Introduction
Gastric cancer is highly prevalent in East Asia,
with 42% of cases occurring in China (1). The median life expectancy of gastric
cancer patients following diagnosis is <1 year; however,
combination chemotherapy treatments have the potential to extend
the survival rate of advanced stage patients (2–8).
Therefore, using chemotherapy in conjunction with effective
targeting of key factors may be beneficial for improving the
clinical outcome of gastric cancer patients. A phase III clinical
trial demonstrated that the use of chemotherapy in conjunction with
a human epidermal growth factor receptor 2 (HER2)-specific
monoclonal antibody (trastuzumab) significantly improved the
overall survival rate of patients with HER2-neu overexpressing
gastroesophageal junction cancer compared with that of chemotherapy
alone (7). However, overexpression
of Her-2 is present in only 10–20% of gastric cancer patients
(9) and therefore, this
combination treatment may not be beneficial for the majority of
patients. Cetuximab (C225), an anti-epidermal growth factor
receptor (EGFR) monoclonal antibody, has been widely used in
combination with chemotherapy for the treatment of various cancer
types, including metastatic colorectal cancers that retain
wild-type K-ras and BRAF genes, squamous cell
carcinoma of the head and neck as well as non-small cell lung
cancer (NSCLC) (10–12). The majority of gastric cancers
overexpress EGFR (13), while
retaining wild-type K-ras and BRAF genes (14,15).
Phase II clinical studies have shown that cetuximab in combination
with chemotherapy delayed the progression of gastric cancer in
patients, with an acceptable response rate (13,16–18).
However, two additional trials failed to demonstrate significant
improvement in overall patient survival with use of the anti-EGFR
antibodies cetuximab or panitumumab in combination with
chemotherapy in advanced gastric cancer patients compared with that
of chemotherapy alone (19,20).
The results of these studies therefore suggested that alternate
mechanisms of resistance to anti-EGFR antibodies existed in gastric
cancer patients.
Numerous key molecules are involved in the EGFR
signal transduction pathway, which is also able to cross-talk with
other signaling pathways. In addition to K-ras and BRAF, other
molecules influence EGFR signaling pathways, including C-Met and
the insulin-like growth factor receptor-I (IGF-IR) signaling
pathway (21–23). IGF-IR is a receptor tyrosine
kinase, which is overexpressed in numerous types of tumor, such as
gastrointestinal carcinomas (24–26).
IGF-IR becomes autophosphorylated following the binding of ligands,
which stimulates its tyrosine kinase activity and subsequently
activates downstream signaling pathways (27). These pathways include the
Ras/Raf/mitogen-activated protein kinase and phosphoinositide
3-kinase/Akt pathways, which are the primary downstream mediators
of EGFR signaling (28). This
therefore suggested that IGF-IR may modulate the sensitivity of
gastric cancer cells to anti-EGFR antibodies.
Resistance to cetuximab was reported to be
associated with overactivation of baseline IGF-IR in human
nasopharyngeal carcinoma cells; in addition, the inhibition of
baseline IGF-IR activation increased sensitivity to cetuximab in
cutaneous squamous cell carcinoma (29). However, the involvement of
cetuximab in the activation of the IGF-IR pathway and inhibition of
the EGFR pathway as well as the role of IGF-IR signaling in
cetuximab resistance in gastric cancer cells has remained to be
elucidated.
The non-receptor tyrosine kinase c-steroid receptor
co-activator (Src) was reported to have a crucial role in IGF-IR
signaling. Numerous studies have indicated that Src may be an
upstream signaling molecule of IGF-IR and EGFR in kidney cells and
epididymal cells (30,31). By contrast, certain studies have
shown that IGF-IR acts upstream of Src in human prostate cancer
DU145 and breast cancer cells (32,33).
Src has also been implicated in chemotherapy resistance in gastric
cancer (34). However, the
involvement of Src in the regulation of IGF-IR signal transduction
and thereby cetuximab sensitivity in gastric cancer cells has
remained to be elucidated.
The aim of the present study was to investigate the
role of cetuximab in the induction of IGF-IR and Src activation in
gastric cancer cells in order to determine the mechanisms
underlying cetuimab resistance.
Materials and methods
Reagents and antibodies
Cetuximab was obtained from Merck KGaA (Darmstadt,
Germany). Src inhibitor
4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]
pyrimidine (PP2) was obtained from Sigma (St. Louis, MO, USA).
IGF-IR inhibitor OSI-906 was purchased from SelleckBio (Houston,
TX, USA). The following antibodies: Anti-EGFR polyclonal antibody,
anti-phospho-EGFR (Tyr1068) polyclonal antibody, anti-phospho-Akt
(Ser473) polyclonal antibody, anti-IGF-IRmonoclonal antibody,
anti-phospho-Src (Y416) polyclonal antibody and anti-phospho-IGF-IR
(Tyr1131) polyclonal antibody, were purchased from Cell Signaling
Technology, Inc.(Danvers, MA, USA). The following antibodies:
Anti-β-actin polyclonal antibody, anti-Akt monoclonal antibody,
anti-extracellular signal-regulated kinase (ERK)1/2 polyclonal
antibody, anti-c-Src monoclonal antibody and anti-phospho-ERK1/2
(Tyr202/Tyr204) polyclonal antibody, were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell cultures
Gastric cancer SGC7901 and MGC803 cells were
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Mutations were not
located in exons 19 or 21 of the EGFR gene in the two gastric
cancer cell lines. The cells were cultured in RPMI-1640 medium
(Gibco-BRL, Grand Island, NY, USA) containing 10% heat-inactivated
fetal bovine serum (FBS; Gibco-BRL), 100 U/ml penicillin and 100
μg/ml streptomycin (Life Technologies, Inc., Carlsbad, CA,
USA) at 37°C under an atmosphere of 95% air and 5% CO2.
Cells were routinely subcultured every two to three days and all
cells used for experimental procedures were in the logarithmic
growth phase.
Small interfering RNA (siRNA)
transfections
IGF-IR siRNAs were obtained from Shanghai Gemma
pharmaceutical technology Co., Ltd (Shanghai, China). IGF-IR siRNA
was synthesized using the primer 5′-GCATGGTAGCCGAAGATTT-3′.
Lipofectamine® 2000 was diluted dropwise into RPMI 1640
and incubated at room temperature for 5 min. IGF-IR siRNA was then
added to the diluted Lipofectamine® 2000 and incubated
for 20 min. Following 48 h of transient transfection, cells were
analyzed using western blot analysis.
Cell viability assay
Cell viability was measured using an MTT assay.
Cells were seeded at 3×104/well in 96-well plates and
incubated overnight. Cells were then exposed to increasing doses of
cetuximab (0.01, 0.1, 1.0 and 10 μg/ml) for 24 h; following
which, 25 μl MTT solution (5 mg/ml) was added to each well
and the cells were incubated for 4 h at 37°C. The cell culture
medium was then removed and the cells were lyzed in 200 μl
dimethylsulphoxide. Optical density was measured at 570 nm using a
microplate reader (Bio-Rad 550; Bio-Rad Laboratories, Hercules, CA,
USA).
Western blot analysis
Cells were washed twice with ice-cold
phosphate-buffered saline and solubilized in 1% Triton lysis buffer
[1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA); 50 mM
Tris-Cl, pH 7.4; 150 mM NaCl; 10 mM EDTA; 100 mM NaF (all purchased
from Sinopharm Chemical Reagent, Shanghai, China); 1 mM
Na3VO4; 1 mM phenylmethylsulfonyl fluoride;
and 2 μg/ml aprotinin (all purchased from Sigma-Alrich)] on
ice and then quantified using the Lowry method (35). Cell lysate proteins were separated
using SDS-PAGE and electrophoretically transferred onto a
nitro-cellulose membrane (Immoblin-P; Millipore, Bedford, MA, USA).
Membranes were blocked using 5% skimmed milk in trimethyl benzene
sulfonyl tetrazole buffer (10 mM Tris-C1, pH 7.4; 150 mM NaCl; 0.1%
Tween 20; all purchased from Sinopharm Chemical Reagent) at room
temperature for 2 h and incubated with anti-EGFR, anti-IGF-IR,
anti-c-Src, anti-ERK1/2, anti-Akt, anti-β-Actin,
anti-phosphor-EGFR(Tyr1068), anti-phospho-IGF-IR (Tyr1131), anti-
phosphor-Src (Y416), anti-phospho-ERK 1/2 (Tyr202/Tyr204) or
anti-phospho-Akt (Ser473) primary antibodies at 4°C overnight. The
secondary anti-rabbit or mouse monoclonal antibodies (Santa Cruz
Biotechnology, Inc.) antibodies (dilution, 1:800) were then added
for 30 min at room temperature. Proteins were detected using an
enhanced chemiluminescence reagent (SuperSignal Western Pico
Chemiluminescent Substrate; Pierce Biotechnology, Rockford, IL,
USA) and visualized using the Electrophoresis Gel Imaging Analysis
System (DNR Bio-Imaging Systems, Ltd., Jerusalem, Israel).
Colony-forming assay
In brief, 300 cells per well were seeded onto
12-well plates. Following adherence to the plates, cells were
exposed to 10 Ig/ml cetuximab, PP2 and OSI-906. On day 14, clones
were air dried without RPMI-1640, then stained for 10 min with
Giemsa stain (Sigma-Aldrich). The clones were then washed with
running water and air dried again. Clones in each well were counted
and images were captured using inverted microscopy (M021; Olympus,
Tokyo, Japan).
Statistical analysis
All experiments were performed in triplicate. Values
are expressed as the mean ± standard deviation. Statistical
comparisons were made by Student’s t-test. SPSS 16.0 software was
used for statistical analysis (International Business Machines,
Armonk,NY, USA) and P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Gastric cancer cell lines are resistant
to cetuximab
In order to evaluate the sensitivity of gastric
cancer cell lines to cetuximab, SGC7901 and MGC803 cells were
treated with increasing concentrations of cetuximab (0.01, 0.1, 1
and 10 μg/ml) for 24, 48 and 72 h. Following treatment with
cetuximab, the two cell lines exhibited minimal growth inhibition
(<10%), which therefore indicated that the cells were
cetuximab-resistant (Fig. 1A) and
the maximal dose of 10 μg/ml cetuximab was therefore used
for the subsequent experiments. As shown in Fig. 1B, the colony forming ability of
gastric cancer cells was not affected by cetuximab treatment.
Furthermore, in order to determine whether cetuximab had a role in
blocking EGFR tyrosine kinase activation, the effect of cetuximab
treatment on EGFR, ERK and Akt phosphorylation was examined. Cells
were incubated with 10 μg/ml cetuximab for 2, 6 and 24 h.
The results demonstrated a marked decrease in EGFR and ERK
phosphorylation; however, Akt phosphorylation remained unchanged
(Fig. 1C). In addition, following
a mutation analysis of K-ras (codons 12 and 13) and
BRAF (exon 15, V600E) genes, no point mutations were
observed in the two cell lines. This therefore indicated that
cetuximab resistance was not associated with the mutation of these
genes (Fig. 1D). Overall, these
results suggested that an alternative pathway mediated cetuximab
resistance via activation of Akt in gastric cancer cells.
Cetuximab induces activation of IGF-IR
and Src in gastriccancer cells
SGC7901 and MGC803 cells were exposed to 10
μg/ml cetuximab for 0.5, 2, 6, 16 and 24 h. Western blot
analysis revealed that IGF-IR phosphorylation was notably increased
in the two cell lines, with peak activation detected at 6 h.
Increased Src phosphorylation was also observed in MGC803 and
SGC7901 cells, with peak activation detected at 6 and 16 h,
respectively (Fig. 2). This
suggested that cetuximab may have induced the activation of IGF-IR
and Src in gastric cancer cells.
Inhibition of IGF-IR activation or
expression increases sensitivity of gastric cancer cells to
cetuximab and reduces Src phosphorylation
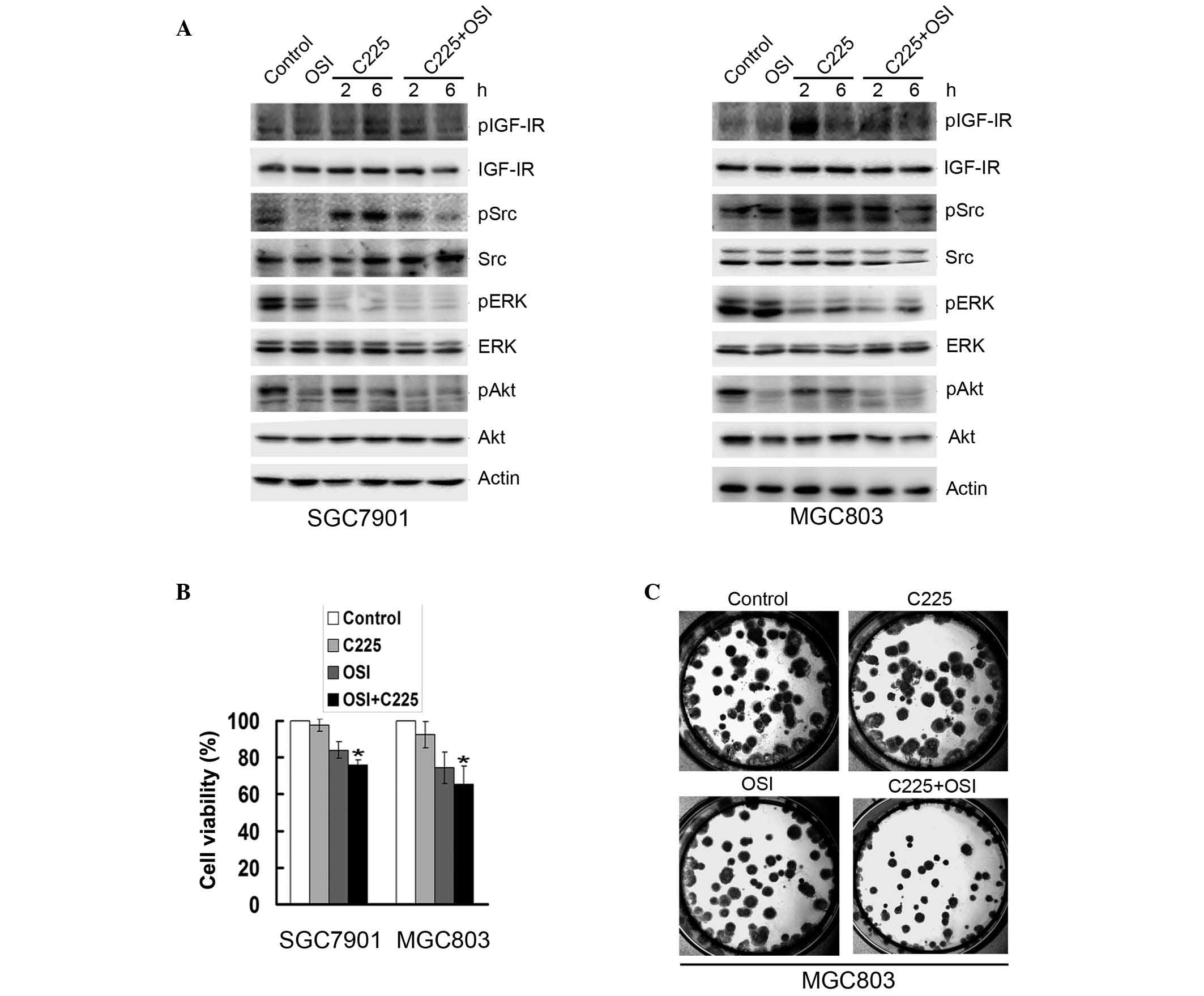
In order to determine whether IGF-IR signaling
induced cetuximab resistance, SGC7901 and MGC803 cells were treated
with 10 μg/ml cetuximab in combination with the tyrosine
kinase dual insulin receptor and IGF-IR inhibitor OSI-906 (10
μM) for 2 and 6 h. OSI-906 inhibited the cetuximab-induced
phosphorylation of Src, IGF-IR and Akt in the two cell lines;
however, ERK activation was not altered (Fig. 3A). Cetuximab treatment in
combination with OSI-906 decreased cell viability in SCG7901 and
MGC803 cells, respectively, by ~25 and 27% compared to treatment
with cetuximab alone (Fig. 3B).
Furthermore, a colony-forming assay of MGC803 cells revealed that
treatment with cetuximab in combination with OSI-906 produced fewer
and smaller colonies than treatment with cetuximab alone (Fig. 3C).
The effect of downregulated IGF-IR gene expression
on downstream signaling in gastric cancer cells was examined using
IGF-IR-specific siRNAs. As shown in Fig. 4A, western blot analysis was used to
confirm the knockdown of IGF-IR. Following exposure to 10
μg/ml cetuximab for 2 h, IGF-IR-depleted cells exhibited
reduced expression of phosphorylated IGF-IR, Src and Akt; however,
ERK phosphorylation remained unchanged (Fig. 4A). Cells transfected with IGF-IR
siRNAs demonstrated significantly reduced survival rates compared
to that of the control cells following exposure to cetuximab for 48
h (Fig. 4B). These results
therefore indicated that cetuximab-induced IGF-IR activation was
responsible for cetuximab resistance and that Src acted downstream
of IGF-IR in gastric cancer cell lines.
 | Figure 4Knockdown of IGF-IR expression
reduces cetuximab-induced Src and Akt phosphorylation as well as
enhances cellular proliferation inhibition rates in gastric cancer
cells. SGC7901 and MGC803 cells were transiently transfected with
IGF-IR siRNAs for 48 h, followed by 10 μg/ml cetuximab for 2
h. (A) Western blot analysis was used to detect the phosphorylation
of IGF-IR, Src, ERK and Akt. (B) Cell viability was assessed using
an MTT assay. *P<0.05, IGF-IR siRNA cells vs. NS
control. IGF-IR, insulin-like growth factor receptor 1; Src,
steroid receptor co-activator; ERK, extracellular signal-related
kinase; p, phosphorylated; C225, cetuximab; siRNA, small
interfering RNA; NS, non-silenced. |
Inhibition of Src restores cetuximab
sensitivity and represses IGF-IR phosphorylation in gastric cancer
cells
In order to investigate the association between Src
and IGF-IR, gastric cancer cells were pretreated with the Src
inhibitor PP2 (10 μM) alone or in combination with cetuximab
for 2 and 6 h. Activation of IGF-IR was then assessed using western
blot analysis. The results revealed that following treatment with
PP2, cetuximab-mediated IGF-IR phosphorylation was markedly
decreased (Fig. 5A). In addition,
gastric cancer cell viability was significantly reduced following
cetuximab treatment in combination with PP2 compared to that of
cetuximab treatment alone (Fig.
5B). Furthermore, the combination treatment reduced colony
formation in MGC803 cells relative to that of treatment with
cetuximab alone (Fig. 5C). These
results therefore showed that cetuximab-induced activation of
IGF-IR was inhibited following the PP2-induced inhibition of Src
activation, indicating that there may be a positive feedback loop
between IGF-IR and Src.
Discussion
Numerous studies have confirmed that the primary
mechanism of cetuximab resistance was via K-ras and
BRAF gene mutations (36–39).
In addition, cetuximab-sensitive gastric cancer cell lines were
reported to significantly reduce EGFR activation following
cetuximab treatment compared with cetuximab-resistant cells
(40). Another study demonstrated
that cetuximab failed to inhibit phosphorylation of EGFR pathways
in a cetuximab-resistant head and neck squamous cell cancer cell
line (41). The results of the
present study indicated that cetuximab resistance occurred in
gastric cancer SGC7901 and MGC803 cells expressing wild-type K-ras
and BRAF. However, these two cell lines exhibited reduced
activation of EGFR and ERK following cetuximab exposure, whereas
Akt activation was not affected. It was therefore suggested that
other pathways may be involved in Akt activation, thereby mediating
cetuximab resistance in gastric cancer cells.
It is widely accepted that EGFR is able to
cross-talk with other signaling factors (42–45).
A recent study demonstrated that tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) was able to activate the EGFR
pathway during TRAIL-induced apoptosis in gastric cancer cells
(46). Morgillo et al
(47) reported that the EGFR
tyrosine kinase inhibitor erlotinib induced heterodimerization of
EGFR/IGF-IR, with activation of IGF-IR and its downstream mediator
Akt in NSCLC cells; in addition, overexpression of IGF-IR has also
been observed in numerous types of human cancers (48) and was shown to be involved in
cisplatin resistance (49).
Furthermore, it was reported that baseline activation of IGF-IR was
correlated with cetuximab resistance (29). The present study found that
baseline levels of phosphorylated IGF-IR in gastric cancer cells
were not increased; however, following exposure to cetuximab there
was a gradual increase in levels of IGF-IR phosphorylation.
Furthermore, treatment with the IGF-IR inhibitor OSI-906 or IGF-IR
siRNAs inhibited activation of IGF-IR and Akt as well as increased
the sensitivity of gastric cancer cells to cetuximab. This
therefore indicated that cetuximab-induced IGF-IR and Akt
activation were involved in cetuximab resistance in gastric
cancer.
In order to further investigate the regulation of
cetuximab-induced IGF-IR activation, Scr activation was then
assessed in the present study. Peterson et al (50) reported that IGF-IR was a substrate
for v-Src. Src activation was found to occur upstream of IGF-IR
transactivation as well as stimulate IGF-dependent proliferation in
HEK293 cells and pancreatic carcinoma cells (30,51).
By contrast, it was reported that IGF induced Src activation in
vascular smooth muscle cells (52). Therefore, the upstream and
downstream association between Src and IGF-IR required further
elucidation. In the present study, cetuximab was shown to
simultaneously induce the activation of IGF-IR and Src. In turn,
inhibition of IGF-IR activation prevented the activation of Src,
while inhibition of Src activation inhibited the activation of
IGF-IR. This therefore provided evidence for a positive feedback
loop between IGF-IR and Src. Furthermore, inhibiting the activation
of IGF-IR as well as Src improved gastric cancer-cell sensitivity
to cetuximab, therefore indicating that cetuximab induced the
activation of IGF-IR and Src, which resulted in cetuximab
resistance in SGC7901 and MGC803 gastric cancer cells.
In conclusion, the results of the present study
demonstrated that cetuximab blocked EGRF while concurrently
inducing activation of IGF-IR and Src. Evidence was provided for a
positive feedback loop between Src and IGF-IR, which activated the
Akt signaling pathway downstream of EGFR, therefore mediating
cetuximab resistance in gastric cancer cells. These present study
contributed evidence towards an explanation for the mechanisms
underlying cetuximab resistance in gastric cancer cells that retain
wild-type K-ras and BRAF genes. In addition, the
results of the present study indicated that inhibition of IGF-IR
activation may be an effective mechanism by which cetuximab
sensitivity may be enhanced in order to improve the effectiveness
of combination chemotherapy in gastric cancer patients.
Acknowledgments
The present study was supported by grants from the
Chinese National Foundation of National Sciences (nos. 81201802,
81172369, 81172198, 81372485 and 81372546), Specialized Research
Fund for the Doctoral Program of Higher Education (nos.
20102104120008 and 20112104110005) and National Science and
Technology Major Project (no. 2013ZX09303002).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Custem E, Moiseyenko VM, Tjulandin S,
et al V325 Study Group: Phase III study of docetaxel and cisplatin
plus fluorouracil compared with cisplatin and fluorouracil as
first-line therapy for advanced gastric cancer: a report of the
V325 Study Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar
|
|
3
|
Cunningham D, Starling N, Rao S, et al
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang YK, Kang WK, Shin DB, et al:
Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as
first-line therapy in patients with advanced gastric cancer: a
randomised phase III noninferiority trial. Ann Oncol. 20:666–673.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang YJ, Van Custem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohtsu A, Shah MA, Van Custem E, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: a randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gómez-Martin C, Garralda E, Echarri MJ, et
al: HER2/neu testing for anti-HER2-based therapies in patients with
unresectable and/or metastatic gastric cancer. J Clin Pathol.
65:751–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Custem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009. View Article : Google Scholar
|
|
11
|
Specenier P and Vermorken JB: Cetuximab in
the treatment of squamous cell carcinoma of the head and neck.
Expert Rev Anticancer Ther. 11:511–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pirker R, Pereira JR, Szczesna A, et al
FLEX Study Team: Cetuximab plus chemotherapy in patients with
advanced non-small-cell lung cancer (FLEX): an open-label
randomised phase III trial. Lancet. 373:1525–1531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pinto C, Di Fabio F, Barone C, et al:
Phase II study of cetuximab in combination with cisplatin and
docetaxel in patients with untreated advanced gastric or
gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J
Cancer. 101:1261–1268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KH, Lee JS, Suh C, et al:
Clinicopathologic significance of the K-ras gene codon 12 point
mutation in stomach cancer. An analysis of 140 cases. Cancer.
75:2794–2801. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moehler M, Mueller A, Trarbach T, et al
German Arbeitsgemeinschaft Internistische Onkologie: Cetuximab with
irinotecan, folinic acid and 5-fluorouracil as first-line treatment
in advanced gastroesophageal cancer: a prospective multi-center
biomarker-oriented phase II study. Ann Oncol. 22:1358–1366. 2011.
View Article : Google Scholar
|
|
17
|
Lordick F, Luber B, Lorenzen S, et al:
Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line
metastatic gastric cancer: a phase II study of the
Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer.
102:500–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinto C, Di Fabio F, Siena S, et al: Phase
II study of cetuximab in combination with FOLFIRI in patients with
untreated advanced gastric or gastroesophageal junction
adenocarcinoma (FOLCETUX study). Ann Oncol. 18:510–517. 2007.
View Article : Google Scholar
|
|
19
|
Lordick F, Kang YK, Chung HC, et al
Arbeitsgemeinschaft Internistische Onkologie and EXPAND
Investigators: Capecitabine and cisplatin with or without cetuximab
for patients with previously untreated advanced gastric cancer
(EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waddel T, Chau I, Cunningham D, et al:
Epirubicin, oxaliplatin, and capecitabine with or without
panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): a randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar
|
|
21
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplification leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bean J, Brennan C, Shih JY, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chakravarti A, Loeffler JS and Dyson NJ:
Insulin-like growth factor receptor I mediates resistance to
antiepidermal growth factor receptor therapy in primary human
glioblastoma cells through continued activation of phosphoinositide
3-kinase signaling. Cancer Res. 62:200–207. 2002.PubMed/NCBI
|
|
24
|
Ouban A, Muraca P, Yeatman T and Coppola
D: Expression and distribution of insulin-like growth factor-1
receptor in human carcinomas. Hum Pathol. 34:803–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pavelić K, Kolak T, Kapitanović S, et al:
Gastric cancer: the role of insulin-like growth factor 2 (IGF 2)
and its receptors (IGF 1R and M6-P/IGF 2R). J Pathol. 201:430–438.
2003. View Article : Google Scholar
|
|
26
|
Shiraishi T, Mori M, Yamagata M, et al:
Expression of insulin-like growth factor 2 mRNA in human gastric
cancer. Int J Oncol. 13:519–523. 1998.PubMed/NCBI
|
|
27
|
Baserga R: The IGF-I receptor in cancer
research. Exp Cell Res. 253:1–6. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuo Q, Shi M, Li L, et al: Development of
cetuximab-resistant human nasopharyngeal carcinoma cell lines and
mechanisms of drug resistance. Biomed Pharmacother. 64:550–558.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oligny-Longpré G, Corbani M, Zhou J, et
al: Engagement of β-arrestin by transactivated insulin-like growth
factor receptor is needed for V2 vasopressin receptor-stimulated
ERK1/2 activation. Proc Natl Acad Sci USA. 109:E1028–E1037. 2012.
View Article : Google Scholar
|
|
31
|
Hamzeh M and Robaire B: Androgens activate
mitogen-activated protein kinase via epidermal growth factor
receptor/insulin-like growth factor 1 receptor in the mouse PC-1
cell line. J Endocrinol. 209:55–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang S, Huang WC, Li P, et al: Combating
trastuzumab resistance by targeting SRC, a common node downstream
of multiple resistance pathways. Nat Med. 17:461–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ligęza J, Ligęza J and Klein A: Growth
factor/growth factor receptor loops in autocrine growth regulation
of human prostate cancer DU145 cells. Acta Biochim Pol. 58:391–396.
2011.
|
|
34
|
Mayer EL and Krop IE: Advances in
targeting SRC in the treatment of breast cancer and other solid
malignancies. Clin Cancer Res. 16:3526–3532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peterson GL: A simplification of the
protein assay method of Lowry et al. which is more generally
applicable. Anal Biochem. 83:346–356. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lièvre A, Bachet JB, Le Corre D, et al:
KRAS mutation status is predictive of response to cetuximab therapy
in colorectal cancer. Cancer Res. 66:3992–3995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Fiore F, Blanchard F, Charbonnier F, et
al: Clinical relevance of KRAS mutation detection in metastatic
colorectal cancer treated by Cetuximab plus chemotherapy. Br J
Cancer. 96:1166–1169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Nicolantonio F, Martini M, Molinari F,
et al: Wild-type BRAF is required for response to panitumumab or
cetuximab in metastatic colorectal cancer. J Clin Oncol.
26:5705–5712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brose MS, Volpe P, Feldman M, et al: BRAF
and RAS mutations in human lung cancer and melanoma. Cancer Res.
62:6997–7000. 2002.PubMed/NCBI
|
|
40
|
Heindl S, Eggenstein E, Keller S, et al:
Relevance of MET activation and genetic alterations of KRAS and
E-cadherin for cetuximab sensitivity of gastric cancer cell lines.
J Cancer Res Clin Oncol. 138:843–858. 2012. View Article : Google Scholar
|
|
41
|
Rebucci M, Peixoto P, Dewitte A, et al:
Mechanisms underlying resistance to cetuximab in the HNSCC cell
line: Role of AKT inhibition in bypassing this resistance. Int J
Oncol. 38:189–200. 2011.
|
|
42
|
Cordero JB, Stefanatos RK, Myant K, et al:
Non-autonomous crosstalk between the Jak/Stat and Egfr pathways
mediates Apc1-driven intestinal stem cell hyperplasia in the
Drosophila adult midgut. Development. 139:4524–4535. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cavallo RA, Cox RT, Moline MM, et al:
Drosophila Tcf and Groucho interact to repress Wingless signalling
activity. Nature. 395:604–608. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brantjes H, Roose J, van De Wetering M and
Clevers H: All Tcf HMG box transcription factors interact with
Groucho-related co-repressors. Nucleic Acids Res. 29:1410–1419.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hasson P and Paroush Z: Crosstalk between
the EGFR and other signalling pathways at the level of the global
transcriptional corepressor Groucho/TLE. Br J Cancer. 96(Suppl):
R21–R25. 2007.PubMed/NCBI
|
|
46
|
Xu L, Zhang Y, Liu J, et al:
TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in
lipid rafts antagonizes TRAIL-induced apoptosis in gastric cancer
cells. Eur J Cancer. 48:3288–3299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Morgillo F, Woo JK, Kim ES, et al:
Heterodimerization of insulin-like growth factor receptor/epidermal
growth factor receptor and induction of survivin expression
counteract the antitumor action of erlotinib. Cancer Res.
66:10100–10111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pollak M: Insulin-like growth factor
physiology and cancer risk. Eur J Cancer. 36:1224–1228. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cortés-Sempere M, de-Miguel MP, Pernía O,
et al: IGFBP-3 methylation-derived deficiency mediates the
resistance to cisplatin through the activation of the IGFIR/Akt
pathway in non-small cell lung cancer. Oncogene. 32:1274–1283.
2013. View Article : Google Scholar
|
|
50
|
Peterson JE, Kulik G, Jelinek T, et al:
Src phosphorylates the insulin-like growth factor type I receptor
on the autophosphorylation sites. Requirement for transformation by
src. J Biol Chem. 271:31562–31571. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Flossmann-Kast BB, Jehle PM, Hoeflich A,
et al: Src stimulates insulin-link growth factor I
(IGF-I)-dependent cell proliferation by increasing IGF-I receptor
number in human pancreatic carcinoma cells. Cancer Res.
58:3551–3554. 1998.PubMed/NCBI
|
|
52
|
Lieskovska J, Ling Y, Badley-Clarke J and
Clemmons DR: The role of Src kinase in insulin-like growth
factor-dependent mitogenic signaling in vascular smooth muscle
cells. J Biol Chem. 281:25041–25053. 2006. View Article : Google Scholar : PubMed/NCBI
|