth and apoptosis
were determined. The present study found that the addition of
cholesterol significantly abrogated α-tomatine-induced growth
inhibition and apoptosis in HL-60 cells. As shown in Fig. 4A, treatment with 2 μM
α-tomatine decreased the number of viable cells by ~50%. The
addition of an equal molar concentration of cholesterol abrogated
the effect of α-tomatine. The addition of an equal molar
concentration of cholesterol also abrogated the effect of tomatine
on the stimulation of apoptosis in HL-60 cells (Fig. 4B).
Effects of TPA on α-tomatine-induced
growth inhibition and apoptosis
The phorbol ester TPA was demonstrated to activate
the nuclear transcription factor, nuclear factor (NF)-κB in cancer
cells, including leukemia cells (22,25,26).
Multiple studies have indicated that NF-κB has an anti-apoptotic
effect and activation of NF-κB protects cancer cells, including
leukemia cells from apoptosis (27–29).
It is therefore important to investigate whether pretreatment with
TPA may protect HL-60 cells from α-tomatine-induced apoptosis. In
initial experiments, HL-60 cells were treated with TPA and its
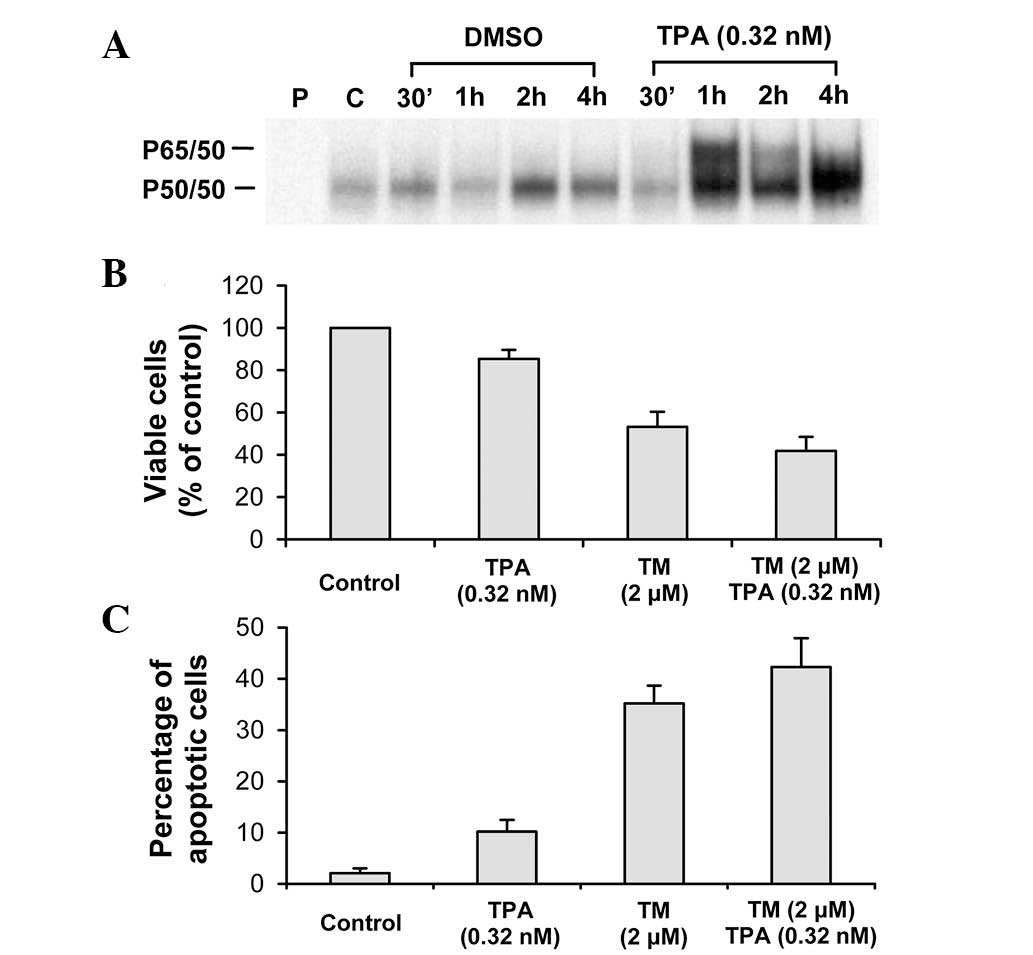
effect on activation of NF-κB was determined using the EMSA. As
shown in Fig. 5A, treatment of
HL-60 cells with TPA for 1 h caused a marked activation of NF-κB.
In subsequent experiments, HL-60 cells were pretreated with TPA for
1 h and then treated with tomatine. However, pretreatment with TPA
failed to protect the cells from α-tomatine-induced growth
inhibition (Fig. 5B) and apoptosis
(Fig. 5C).
Effect of α-tomatine on the growth of
HL-60 xenograft tumors
Female SCID mice with subcutaneous HL-60 xenograft
tumors were injected IP with vehicle (5 μl/g body weight) or
α-tomatine (5 mg/kg; 5 μl vehicle/g) three times a week for
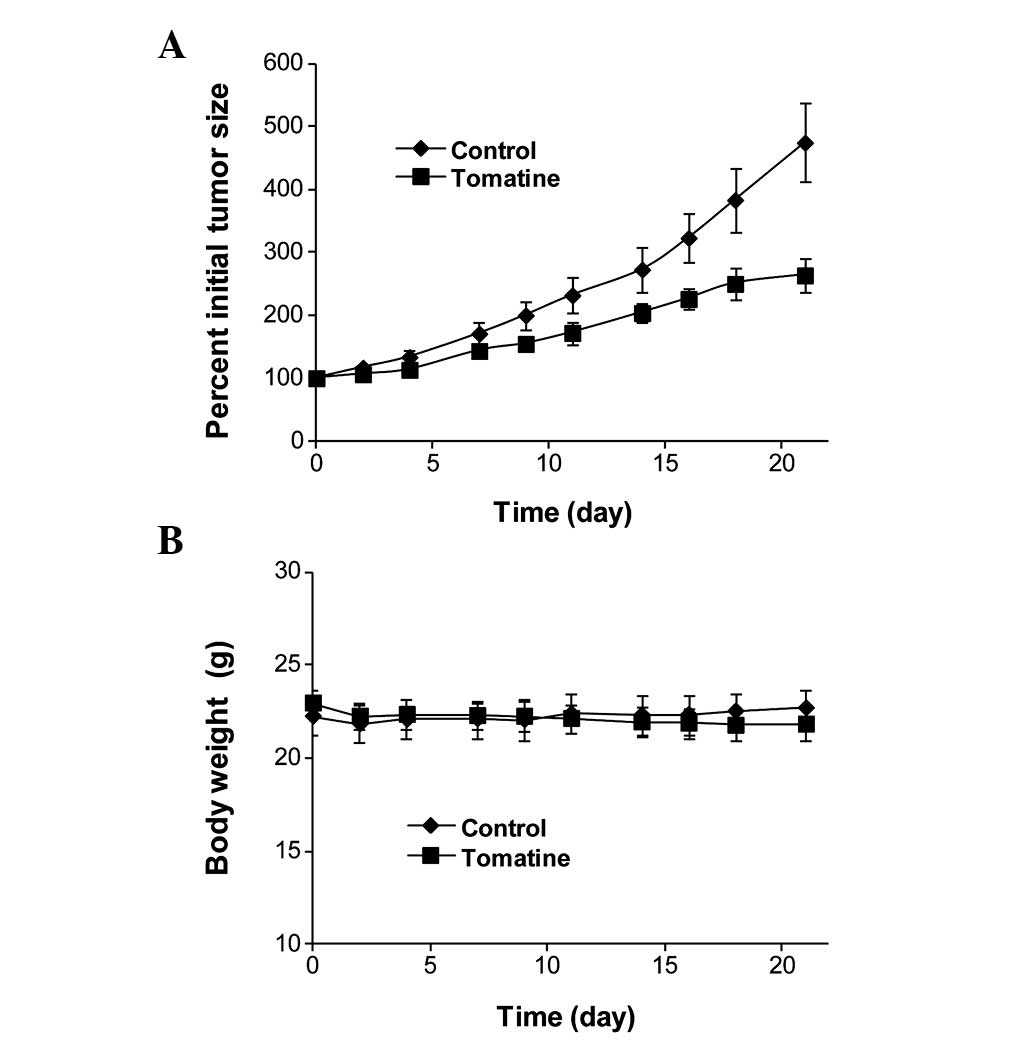
3 weeks. As shown in Fig. 6,
treatment with α-tomatine significantly inhibited the growth of
HL-60 tumors. The mean ± standard error of the mean (SEM) for the
percentage of the initial tumor size after 3 weeks of treatment was
475.2±62.2 for the control group and 263.9±25.7 for the
α-tomatine-treated group. Statistical analysis using Student’s
t-test revealed that the difference in the percentage of the
initial tumor size between the control group and the
α-tomatine-treated group were statistically significant
(P<0.01). The mean ± SEM for the body weight (g) was 22.7±0.9
for the vehicle-treated control group and 21.8±1.0 for the
α-tomatine-treated group. Statistical analysis using Student’s
t-test revealed that the difference in the body weight between the
control group and the treatment group was not statistically
significant (P>0.05).
Discussion
Glycoalkaloids are nitrogen-containing secondary
plant metabolites found in numerous plants, including potatoes and
tomatoes (30). The glycoalkaloid
α-tomatine has been hypothesized to be involved in host-plant
resistance against phytopathogens and has a variety of
pharmacological and toxicological properties in animals and humans
(5). α-tomatine has previously
been found to exert anticancer activities (13–17,31).
In the present study, the effect of α-tomatine and its aglycone,
tomatidine was determined in myeloid leukemia HL-60 cells. Although
it has previously been reported that α-tomatine inhibited growth
and induced apoptosis in leukemia cells, the effect of tomatidine
on leukemia cells has not been investigated. In the present study
it was demonstrated for the first time, to the best of our
knowledge, that α-tomatine, but not tomatidine inhibited growth and
induced apoptosis in HL-60 cells indicating the importance of the
glycone compartment of this compound. It was also found that
α-tomatine was as potent as the widely used anti-leukemia drug
Ara-C for inhibiting the growth and stimulating the apoptosis of
HL-60 cells.
The mechanisms by which α-tomatine induces growth
inhibition and apoptosis in myeloid leukemia cells remain to be
elucidated. A previous study revealed that treatment with
α-tomatine resulted in activation of Bak and Mcl-1s, and caused
release of apoptosis inducing factor and suppression of survivin
(16). Other studies demonstrated
that α-tomatine inhibited the NF-κB and
phosphatidyl-inositol-3-kinase/Akt signaling pathways activation in
lung and prostate cancer cells (13,15).
However, the primary cellular target(s) for α-tomatine and its
mechanisms for modulating apoptosis-associated pathways remain to
be elucidated. Previous in vitro studies revealed that
α-tomatine forms a robust complex with cholesterol in aqueous media
(23,24) and α-tomatine was used as a
cholesterol probe (32). Keukens
et al (33) demonstrated
that α-tomatine and other glycoalkaloids interacted with
membrane-associated cholesterol. In the present study, it was
investigated whether the interaction of α-tomatine with cell
membrane-associated cholesterol is essential for its effects on
leukemia cells. It was found that inhibiting the interaction of
α-tomatine with cell membrane-associated cholesterol by addition of
equal molar concentrations of free cholesterol in the medium
abrogated the effect of α-tomatine. This is the first study, to the
best of our knowledge, indicating that the interaction of tomatine
with cholesterol is important for mediating its effect on growth
and apoptosis in leukemia cells. The interaction of α-tomatine with
cholesterol and the disruptive effect on the cell membrane may be
one of the mechanisms by which α-tomatine induces apoptosis in
HL-60 cells. It is also possible that the formation of complexes of
α-tomatine and cholesterol may modulate the responsiveness of cell
membrane receptors to growth stimuli and thus decrease the growth
of HL-60 cells.
To further investigate whether the glycone group is
required for α-tomatine to inhibit growth and induce apoptosis, the
effects of α-tomatine with its aglycone tomatidine on HL-60 cells
were investigated. Although previous studies have revealed that
tomatidine was able to interact and form a complex with cell
membrane-associated cholesterol (23,24),
it was found that tomatidine had little effect on growth inhibition
and apoptosis at the doses assessed. Other studies have
demonstrated that glycoalkaloids caused membrane disruption leading
to the release of peroxidase previously enclosed in lipid vesicles
(33,34). It was observed that α-tomatine
increased the fluorescence-measured membrane permeability of frog
embryos while tomatidine had a weak effect (5). α-tomatine also decreased active
transport via the activity of the sodium-potassium pump in frog
skin, however, tomatidine had no effect on frog skin (5). These results, together with the
present findings suggest that the glycone component is important
for mediating the effects of α-tomatine on growth inhibition and
apoptosis. In additional studies, it was investigated whether
pre-treatment with the phorbol ester TPA may protect HL-60 cells
from apoptosis induced by α-tomatine. Although the present study
demonstrated that treatment with TPA resulted in a marked
activation of NF-κB, pre-treatment with TPA did not protect HL-60
cells from α-tomatine-induced apoptosis. This result suggests that
α-tomatine may also trigger pathways that are not associated with
NF-κB to induce apoptosis in HL-60 cells.
The present study also demonstrated an in
vivo effect of α-tomatine. Treatment of SCID mice with IP
injection of α-tomatine three times a week significantly inhibited
the growth of HL-60 cells in vivo. At the dose of 5 mg/kg
body weight used in the present study, α-tomatine appeared to be
non toxic as no body weight loss and no abnormalities in the major
organs were observed in the animals. Previous studies revealed that
α-tomatine was not toxic when consumed orally by rats (35–37).
When administered intravenously, it had a median lethal dose value
equal to 18 mg/kg body weight (35,36).
Nishie et al (38) revealed
that IP injection of α-tomatine (30–100 mg/kg) in rabbits produced
neither fatalities nor abnormal ECG signals. Although the plasma
concentrations of α-tomatine in these studies were not known, it is
reasonable to assume that blood concentration of α-tomatine at
micromolar level may be achievable without severe toxicity. Further
studies are required to establish the plasma levels of α-tomatine
in association with its inhibitory effect on leukemia and other
types of cancer in suitable animal models.
In conclusion, the present study demonstrated that
α-tomatine had a marked inhibitory effect on growth and a marked
stimulatory effect on apoptosis in human myeloid leukemia HL-60
cells. α-tomatine also significantly inhibited the in vivo
growth of HL-60 cells in the SCID mouse xenograft model. The
present study suggests that cell membrane-associated cholesterol
serves as a primary target for mediating the effect of α-tomatine
in leukemia cells. The present study also indicates that the
glycone component is critical for α-tomatine to convey signals for
growth inhibition and apoptosis. The results from the present study
indicate that α-tomatine may be a candidate for the development of
novel anti-leukemia agents.
Acknowledgments
The present study was supported by the Guangdong
Province Leadership Grant, China National Science Foundation (grant
nos. 81272452, 21102020 and 21272043) and the Rutgers Cancer
Institute of New Jersey (grant no. CCSG P30-CA072720 RSD).
References
|
1
|
Willker W and Leibfritz D: Complete
assignment and conformational studies of tomatine and tomatidine.
Magn Reson Chem. 30:645–650. 1992. View Article : Google Scholar
|
|
2
|
Friedman M: Tomato glycoalkaloids: role in
the plant and in the diet. J Agric Food Chem. 50:5751–5780. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roddick JG: The steroidal glycoalkaloid
α-tomatine. Phytochemistry. 13:9–25. 1974. View Article : Google Scholar
|
|
4
|
Lairini K, Perez-Espinosa A, Pineda M and
Ruiz-Rubio M: Purification and characterization of tomatinase from
Fusarium oxysporum f. sp lycopersici. Appl Environ Microbiol.
62:1604–1609. 1996.PubMed/NCBI
|
|
5
|
Blankemeyer JT, White JB, Stringer BK and
Friedman M: Effect of α-tomatine and tomatidine on membrane
potential of frog embryos and active transport of ions in frog
skin. Food Chem Toxicol. 35:639–646. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiratko J: Comparison of antifungal
activity of tomatine and tomato extract. Ochrani Rostlenia.
29:93–98. 1993.
|
|
7
|
Chan HT and Tam SYT: Toxicity of
α-tomatine to larvae of the Mediterranean fruit fly (Diptera:
Tephrididae). J Economic Entomology. 78:305–307. 1985. View Article : Google Scholar
|
|
8
|
Chu YI and Lu FM: The ovicidal effect of
tomatine against deposited eggs of the diamondback moth, Plutella
xylostella L. Chinese Journal of Entomology. 12:213–216. 1992.
|
|
9
|
Weissenberg M, Levy A, Svoboda JA and
Ishaaya I: The effect of some Solanum steroidal alkaloids and
glycoalkaloids on larvae of the red flour beetle, Tribolium
castaneum, and the tobacco hornworm, Manduca sexta. Phytochemistry.
47:203–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chataing B, Concepcion JL, Lobaton R and
Usubillaga A: Inhibition of Trypanosoma cruzi growth in vitro by
Solanum alkaloids: a comparison with ketoconazole. Planta Med.
64:31–36. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bergers Lee WW and Alink GM: Toxic effect
of the glycoalkaloids solanine and tomatine on cultured neonatal
rat heart cells. Toxicol Lett. 6:29–32. 1980. View Article : Google Scholar
|
|
12
|
Lee KR, Kozukue N, Han JS, et al:
Glycoalkaloids and metabolites inhibit the growth of human colon
(HT29) and liver (HepG2) cancer cells. J Agric Food Chem.
52:2832–2839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shieh JM, Cheng TH, Shi MD, et al:
α-Tomatine suppresses invasion and migration of human non-small
cell lung cancer NCI-H460 cells through inactivating FAK/PI3K/Akt
signaling pathway and reducing binding activity of NF-κB. Cell
Biochem Biophys. 60:297–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih YW, Shieh JM, Wu PF, et al:
α-tomatine inactivates PI3K/Akt and ERK signaling pathways in human
lung adenocarcinoma A549 cells: effect on metastasis. Food Chem
Toxicol. 47:1985–1995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee ST, Wong PF, Cheah SC and Mustafa MR:
α-tomatine induces apoptosis and inhibits nuclear factor-κB
activation on human prostatic adenocarcinoma PC-3 cells. PLoS One.
6:e189152011. View Article : Google Scholar
|
|
16
|
Chao MW, Chen CH, Chang YL, et al:
α-Tomatine-mediated anti-cancer activity in vitro and in vivo
through cell cycle- and caspase-independent pathways. PLoS One.
7:e440392012. View Article : Google Scholar
|
|
17
|
Yang YW, Wu CA and Morrow WJ: The
apoptotic and necrotic effects of tomatine adjuvant. Vaccine.
22:2316–2327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Collins SJ: The HL-60 promyelocytic
leukemia cell line: proliferation, differentiation, and cellular
oncogene expression. Blood. 70:1233–1244. 1987.PubMed/NCBI
|
|
19
|
Semizarov D, Glesne D, Laouar A, et al: A
lineage-specific protein kinase crucial for myeloid maturation.
Proc Natl Acad Sci USA. 95:15412–15417. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng X, Chang RL, Cui XX, et al:
Synergistic effects of clinically achievable concentrations of
12-O-tetradecanoylphorbol-13-acetate in combination with all-trans
retinoic acid, 1α,25 dihydroxyvitamin D3, and sodium butyrate on
differentiation in HL-60 cells. Oncol Res. 12:419–427. 2000.
|
|
21
|
Zheng X, Cui XX, Khor TO, et al:
Inhibitory effect of a γ-tocopherol-rich mixture of tocopherols on
the formation and growth of LNCaP prostate tumors in
immunodeficient mice. Cancers (Basel). 3:3762–3772. 2011.
View Article : Google Scholar
|
|
22
|
Hansson A, Marín YE, Suh J, et al:
Enhancement of TPA-induced growth inhibition and apoptosis in
myeloid leukemia cells by BAY 11–7082, an NF-κB inhibitor. Int J
Oncol. 27:941–948. 2005.PubMed/NCBI
|
|
23
|
Micich TJ: Behavior of polymer-supported
tomatine toward cholesterol in the presence and absence of butter
oil. J Agricu Food Chem. 39:1610–1613. 1993. View Article : Google Scholar
|
|
24
|
Roddick JG: Complex formation between
solanaceous steroidal glycoalkaloids and free sterols in vitro.
Phytochemistry. 18:1467–1470. 1979. View Article : Google Scholar
|
|
25
|
Maiti A, Cuendet M, Kondratyuk T, et al:
Synthesis and cancer chemopreventive activity of zapotin, a natural
product from Casimiroa edulis. J Med Chem. 50:350–355. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamiya T, Makino J, Hara H, et al:
Extracellular-superoxide dismutase expression during monocytic
differentiation of U937 cells. J Cell Biochem. 112:244–255. 2011.
View Article : Google Scholar
|
|
27
|
Bortul R, Tazzari PL, Cappellini A, et al:
Constitutively active Akt1 protects HL60 leukemia cells from
TRAIL-induced apoptosis through a mechanism involving NF-κB
activation and cFLIP(L) up-regulation. Leukemia. 17:379–389. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-κB. J Clin
Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cuní S, Pérez-Aciego P, Pérez-Chacón G, et
al: A sustained activation of PI3K/NF-κB pathway is critical for
the survival of chronic lymphocytic leukemia B cells. Leukemia.
18:1391–1400. 2004. View Article : Google Scholar
|
|
30
|
Friedman M and McDonald GM: Potato
glycoalkaloids: chemistry, analysis, safety, and plant physiology.
Crit Rev Plant Sci. 16:55–132. 1997. View Article : Google Scholar
|
|
31
|
Friedman M: Anticarcinogenic,
cardioprotective, and other health benefits of tomato compounds
lycopene, α-tomatine, and tomatidine in pure form and in fresh and
processed tomatoes. J Agric Food Chem. 61:9534–9550.
2013.PubMed/NCBI
|
|
32
|
Severs NJ and Simms HL: Failure of filipin
to detect cholesterol-rich domains in smooth muscle plasma membane.
Nature. 303:637–638. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Keukens EAJ, de Vrije T, van den Boom C,
et al: Molecular basis of glycoalkaloid induced membrane
disruption. Biochim Biophys Acta. 1240:216–228. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roddick JG, Rijnenberg AL and Osman SF:
Synergistic interaction between potato glycoalkaloids α-solanine
and α-chaconine in relation to destabilization of cell membranes:
Ecological implications. J Chem Ecol. 14:889–902. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cayen MN: Effect of dietary tomatine on
cholesterol metabolism in the rat. J Lipid Res. 12:482–490.
1971.PubMed/NCBI
|
|
36
|
Friedman M, Henika PR and Mackey BE:
Feeding of potato, tomato and eggplant alkaloids affects food
consumption and body and liver weights in mice. J Nutr.
126:989–999. 1996.PubMed/NCBI
|
|
37
|
Wilson RH, Poley GW and De Eds F: Some
pharmacologic and toxicologic properties of tomatine and its
derivatives. Toxicol Appl Pharmacol. 3:39–48. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishie K, Norred WP and Swain AP:
Pharmacology and toxicology of chaconine and tomatine. Res Comm
Chem Pathol Pharm. 12:657–668. 1975.
|