Introduction
Despite major advances in the treatment of coronary
artery disease, acute myocardial infarction (AMI) remains the
leading cause of human mortality worldwide (1). AMI is characterized by a sudden
reduction in blood and oxygen supply to the heart, irreversible
muscle damage and cardiomyocyte death, resulting in the formation
of an infarct zone containing nonfunctional myocytes, which are
remodeled into scar tissue (2).
The limited ability of the damaged heart to regenerate and replace
the damaged myocardium leads to the progression of cardiac
decompensation and heart failure. The development of cell-based
therapeutic strategies has focused on repairing damaged vascular
and cardiac tissue (2).
Adipose-derived mesenchymal stem cells (ADSCs) are advantageous for
therapeutic approaches due to their ease of acquirement and low
levels of immunosuppression (3,4).
Although the majority of animal and preliminary human studies
involving the transplantation of ADSCs have exhibited an overall
improvement in cardiac function (5,6),
several significant problems remain, which limit the use of ADSCs
in the treatment of AMI, including low rates of cell homing,
retention and survival in the injured myocardium (7). The migration and homing of ADSCs to
the ischemic site is a prerequisite and is crucial for myocardial
repair following transplantation (8). The stromal cell-derived factor-1α
(SDF-1α) chemokine and its unique receptor, CXC chemokine receptor
4 (CXCR4), are important in the mobilization and recruitment of
ADSCs (9). A previous study
demonstrated that SDF-1α, secreted following tissue damage induced
the migration, homing and retention of CXCR4+ cells to
the ischemic area (10). Of note,
the increased expression of SDF-1α/CXCR4 leads to increased homing
of transplanted stem cells to the infarcted myocardium and improves
ventricular function (11). These
previous findings suggest that the SDF-1α/CXCR4 cascade may be an
important therapeutic target of cell‑based therapy following AMI.
Therefore, there is a focus on identifying ways to regulate the
expression of SDF-1α/CXCR4 (12–14).
Exendin-4 (Ex-4) is a glucagon-like peptide-1
receptor agonist, which has cell protective effects, including the
inhibition of cardiomyocyte apoptosis (15), promoting the proliferation and
regeneration of β-cells (16) and
increasing the migration of human umbilical vein endothelial cells,
observed in in vitro scratch wound assays (17). However, the effects of Ex-4 on the
SDF-1α/CXCR4 cascade and the migration of ADSCs remain to be
elucidated.
The present study aimed to investigate whether Ex-4
affected the migration of ADSCs to conditioned medium, derived from
neonatal rat ventricular cardiomyocytes (NRVC-CM), via the
SDF-1α/CXCR4 cascade.
Materials and methods
Ethics
The present study was performed in accordance with
the Declaration of Helsinki and the guidelines of the Ethical
Committee of the Chinese People’s Liberty Army (PLA) General
Hospital (Beijing, China).
Isolation, culture and characterization
of ADSC and NRVCs
The ADSCs used in the present study were obtained
from a total of five male Sprague-Dawley rats (60–80 g) obtained
from the Laboratory Animal Center, Chinese PLA General Hospital, as
described previously, with minor modifications, including the
adjustment of collagenase I concentration to 0.1% (v/v) and the
dose of trypsin to 0.05% (v/v) (18). Rats were sacrificed by cervical
dislocation immediately following obtainment. Briefly, the white
adipose tissue from the inguinal region was washed three times with
phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO, USA)
and digested using collagenase I (5 ml; Sigma-Aldrich) and trypsin
(5 ml; Sigma-Aldrich) at 37°C for 40–45 min with continuous
agitation at 100 rpm using a MYP11–2 magnetic stirrer (Shanghai Wei
Ling Scientific Instrument Co., Ltd., Shanghai, China). The
digested tissue was filtered through 70-μm filters (BD
Biosciences, Franklin Lakes, NJ, USA), followed by centrifugation
for 10 min at 400 × g and resuspended in L-Dulbecco’s modified
Eagle’s medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Hyclone Laboratories,
Inc., Logan, UT, USA), 100 units/ml penicillin and 100 mg/ml
streptomycin (Sigma-Aldrich). The cell fraction was cultured at
37°C in 5% CO2 and the medium was refreshed every three
days. The subsequent experiments were performed with ADSCs between
the fourth and fifth passages.
The NRVCs were isolated by enzymatic dissociation,
where the heart tissue was treated with 0.1% (w/v) collagenase for
20 min at 37°C, and then incubated with 0.25% (w/v) trypsin
overnight at 4°C. The cells were subsequently plated at a density
of 5×105 cells/ml in DMEM supplemented with 15% (v/v)
fetal calf serum (Hyclone Laboratories, Inc.) at 37°C and 5% (v/v)
CO2, and cultured according to a standard procedure, as
previously described (19). The
NRVCs were maintained in H-DMEM (Gibco Life Technologies)
supplemented with 20% FBS and antibiotics (100 U/ml−1
penicillin and 100 mg/ml−1 streptomycin) at a density of
2×104 cells/cm−2 in a humidified incubator.
After 48 h incubation, the cardiomyocytes were confluent and were
beating spontaneously. The morphological characteristics of ADSCs
and NRVCs were evaluated by inverted microscope (magnification,
×40; BX51; Olympus Corp., Tokyo, Japan).
Flow cytometry
The ADSCs were harvested in the fourth passage to
detect surface antigens. Prior to immunostaining, the cells were
washed twice with PBS. The cells were then incubated with anti-rat
fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies
CD29 (1:500; 561796; BD Biosciences), CD31 (1:200; bs-0468R-FITC;
Bioss Inc., Woburn, MA, USA), CD34 (1:200; bs-2038R-FITC; Bioss
Inc.), CD45 (1:500; 561867; BD Biosciences) and CD90 (1:200;
bs-0778R-FITC; Bioss Inc.) at concentrations specified by the
manufacturer. FITC-labeled immunoglobulin(Ig)G-stained cells were
used as negative controls.
Following treatment with various concentrations of
Ex-4 (0, 1, 5, 10 and 20 nm/l; Sigma-Aldrich) for 24 h, the ADSCs
at passage 4 were used to detect the expression of CXCR4. The cells
were washed with PBS and each group of cells was stained with
either FITC-labeled CXCR4 (Bioss, Inc., Woburn, MA, USA) or
FITC-labeled IgG for 30 min at room temperature. Flow cytometric
analyses were performed using a BD FACSCalibur™ flow cytometer (BD
Biosciences), as previously described (3).
MTT proliferation assay
An MTT assay was performed to examine cell
proliferation. ADSCs were seeded in a 96-well plate at a density of
1×103 cells/well and treated with various concentrations
of Ex-4 (0–20 nm/l) in triplicate for seven days at 37°C, with 5%
CO2. Each day, 20 μl MTT (5 mg/ml PBS; pH7.4;
Sigma-Aldrich) was added to the cells for 4 h. The supernatants
were then discarded and 100 μl dimethyl sulfoxide
(Sigma-Aldrich) was added to each well for 10 min. The optical
density (OD) of the samples was measured at an absorbance of 490 nm
(Epoch 2; BioTek Instruments, Inc., Winooski, VT, USA). The assay
was repeated three times.
Western blot analysis
The cells were washed with ice-cold PBS and lysed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) supplemented with protease inhibitor
phenylmethanesulfonyl fluoride (1 nM/l; Beyotime Institute of
Biotechnology). The proteins (60–80 μg) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were then
blocked with tris-buffered saline plus 0.1% Tween® 20
(TBST; Sigma-Aldrich) with 5% non-fat milk for 90 min at room
temperature. Following blocking, the membranes were incubated with
the following primary antibodies: Polyclonal CXCR4 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; 1:250), total Akt, (Santa
Cruz Biotechnology, Inc.; 1:1,000), phosphorylated (p-)Akt (Santa
Cruz Biotechnology, Inc.; 1:1,000), SDF-1α, (Abcam, Cambridge, MA,
USA; 1:1,000) and β-actin (Santa-Cruz Biotechnology, Inc.; 1:2,000)
in TBST containing 5% non-fat milk at 4°C overnight. The membranes
were then washed with TBST and incubated for 1 h with horseradish
peroxidase-conjugated secondary antibodies. The blots were
visualized using enhanced chemiluminescence reagents (BeyoECL Plus;
Beyotime Institute of Biotechnology). The mean densities of the
bands were represented as the OD in units per square millimeter and
normalized to that of β-actin (Quantity One, version 4.6.2; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
ELISA detection of SDF-1α
The NRVCs were cultured with various concentrations
of Ex-4 (0–20 nm/l) for 24 h at 37°C and the expression of SDF-1α
in the culture supernatant was measured using an ELISA kit (cat.
no. R6782; Biotang, Inc., Lexington, MA, USA). The sample (50
μl culture supernatant) was added into 50 μl dilution
buffer (phosphate buffer solution plus 0.05% Tween 20, pH 7.2–7.4;
Sigma-Aldrich) in each well and it was washed twice. Subsequently,
the detection antibody (anti-rat SDF-1α polyclonal antibodies, 2
μg/ml, cat. no. ab9797; Abcam) was added and it was
incubated for 1 h at room temperature. The substrate and stop
solutions were then added into each well. Immediately following
this, the optical density of each well was determined using the
microplate reader set to a wavelength of 450 nm (Epoch 2; BioTek
Instruments, Inc.).
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and was
reverse transcribed into a total of 1 μl (60 ng/μl)
cDNA using a One-Step RT-PCR kit (TransGen Biotech Co., Ltd.,
Beijing, China), according to the manufacturer’s instructions.
Quantification of gene expression was performed using an ABI PRISM
7500 Sequence Detection system (Applied Biosystems Life
Technologies, Foster City, CA) with SYBR® Green (TransGen Biotech
Co., Ltd.). The relative mRNA expression levels of SDF-1α and CXCR4
were normalized to that of β-actin using the 2−ΔΔCT
method (20). The following primer
sequences were used (Shanghai GenePharma Co., Ltd., Shanghai,
China): Rat CXCR4, forward 5′-GCTGAGGAGCATGACAGACA-3′ and reverse
5′-GAT GAAGGCCAGGATGAGAA-3′ (21);
rat SDF-1α, forward 5′-CTGTTGTGCTTACTTGTTTAAGGCTTTGTC-3′ and
reverse 5′-GACGCCAAGGTCGTCGGT-3′ (22) and rat β-actin, forward
5′-GCTACAGCTTCACCACCACA-3′ and reverse 5′-GCCATCTCTTGCTCGAAGTC-3′.
The cycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec and 72°C for 35 sec, for telomere
PCR. The experiments were repeated three times with triplicates of
each sample.
Cell chemotaxis assay
The migration of the ADSCs was evaluated using a
24-well Transwell plate with an 8 μm pore size (Corning,
Inc., Corning, NY, USA). Briefly, 2 ml normal NRVC-CM (Nor-CM) and
2 ml NRVC-CM induced with Ex-4 (Ex-4-CM) were collected and placed
in the lower chamber of the plate and 105 ADSCs, with or
without the aforementioned treatment of Ex-4, were added to the
upper chamber in serum-free DMEM. The chemotaxis chambers were then
incubated for 12 h at 37°C, followed by removal of the
non-migrating cells from the upper chamber and fixing of the
migrated cells in methanol for 15 min at room temperature prior to
staining with 0.05% crystal violet for 15 min at room temperature
in the dark. To quantify the levels of chemotaxis, the number of
cells that had migrated through to the underside of the insert
membranes were counted in at least five randomly selected fields
using a BX51 microscope (magnification, ×100; Olympus Corp.). The
data were expressed as the ratio of the experimental samples to the
control samples × 100.
Pre-treatment of reagents
Prior to the western blot analysis and migration
assay, the cardiomyocytes were pretreated with or without PI3K
inhibitor (LY294002; 20 μm/l; Cell Signaling Technology,
Inc., Danvers, MA, USA) for 2 h prior to treatment with Ex-4 (20
nm/l) for 24 h. Following treatment, the conditioned medium was
collected and used for subsequent experiments. In addition, prior
to western blotting and migration assay, the ADSCs were incubated
with or without either LY294002 (30 μm/l) for 2 h or an
SDF-1α/CXCR4 cascade antagonist (AMD3100; Abcam; 5 μg/ml)
(23) for 1 h, prior to treatment
with Ex-4 (20 nm/l) for 24 h.
Statistical analysis
SPSS for Windows version 15.0 (SPSS Inc., Chicago,
IL, USA) was used for statistical analyses. The data are expressed
as the mean ± standard deviation. One-way analysis of variance was
used to determine statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological characterization of the
ADSCs and NRVCs
The ADSCs cultured in medium exhibited a
spindle-shaped or fibroblast-like morphology (Fig. 1A). After 48 h in culture, the
cardiomyocytes were confluent and beating spontaneously (Fig. 1B). In addition, flow cytometry
revealed that the ADSCs were positive for the CD29 and CD90
mesenchymal stem cell markers, but were negative for the CD45 and
CD34 hematopoietic lineage markers and the CD31 endothelial marker
(Fig. 1C). These findings are
concordant with those of a previous study (24).
Effects of Ex-4 on the proliferation of
ADSCs
A dose-dependent increase in the proliferative
capacity of the ADSCs was observed following treatment with Ex-4
(Fig. 2). The optimal
concentration of Ex-4 in stimulating the proliferation of ADSCs was
10 nm. The proliferation of the ADSCs was markedly higher in the
cells treated with 20 nm Ex-4 compared with the other groups
between 1 and 4 days, but was lower compared with the 10 nm group
between 4 and 7 days. Furthermore, no significant differences were
observed in the proliferative ability of the cells between the 1 nm
group and the normal control group; and, during the first 24 h, no
statistical differences were observed between any of the
groups.
Ex-4 upregulates the expression of CXCR4
in ADSCs and the production of SDF-1α in NRVCs
Since no significant difference was observed in the
proliferative ability of the cells following treatment with or
without Ex-4 for 24 h, the cells were treated with Ex-4 for 24 h in
the subsequent experiments to exclude the effects of proliferation
on the results. The ADSCs were treated with various concentrations
(0–20 nm/l) of Ex-4 for 24 h, following which the mRNA expression
levels of CXCR4 were analyzed by RT-qPCR. Treatment with Ex-4
caused a dose-dependent increase in the mRNA expression levels of
CXCR4, which was highest in the cells treated with 20 nm Ex-4
(Fig. 3A). Furthermore, to confirm
whether the upregulation of CXCR4 mRNA resulted in increased
protein translation, the protein expression levels were measured on
the cell surface (Fig. 3B) and
intracellularly (Fig. 3C) and by
western blot analysis and flow cytometry, respectively. Consistent
with the enhanced mRNA expression levels of CXCR4, the western blot
analysis revealed that the ADSCs also exhibited higher protein
expression levels of CXCR4 following treatment with Ex-4 (Fig. 3C). These results suggested that the
treatment of ADSCs with Ex-4 enhanced the mRNA and protein
expression of CXCR4.
 | Figure 3Effects of Ex-4 on the expression
levels of CXCR4 in ADSCs and the secretion of SDF-1α in NRVCs. (A)
Reverse transcription quantitative polymerase chain reaction was
used to determine the mRNA expression levels of CXCR4 and SDF-1α in
the ADSCs and NRVCs, respectively, following treatment with various
concentrations of Ex-4 (0–20 nm/l) for 24 h. (B) Flow cytometry was
used to analyze the levels of CXCR4 in the ADSCs at passage 4
following treatment with Ex-4. Red, ADSCs treated with various
concentrations of Ex-4 for 24 h and then incubated with
FITC-labeled CXCR4; green, ADSCs treated with various
concentrations of Ex-4 for 24 h and then incubated with
FITC-labeled immunoglobulin G.Western blot analysis was performed
to detect the protein expression levels of (C) CXCR4 and (D)
SDF-1α. Flow cytometric analysis of the (E) percentage of CXCR4
positive cells in the ADSCs at passage 4 and the (F) concentration
of SDF-1α in the supernatant of culture medium obtained from NRVCs.
Values are expressed as the mean ± standard deviation. *P<0.05,
vs. normal group. ADSC, adipose-derived stem cell; NRVC, neonatal
rat ventricular cardiomyocyte; Ex-4, exendin-4; CXCR4, CXC
chemokine receptor 4; SDF-1α, stromal cell-derived factor-1α; Nor,
normal control (0 nm/l Ex-4). |
The present study also aimed to determine whether
treatment with Ex-4 upregulated the expression of SDF-1α in the
NRVCs. The cells were incubated with Ex-4 (0–20 nm/l) for 24 h and
the mRNA and protein expression levels of SDF-1α were then analyzed
by RT-qPCR, western blot analysis and ELISA. The mRNA expression
levels of SDF-1α, relative to the levels of a constitutively
expressed control gene, were ~three times higher in the 20 nm group
compared with the normal group (Fig.
3A). The protein expression levels exhibited a similar pattern
to the mRNA expression levels, as determined by western blotting
(Fig. 3D). In addition, flow
cytometric analysis of the two groups of cells revealed an
increased number of CXCR4-positive cells in the P4 ADSCs
compared with the control group, peaking at >6-fold higher
(16.92±2.59% in the 20 nm group, vs 2.73±0.35% in the normal group
(Fig. 3E) and increased protein
expression levels of autocrine SDF-1α following incubation of the
NRVCs with Ex-4 (Fig. 3F). The
concentration of SDF-1α in the supernatant of the culture medium
was higher in the cells treated with 20 nm Ex-4 compared with the
normal group (3.8±0.19 vs. 2.03±0.14 ng/ml; P<0.05; n=3).
However, no statistical difference was observed between the cells
treated with 1 nm Ex-4 and the normal group.
Ex-4 promotes the migration of ADSCs to
NRVC-CM
A chemotaxis assay was performed in order to confirm
whether alterations in the expression levels of SDF-1α and CXCR4 in
ADSCs and NRVC-CM, following treatment with Ex-4, promotes
chemoattraction. Treatment of ADSCs with 20 nm Ex-4 for 24 h
resulted in a marked increase in the migration of ADSCs in response
to Nor-CM (Fig. 4). In addition,
the ADSCs were more attracted to Ex-4-CM than Nor-CM. Notably, in
the presence of Ex-4-CM, the Ex-4-ADSCs had the highest migratory
response. These data indicated that Ex-4 improved the migration of
ADSCs and enhanced the chemotactic abilities of the NRVC-CM. To
confirm whether Ex-4 increased the migration of ADSCs to NRVC-CM
via upregulation of the SDF-1α/CXCR4 cascade, the ADSCs were
pretreated with AMD3100 (5 μg/ml), a SDF-1α/CXCR4 cascade
antagonist, which inhibits the binding of SDF-1α to CXCR4 As
expected, the increased chemotactic response of the Ex-4-ADSCs to
the Ex-4-CM was markedly inhibited by pretreatment with AMD3100
(Fig. 4). These results suggested
that Ex-4-mediated cell migration was dependent on the interaction
between SDF-1α and CXCR4 and that the increased expression of CXCR4
in the Ex-4-ADSCs and of SDF-1α in the Ex-4-CM were responsible for
the upregulation in ADSC migration.
 | Figure 4Effects of Ex-4 on the migration of
ADSCs to NRVC-CM. Ex-4-CM and Nor-CM were obtained from the NRVCs,
which were added to the lower chamber of a Transwell plate. ADSCs
treated with Ex-4 (Ex-ADSCs), untreated ADSCs, and Ex-ADSCs
pretreated with AMD3100 (5 μg/ml) were placed in the upper
chamber. Following a 12 h incubation at 37°C, the non-migrating
ADSCs were removed and the migrated cells were stained with 0.05%
crystal-violet, followed by observation under a fluorescence
microscope. The data are expressed as the ratio of the experimental
samples to the normal samples which were designated as 100%. (A)
Treatment with Ex-4 increased the migration of ADSCs to NRVC-CM and
this effect was reversed by pretreatment with AMD3100
(magnification, ×100). (B) Relative percentage of migrated cells in
the experimental groups compared with the normal group. Results are
representative of three separate experiments. Values are expressed
as the mean ± standard deviation.*P<0.05, vs. normal group;
#P<0.05, compared with the Ex-ADSCs and Ex-4-CM
groups. ADSC, adipose-derived stem cell; NRVC, neonatal rat
ventricular cardiomyocte; CM, conditioned medium; Ex-4, exendin-4;
AMD. AMD3100; Nor, normal control (Nor-CM). |
Ex-4-mediated upregulation of the
SDF-1α/CXCR4 cascade is dependent on the PI3K/Akt pathway in ADSCs
and NRVCs
Since the increased secretion of SDF-1α and
expression of CXCR4 is regulated by the PI3K/Akt pathway (25) and Akt is a downstream target of
Ex-4 (26), the present study
hypothesized that the PI3K/Akt pathway may contribute to the
Ex-4-mediated expression of SDF-1α and CXCR4 in the ADSCs and
NRVCs, respectively. To confirm this hypothesis, the expression
levels of p-Akt were examined in the two cell lines following
treatment with Ex-4. Treatment with Ex-4 increased the protein
expression levels of p-Akt in the two cell types (Fig. 5A and B). Furthermore, a PI3K/Akt
pathway inhibitor was used to determine the role of the PI3K/Akt
pathway on the expression of SDF-1α and CXCR4 in the ADSCs and
NRVCs, respectively. The cells were pretreated for 2 h with the
LY294002 PI3K/Akt inhibitor, followed by treatment with Ex-4 (20
nm/l) for 24 h. The protein expression levels of p-Akt were
markedly inhibited following treatment with LY-294002 in the ADSCs
and NRVCs (Fig. 5A and B) and
treatment with the PI3K/Akt inhibitor significantly inhibited the
Ex-4-mediated upregulation of SDF-1α and CXCR4 in the ADSCs and
NRVCs, respectively (Fig. 5CF).
These results confirmed that Ex-4 induced an upregulation in the
expression levels of SDF-1α and CXCR4 via the PI3K/Akt pathway.
 | Figure 5Western blot analysis of the protein
expression levels of t-Akt, p-Akt, CXCR4 and SDF-1α in the ADSCs
and NRVCs treated with Ex-4 and/or LY. β-actin was used as an
internal reference protein. (A) Changes in the protein expression
levels in ADSCs; (B) Changes in the protein expression levels in
the NRVCs. (C and D) Relative changes in the protein expression
levels of p-Akt and CXCR4 in ADSCs. (E and F) Relative changes in
the protein expression levels of p-Akt and SDF-α in NRVCs. Values
are expressed as the mean ± standard deviation. *P<0.05, vs.
ctrl. ADSC, adipose-derived stem cells; NRVC, neonatal rat
ventricular cardiomyocyte; Ex-4, exendin-4; CXCR4, CXC chemokine
receptor 4; SDF-1α, stromal cell-derived factor-1α; t-Akt, total
Akt; p-Akt, phosphorylated Akt; LY, LY294002; ctrl, control
(untreated). |
PI3K/Akt pathway is involved in the
migratory response of ADSCs to NRVC-CM
The present study also aimed to identify the
potential role of the PI3K/Akt pathway on the migration of the
ADSCs to the NRVC-CM. The NRVCs were pretreated with or without the
LY294002 PI3K/Akt inhibitor (20 μm/ml) and then treated with
Ex-4 (20 nm/l) for 24 h. The NRVC-CM were collected and a Transwell
assay was performed. Fewer ADSCs translocated through the membrane
following pretreatment of the NRVCs with LY294002 (Fig. 6). In addition, the ADSCs were
pretreated with LY294002 (30 μm/ml) and, as expected, the
number of migratory ADSCs decreased. When the NRVCs and ADSCs were
pretreated with LY294002 for 2 h, followed by treatment with Ex-4
for 24 h, the migratory response of the ADSCs to the NRVC-CM was
minimal. These results indicated that inhibition of the PI3K/Akt
pathway partially impaired the effects of Ex-4 on the migration of
the ADSCs to the NRVC-CM.
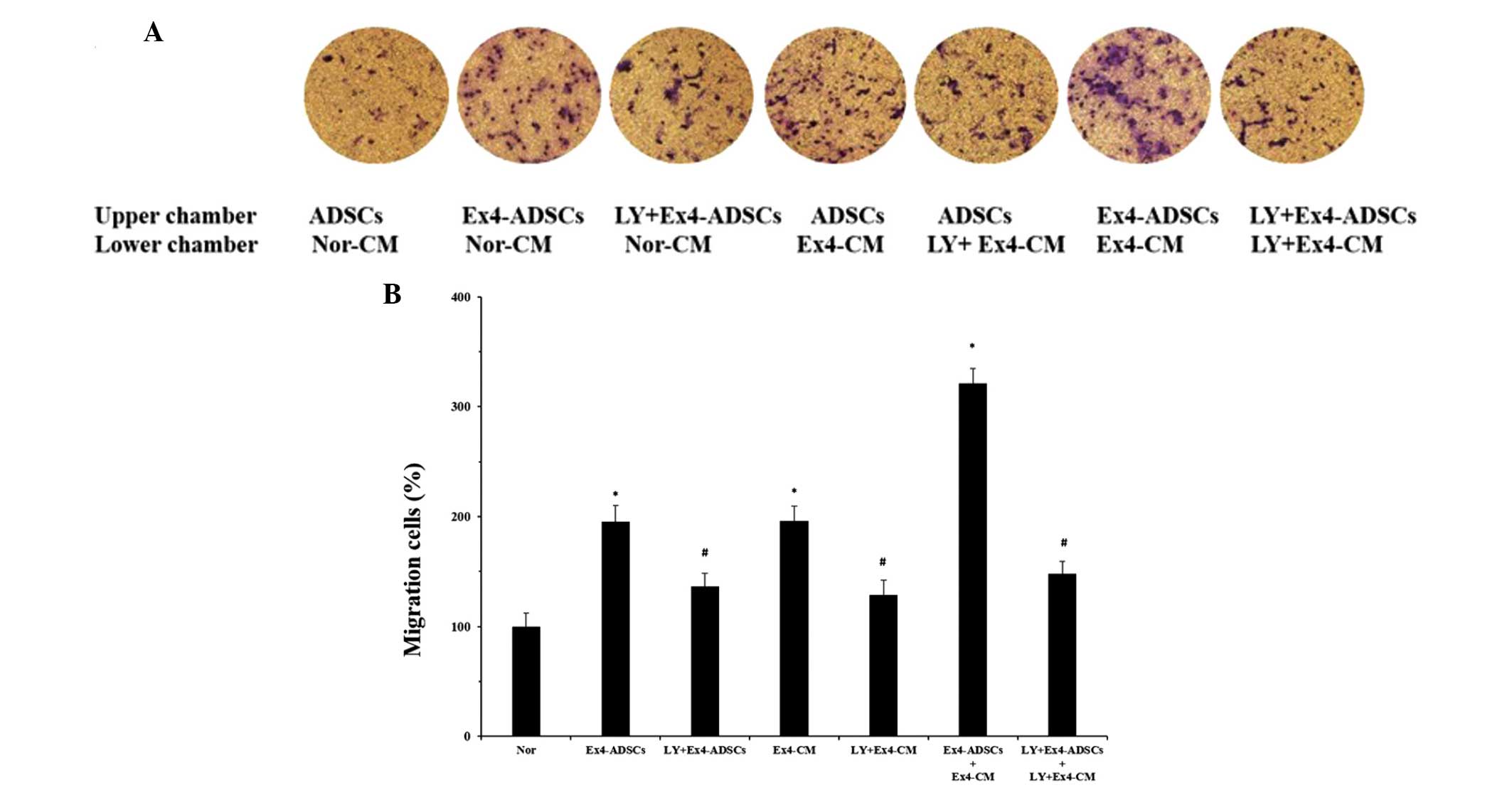 | Figure 6Effects of the PI3K/Akt pathway on
the migration of ADSCs to NRVC-CM. The NRVCs were treated with or
without the LY294002 for 2 h, followed by treatment with Ex-4 (20
nm) for 24 h. Ex-4-CM and LY+Ex-4-CM, were collected from the NRVCs
and added to the lower chamber of a Transwell plate. The same
treatment were applied to the ADSCs and the Ex-4-ADSCs and
LY+Ex-4-ADSCs were added to the upper chamber. Untreated ADSCs and
Nor-CM were defined as the Nor groups. After 12 h incubation at
37°C, the migrated cells were observed in at least five randomly
selected fields. (A) PI3K/Akt pathway was involved in the
Ex-4-mediated migration of ADSCs to NRVC-CM, since pretreatment
with LY294002 partially inhibited the heightened migratory response
induced by Ex-4 (magnification, ×100). (B) Data are expressed as
the ratio of migrated cells in the experimental groups relative to
the Nor group. Results are representative of three separate
experiments. Values are expressed as the mean ± standard
deviation.*P<0.05, vs. control group; #P<0.05, vs.
Ex-4 treatment groups, including Ex4-ADSCs, Ex4-CM, and
Ex4-ADSCs+Ex4-CM. ADSC, adipose-derived stem cell; NRVC, neonatal
rat ventricular cardiomyocyte; CM, conditioned medium; Ex-4,
exendin-4; PI3K, phosphoinositide 3-kinase; LY, LY294002; Nor,
normal control group. |
Discussion
Stem cell transplantation has recently emerged as a
promising tool for the treatment of AMI and ADSCs appear to be a
suitable candidate for stem cell therapy. However, despite the
improved cardiac function and reduced infarct size observed
following injection of ADSCs, the clinical benefits and long-term
outcomes remain under debate (27–29).
The major obstacle in ADSC therapy is the washout of transplanted
cells from the heart (30). The
magnitude of cell washout may depend on the presence of cell
traffcking and/or homing factors in transplanted cells and the
heart. The SDF-1α/CXCR4 cascade has previously been identified as a
key factor in the recruitment of stem cells to areas of injured
tissue in multiple organ systems (31–33),
which is fundamental in stem cell therapy following AMI (34). Briefly, on binding to CXCR4, SDF-1α
induces the mobilization of calcium, decreases levels of cyclic AMP
within the cells and activates several signaling pathways (35). Ultimately, SDF-1α-bound CXCR4
causes cytoskeletal rearrangement, adhesion to endothelial cells
and the polarized migration of cells to specific organs (36,37).
Although SDF-1α is predominantly secreted by endothelial cells and
upregulated in areas of infarction in short periods of time
(9,31), CXCR4 is expressed only in the early
passage of ADSCs (38). Therefore,
several strategies have been attempted to augment or stabilize the
expression of SDF-1α and CXCR4, including genetic modification and
altering culture conditions (14,39,40).
However, the high cost and adverse side effects of these strategies
have limited their application. The present study used a novel
drug, Ex-4, to alter the SDF-1α/CXCR4 cascade in cardiomyocytes and
ADSCs, and its effects on the SDFA-1α/CXCR4 cascade and migration
of ADSCs were observed. Ex-4 is an antidiabetic agent, which
reduces hyperglycemia through increased glucose-dependent insulin
secretion, glucagon suppression, delayed gastric emptying and
appetite suppression (41). It has
been demonstrated that Ex-4 is important for cardiopotection,
including the reduction in infarction size, improvement in left
ventricular ejection fraction (42) and reversion of cardiac remodeling
(43). The present study revealed
that treatment with Ex-4 significantly increased the secretion of
SDF-1α from cardiomyocytes and increased the number of
CXCR4+ ADSCs. Furthermore, an increased number of ADSCs
migrated to the NRVC-CM following treatment with Ex-4. These
results demonstrated that Ex-4 was capable of upregulating the
SDF-1α/CXCR4 cascade and the chemotaxis of ADSCs to NRVC-CM.
Therefore, Ex-4 may be a mediator with an important role in
advancing the migration of ADSCs. It was previously demonstrated
that SDF-1α alone has cardioprotective effects without the
involvement of stem cells (44,45).
Following direct administration of SDF-1α into the left ventricle
cavity in vivo, SDF-1α activates the reperfusion injury
signaling kinase pathway in cardiomyocytes, resulting in the
recruitment of the anti-apoptotic kinases, extracellular
signal-regulated kinase (44), Akt
(44) and signal transducer and
activator of transcription 3 (45). This promotes an anti-apoptotic
response, which confers protection against ischemia/reperfusion
damage, as part of the intrinsic repair mechanism following AMI
(46). The present study
demonstrated that Ex-4 improved the autocrine functions of SDF-1α
in cardiac myocytes, which enhanced the endogenous repair system to
preserve cardiac performance following AMI. Further studies are
required to confirm whether the upregulation of SDF-1α production
in cardiomyocytes in vivo is sufficient to ameliorate
cardiac function following AMI.
Li et al (9)
previously demonstrated that the expression of CXCR4 declined
between 51.4% in P0 ADSCs and 2.54% in P3
ADSCs in vitro, which may affect the homing and reparative
potential of ADSCs, impairing the efficacy of ADSC-based therapy on
ischemic or injured tissue (9).
The results of the present study demonstrated that P4
ADSCs with a weak migratory response contained only 2.73%
CXCR4+ cells, which was consistent with the results of
the previous study (9). However,
following treatment with Ex-4, the expression of CXCR4 in the
P4 ADSCs increased in a dose-dependent manner, resulting
in a higher number of ADSCs in the lower Transwell chamber. These
results indicated that exposure of ADSCs to Ex-4 may increase the
expression of CXCR4, functionally contributing to the increased
migration of ADSCs. This suggested that Ex-4 may be used as an
adjuvant to optimize the phenotype and subsequent function of ADSCs
in vitro, however, whether the change in phenotype and
function of ADSCs is associated with the increased efficiency of
cell-based therapy requires further investigation.
It may be hypothesized that increased expression of
the SDF-1α/CXCR4 cascade may explain the modulatory role of Ex-4 in
the migration of ADSCs; and the increased expression of
SDF-1α/CXCR4 may be responsible for upregulating the migration of
ADSCs to NRVC-CM. To assess this hypothesis, the ADSCs were
pretreated with AMD3100, a specific antagonist of the SDF-1α/CXCR4
cascade. As expected, treatment with AMD3100 inhibited the
upregulation of Ex-4-induced ADSC migration. These results
indicated that the Ex-4-mediated improvement in the chemotaxis of
the ADSCs to the NRVC-CM was due to enhancement of the SDF-1α/CXCR4
cascade. These findings were concordant with those of previous
studies, which reported that the SDF-1α/CXCR4 cascade contributed
to cell mobilization and the homing of hematopoietic and
mesenchymal stem cells (45–50).
Numerous studies have focused on the benefits of Ex-4 in the
treatment of AMI and post-MI, whereas the present study
demonstrated the significant role of Ex-4 in enhancing the
SDF-1α/CXCR4 cascade and the subsequent migration of ADSCs, which
may assist in improving the grafting of stem cells in clinical
transplantation. However, the underlying mechanisms remain to be
fully elucidated.
Akt is an important mediator of stem cell survival
(51), growth and paracrine
mechanisms (52), which regulates
various biological responses by phosphorylating a number of
substrates. Previous studies have demonstrated that the
cardioprotective effects of Ex-4 are associated with the activation
of the PI3K/Akt signaling pathway (26,53).
Coincidentally, the PI3K/Akt signaling pathway has been observed to
regulate the secretion of SDF-1α and the expression of CXCR4
(54–57). Therefore, whether Akt is important
in the Ex-4-induced expression of SDF-1α/CXCR4 and the upregulation
of ADSC migration to NRVC-CM. The results revealed increased
expression levels of p-Akt in the two types of cells, with
increased expression of SDF-1α/C XCR4 following treatment with
Ex-4, whereas in normal untreated groups with low expression levels
of p-Akt, the expression of SDF-1α/CXCR4 expression was minimal.
However, this effect was inhibited by treatment with the LY294002
PI3K/Akt inhibitor, suggesting the importance of the PI3K/Akt
signaling pathway in Ex-4-induced SDF-1α/CXCR4 expression. In
addition, treatment with LY294002 reduced the migration of the
ADSCs to the NRVC-CM, suggesting that the PI3K/Akt signaling was
involved in the process of ADSC migration to the NRVC-CM. These
results revealed that the PI3K/Akt-SDF-1α/CXCR4 pathway was
essential for the Ex-4-induced migration of ADSCs, suggesting
potential therapeutic applications for Ex-4, including the
facilitation of stem cell recruitment and homing.
In conclusion, the present study provided novel
insight into potential methods to improve the migration of ADSCs
and identified several key signaling molecules involved in this
process, including PI3K/Akt and SDF-1α/CXCR4. The PI3K/Akt pathway
was found to be responsible for the Ex-4-induced upregulation of
the SDF-1α/CXCR4 cascade, which represents the final mediator of
ADSC migration and, therefore, offers a potential mechanism to
maximize the effectiveness of ADSC-based therapy. However, the
detailed genetic changes underlying Ex-4, and whether the increased
migratory capacity of the ADSCs observed in vitro is
associated with improved recruitment and homing of transplanted
cells in vivo requires further investigation.
Acknowledgments
The authors of the present study would like to thank
the Institute of Basic Medicine Science and Cardiology Lab of the
Chinese PLA General Hospital for assistance. The present study was
supported by grants from the National 863 high technology R&D
Program (no. 2011AA020101) and the National Natural Science
Foundation of China (nos. 81270186, 81170177, 81441008,
81400229).
References
|
1
|
Wallentin L, Kristensen SD, Anderson JL,
et al: How can we optimize the processes of care for acute coronary
syndromes to improve outcomes? Am Heart J. 168:622–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Segers VF and Lee RT: Stem-cell therapy
for cardiac disease. Nature. 451:937–942. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bai X, Yan Y, Song YH, et al: Both
cultured and freshly isolated adipose tissue-derived stem cells
enhance cardiac function after acute myocardial infarction. Eur
Heart J. 31:489–501. 2010. View Article : Google Scholar
|
|
4
|
Strioga M, Viswanathan S, Darinskas A,
Slaby O and Michalek J: Same or not the same? Comparison of adipose
tissue-derived versus bone marrow-derived mesenchymal stem and
stromal cells. Stem Cells Dev. 21:2724–2752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harasymiak-Krzyżanowska I, Niedojadło A,
Karwat J, et al: Adipose tissue-derived stem cells show
considerable promise for regenerative medicine applications. Cell
Mol Biol Lett. 18:479–493. 2013. View Article : Google Scholar
|
|
6
|
Konno M, Hamabe A, Hasegawa S, et al:
Adipose-derived mesenchymal stem cells and regenerative medicine.
Dev Growth Differ. 55:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mazo M, Gavira JJ, Pelacho B and Prosper
F: Adipose‑derived stem cells for myocardial infarction. J.
Cardiovasc Transl Res. 4:145–153. 2011. View Article : Google Scholar
|
|
8
|
Chavakis E and Dimmeler S: Homing of
progenitor cells to ischemic tissues. Antioxid Redox Signal.
15:967–980. 2011. View Article : Google Scholar
|
|
9
|
Li Q, Zhang A, Tao C, Li X and Jin P: The
role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose
tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res
Commun. 441:675–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cencioni C, Capogrossi MC and Napolitano
M: The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc
Res. 94:400–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang Y, Chen X, Xu M, Zhang LY and Xiang
F: Chemokine stromal cell-derived factor 1/CXCL12 increases homing
of mesenchymal stem cells to injured myocardium and
neovascularization following myocardial infarction. Chin Med J
(Engl). 122:183–187. 2009.
|
|
12
|
Haider H, Jiang S, Idris NM and Ashraf M:
IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow
stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4
signaling to promote myocardial repair. Circ Res. 103:1300–1308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho HH, Kyoung KM, Seo MJ, Kim YJ, Bae YC
and Jung JS: Overexpression of CXCR4 increases migration and
proliferation of human adipose tissue stromal cells. Stem Cells
Dev. 15:853–864. 2006. View Article : Google Scholar
|
|
14
|
Wang K, Zhao X, Kuang C, et al:
Overexpression of SDF-1α enhanced migration and engraftment of
cardiac stem cells and reduced infarcted size via CXCR4/PI3K
pathway. PLoS One. 7:e439222012. View Article : Google Scholar
|
|
15
|
Chang G, Zhang D, Yu H, et al:
Cardioprotective effects of exenatide against oxidative
stress-induced injury. Int J Mol Med. 32:1011–1020. 2013.PubMed/NCBI
|
|
16
|
Li Y, Cao X, Li LX, et al: beta-Cell Pdx1
expression is essential for the glucoregulatory, proliferative, and
cytoprotective actions of glucagon-like peptide-1. Diabetes.
54:482–491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang HM, Kang Y, Chun HJ, Jeong JW and
Park C: Evaluation of the in vitro and in vivo angiogenic effects
of exendin-4. Biochem Biophys Res Commun. 434:150–154. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyahara Y, Nagaya N, Kataoka M, et al:
Monolayered mesenchymal stem cells repair scarred myocardium after
myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Liu J, Liu X, et al: Panax
notoginseng saponins inhibit ischemia-induced apoptosis by
activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol.
137:263–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma X, Gao Y, Fan Y, et al: Overexpression
of E2F1 promotes tumor malignancy and correlates with TNM stages in
clear cell renal cell carcinoma. PLoS One. 8:e734362013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang D, Fan GC, Zhou X, et al:
Over-expression of CXCR4 on mesenchymal stem cells augments
myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol.
44:281–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonaros N, Sondermeijer H, Wiedemann D, et
al: Downregulation of the CXC chemokine receptor 4/stromal
cell-derived factor 1 pathway enhances myocardial
neovascularization, cardiomyocyte survival, and functional recovery
after myocardial infarction. J Thorac Cardiovasc Surg. 142:687–696.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YB, Liu YF, Lu XT, et al: Rehmannia
glutinosa extract activates endothelial progenitor cells in a rat
model of myocardial infarction through a SDF-1 α/CXCR4 cascade.
PLoS One. 8:e543032013. View Article : Google Scholar
|
|
24
|
Yang J, Zhang H, Zhao L, Chen Y, Liu H and
Zhang T: Human adipose tissue-derived stem cells protect impaired
cardiomyocytes from hypoxia/reoxygenation injury through
hypoxia-induced paracrine mechanism. Cell Biochem Funct.
30:505–514. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Qu J, Shelat H, Gao S, Wassler M and
Geng YJ: Clusterin induces CXCR4 expression and migration of
cardiac progenitor cells. Exp Cell Res. 316:3435–3442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Timmers L, Henriques JP, de Kleijn DP, et
al: Exenatide reduces infarct size and improves cardiac function in
a porcine model of ischemia and reperfusion injury. J Am Coll
Cardiol. 53:501–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strauer BE and Steinhoff G: 10 years of
intracoronary and intramyocardial bone marrow stem cell therapy of
the heart: from the methodological origin to clinical practice. J
Am Coll Cardiol. 58:1095–1104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Templin C, Lüscher TF and Landmesser U:
Cell‑based cardiovascular repair and regeneration in acute
myocardial infarction and chronic ischemic cardiomyopathy-current
status and future developments. Int J Dev Biol. 55:407–417. 2011.
View Article : Google Scholar
|
|
29
|
Krane M, Wernet O and Wu SM: Promises and
pitfalls in cell replacement therapy for heart failure. Drug Discov
Today Dis Mech. 7:e109–e115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dow J, Simkhovich BZ, Kedes L and Kloner
RA: Washout of transplanted cells from the heart: a potential new
hurdle for cell transplantation therapy. Cardiovasc Res.
67:301–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Askari AT, Unzek S, Popovic ZB, et al:
Effect of stromal-cell-derived factor 1 on stem-cell homing and
tissue regeneration in ischaemic cardiomyopathy. Lancet.
362:697–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosenkranz K, Kumbruch S, Lebermann K, et
al: The chemokine SDF-1/CXCL12 contributes to the ‘homingʼ of
umbilical cord blood cells to a hypoxic-ischemic lesion in the rat
brain. J Neurosci Res. 88:1223–1233. 2010.
|
|
33
|
Stokman G, Stroo I, Claessen N, Teske GJ,
Florquin S and Leemans JC: SDF-1 provides morphological and
functional protection against renal ischaemia/reperfusion injury.
Nephrol Dial Transplant. 25:3852–3859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Penn MS: SDF-1:CXCR4 axis is fundamental
for tissue preservation and repair. Am J Pathol. 177:2166–2168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sotsios Y, Whittaker GC, Westwick J and
Ward SG: The CXC chemokine stromal cell-derived factor activates a
Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol.
163:5954–5963. 1999.PubMed/NCBI
|
|
36
|
Hillyer P, Mordelet E, Flynn G and Male D:
Chemokines, chemokine receptors and adhesion molecules on different
human endothelia: discriminating the tissue-specific functions that
affect leucocyte migration. Clin Exp Immunol. 134:431–441. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alsayed Y, Ngo H, Runnels J, et al:
Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent
migration and homing in multiple myeloma. Blood. 109:2708–2717.
2007.
|
|
38
|
Honczarenko M, Le Y, Swierkowski M, Ghiran
I, Glodek AM and Silberstein LE: Human bone marrow stromal cells
express a distinct set of biologically functional chemokine
receptors. Stem Cells. 24:1030–1041. 2006. View Article : Google Scholar
|
|
39
|
Cheng Z, Ou L, Zhou X, et al: Targeted
migration of mesenchymal stem cells modified with CXCR4 gene to
infarcted myocardium improves cardiac performance. Mol Ther.
16:571–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi M, Li J, Liao L, et al: Regulation of
CXCR4 expression in human mesenchymal stem cells by cytokine
treatment: role in homing efficiency in NOD/SCID mice.
Haematologica. 92:897–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tahrani AA, Bailey CJ, Del Prato S and
Barnett AH: Management of type 2 diabetes: new and future
developments in treatment. Lancet. 378:182–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davidson MH: Cardiovascular effects of
glucagonlike peptide-1 agonists. Am J Cardiol. 108:33B–41B. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Monji A, Mitsui T, Bando YK, Aoyama M,
Shigeta T and Murohara T: Glucagon-like peptide-1 receptor
activation reverses cardiac remodeling via normalizing cardiac
steatosis and oxidative stress in type 2 diabetes. Am J Physiol
Heart Circ Physiol. 305:H295–H304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu X, Dai S, Wu WJ, et al: Stromal cell
derived factor-1 alpha confers protection against myocardial
ischemia/reperfusion injury: role of the cardiac stromal cell
derived factor-1 alpha CXCR4 axis. Circulation. 116:654–663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang C, Gu H, Zhang W, Manukyan MC, Shou
W and Wang M: SDF-1/CXCR4 mediates acute protection of cardiac
function through myocardial STAT3 signaling following global
ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol.
301:H1496–H1505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hausenloy DJ, Lecour S and Yellon DM:
Reperfusion injury salvage kinase and survivor activating factor
enhancement prosurvival signaling pathways in ischemic
postconditioning: two sides of the same coin. Antioxid Redox
Signal. 14:893–907. 2011. View Article : Google Scholar
|
|
47
|
Zou YR, Kottmann AH, Kuroda M, Taniuchi I
and Littman DR: Function of the chemokine receptor CXCR4 in
haematopoiesis and in cerebellar development. Nature. 393:595–599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peled A, Petit I, Kollet O, et al:
Dependence of human stem cell engraftment and repopulation of
NOD/SCID mice on CXCR4. Science. 283:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ji JF, He BP, Dheen ST and Tay SS:
Interactions of chemokines and chemokine receptors mediate the
migration of mesenchymal stem cells to the impaired site in the
brain after hypoglossal nerve injury. Stem Cells. 22:415–427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Odemis V, Boosmann K, Dieterlen MT and
Engele J: The chemokine SDF1 controls multiple steps of myogenesis
through atypical PKCzeta. J Cell Sci. 120:4050–4059. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mangi AA, Noiseux N, Kong D, et al:
Mesenchymal stem cells modified with Akt prevent remodeling and
restore performance of infarcted hearts. Nat Med. 9:1195–1201.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
González N, Acitores A, Sancho V, Valverde
I and Villanueva-Peñacarrillo ML: Effect of GLP-1 on glucose
transport and its cell signalling in human myocytes. Regul Pept.
126:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu N, Tian J, Cheng J and Zhang J:
Migration of CXCR4 gene-modified bone marrow-derived mesenchymal
stem cells to the acute injured kidney. J Cell Biochem.
114:2677–2689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim SJ, Lee Y, Kim NY, et al: Pancreatic
adenocarcinoma upregulated factor, a novel endothelial activator,
promotes angiogenesis and vascular permeability. Oncogene.
32:3638–3647. 2013. View Article : Google Scholar
|
|
56
|
McCaig AM, Cosimo E, Leach MT and Michie
AM: Dasatinib inhibits CXCR4 signaling in chronic lymphocytic
leukaemia cells and impairs migration towards CXCL12. PLoS One.
7:e489292012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu H, Xue W, Ge G, et al: Hypoxic
preconditioning advances CXCR4 and CXCR7 expression by activating
HIF-1α in MSCs. Biochem Biophys Res Commun. 401:509–515. 2010.
View Article : Google Scholar : PubMed/NCBI
|




















