Introduction
Venous thromboembolism (VTE) is a severe health
problem with pathogenic contributions from genetic and
environmental components, which have been poorly investigated in
non-European populations and those outside North America (1,2).
Although ethnic differences in the occurrence of VTE have been
reported for several years and the risk of genetic and
environmental factors is known to vary depending on ethnicity, the
impact of these differences on the incidence of VTE remains to be
clarified (3). Outside North
America and Europe, data concerning the risk factors for VTE
incidence are limited. In addition, 30% of VTE incidence remains
unexplained, however, in previous years studies have focused on
inflammatory factors, their gene polymorphisms and the progression
of VTE (4–8).
Interleukin-6 (IL-6) is a circulating cytokine known
to be affected by a number of environmental and genetic factors,
which subsequently affects individual susceptibility to VTE. IL-6
regulates the inflammatory reaction in vessel walls, accelerates
bone restoration and is critical in atherogenesis and thrombosis
(9–13). IL-6 (572G/C) polymorphisms are
associated with elevated IL-6 production or protein expression
in vivo and in vitro. The C allele of -572G/C is
associated with increased concentrations of IL-6 (14). Elevated levels of IL-6 are
associated with a 2-fold risk of developing VTE and recurrent VTE
(15). Consequently, it was
hypothesized that IL-6 genetic polymorphisms may represent a
candidate gene for VTE. Empirical data from different ethnic
populations have indicated that genetic factors are closely
associated with the development of VTE (16). The frequency of the -572C allele is
reported to be 0.0055 in the English population and 0.0019 in the
Scottish population (17),
compared with a frequency of 0.15 in the Indian population
(18) and 0.419 in the Han Chinese
population (9). Zheng et al
reported that the -572G/C polymorphism of the IL-6 gene may
possibly lead to the development of coronary heart disease
(19). The total Uyghur population
was 8.4 million in 2000 and 99.4% of the total population of
Uyghurs live in the Xinjiang Uyghur Autonomous Region, which is
situated in the center of Asia (20). As stated in our preliminary
research, the main reason for the difference in prevalence of VTE
is possibly the difference in diet between the Chinese Han and
Uyghur populations. Compared with the Han population, the Uyghur
population consumed more pasta, meat and milk products. Thus, the
prevalence of obesity, particularly central obesity in the Uyghur
population was higher compared with that in the Han Chinese
population (21). There are >13
ethnic groups living in this area. A number of ethnicities,
including the Uyghur (48%), Han (38%) and Kazakh people (7%), are
present in this area (22). Due to
the religious beliefs of the Uyghur population, lifestyle, customs
and marital practices are different from other ethnic groups in
China. This population has lived in a relatively fixed location for
significant time periods, leading to the development of a
population with a number of stable genetic features. Multiple
studies have also identified that the Uyghur allele distribution is
distinctly different from that of other populations in Northwest
China, including Han, Hui and Mongolian, which therefore indicates
that the prevalence of VTE differs between these populations
(23–26). Several studies have reported that
the main risk factor for coronary artery disease (27), hypertension (28) and atrial fibrillation (29) is different among these ethnic
groups in Xinjiang. To date, to the best of our knowledge, no data
are available regarding the mutations in the IL-6 gene hypothesized
to be responsible for the prevalence of VTE in the Chinese Uyghur
population. Therefore, screening for possible mutations and
polymorphisms of the IL-6 gene was performed and the association
between the genotypes of this gene and the progression of VTE in
the Chinese Han and Chinese Uyghur population was assessed.
Materials and methods
Study population
The present study included two patient populations
(Han and Uyghur) with documented VTE. A total of 160 Han patients
and 86 Uyghur patients diagnosed with VTE at the First Affiliated
Hospital of Xinjiang Medical University (Xinjiang, China) between
2008 and 2010 were recruited (Table
I). The Han and Uyghur populations were termed the first and
second VTE group, respectively. The number of patients with
pulmonary embolism (PE) alone, deep vein thrombosis (DVT) alone and
PE-DVT were 101, 23 and 36 in the first VTE group and 40, 18 and 28
in the second VTE group, respectively. Patients with PE were
diagnosed according to the results of pulmonary CT angiography,
pulmonary artery magnetic resonance imaging and autopsy. Patients
with DVT were diagnosed according to the results of compressed
ultrasound and venography of veins in the lower limbs. The patients
eligible for inclusion criteria, were successively selected using
standardized criteria (29).
Patients in the control group at the time of enrollment did not
have a personal or family history of VTE. Systemic arterial
hypertension was defined as a systolic blood pressure of ≥140 mmHg
and/or a diastolic blood pressure of ≥90 mm Hg, on at least two
different occasions or patients receiving antihypertensive
treatment. Patients that recorded habitual smoking in the previous
6 months were considered to be current smokers. For each VTE case
group, healthy participants matched for ethnicity, gender and age
were used as the controls. The control subjects were recruited from
the Medical Centre of the First Affiliated Hospital of Xinjiang
Medical University. The present study was authorized by the Ethics
Committee of the First Affiliated Hospital of Xinjiang Medical
University. Written informed consent was obtained from all
participants.
 | Table IClinical characteristics and blood
parameters measured in the study subjects of the Uyghur and Han
population. |
Table I
Clinical characteristics and blood
parameters measured in the study subjects of the Uyghur and Han
population.
| Characteristic | Uyghur
| Han
|
|---|
| VTE (n=86) | Control (n=122) | P-value | VTE (n=160) | Control (n=170) | P-value |
|---|
| Male (%) | 41 (47.67) | 64 (52.46) | 0.574 | 76 (47.50) | 91 (53.53) | 0.322 |
| Age (years) | 51.61±13.73 | 53.52±13.64 | 0.386 | 57.41±13.25 | 55.82±11.83 | 0.291 |
| Smoking (%) | 43 (50) | 43 (35.25) | 0.045 | 91 (56.88) | 65 (38.24) | 0.001 |
| Drinking (%) | 55 (63.95) | 86 (70.49) | 0.000 | 70 (43.75) | 53 (31.18) | 0.023 |
| Hypertension (%) | 24 (27.91) | 28 (22.95) | 0.422 | 102 (63.75) | 90 (52.94) | 0.058 |
| Diabetes (%) | 18 (20.93) | 14 (11.48) | 0.079 | 33 (20.63) | 23 (13.53) | 0.106 |
| Obesity (%) | 45 (52.33) | 38 (31.15) | 0.004 | 70 (43.75) | 53 (31.18) | 0.023 |
| BMI (kg/m2) | 27.61±3.13 | 29.32±2.76 | 0.006 | 25.90±3.63 | 24.52±2.74 | 0.000 |
| Cr
(μmol/l)a | 71 (55, 93) | 87 (78, 98) | 0.531 | 81 (63,100) | 88 (74, 99) | 0.898 |
| UA
(μmol/l)a | 251 (177, 336) | 312 (251, 375) | 0.314 | 317 (208,371) | 315 (272,
354.) | 0.548 |
| TG
(μmol/l)a | 1.21
(0.85,1.51) | 1.86 (1.18,
2.35) | 0.000 | 1.39
(0.90,1.73) | 1.60 (1.14,
2.03) | 0.001 |
| TC
(μmol/l) | 3.81±1.27 | 4.16±1.91 | 0.046 | 4.16±1.0 | 3.81±0.92 | 0.002 |
| HDL
(μmol/l) | 0.95±0.38 | 0.97±0.25 | 0.701 | 1.05±0.29 | 0.90±0.51 | 0.003 |
| LDL-C
(μmol/l) | 2.43±0.78 | 2.50±0.76 | 0.561 | 2.47±0.77 | 2.54±1.06 | 0.499 |
| CK (U/l)a | 51.21 (29.62,
72.11) | 68.01(45.52,
91.58) | 0.412 | 53.55 (28.53,
75.22) | 65.08 (54.31,
82.53) | 0.541 |
| CK-MB (U/l)a | 10.02 (7.36,
14.81) | 11.91 (8.72,
15.63) | 0.665 | 9.76
(5.51,14.91) | 12.95
(8.73,15.12) | 0.661 |
| FIB (g/l) | 6.47±2.74 | 3.61±1.22 | 0.054 | 6.61±4.52 | 3.52±1.51 | 0.067 |
| LDH (U/l)a | 220 (155, 273) | 164 (127,187) | 0.412 | 222 (179,269) | 137(121,161) | 0.871 |
| ALP (U/l)a | 78.31 (68.14,
101.12) | 75.51 (67.01,
83.82) | 0.073 | 83.48 (62.24,
97.13) | 63.52(52.71,
80.28) | 0.061 |
| Leptin
(μg/l) | 11.81±3.26 | 3.81±1.07 | 0.031 | 13.81±6.11 | 3.79±1.55 | 0.025 |
| IL-6 (pg/ml) | 61.14
(31.3–94.27) | 68.6
(39.22–106.94) | 0.002b | 65.1
(30.83–93.65) | 64.5
(40.44–112.83) | 0.002b |
| CRP (pg/ml) | 27.82
(21.12–36.43) | 21.60
(15.53–24.26) | 0.000b | 26.7
(21.23–35.14) | 22.56
(15.35–25.32) | 0.000b |
Blood sampling
Following overnight fasting for 12 h, venous blood
was collected in ethylenediaminetetraacetic acid. The samples were
promptly centrifuged at 1,006.2 × g for 10 min following collection
and stored at −20°C. The lymphocyte secretion medium was used to
isolate white blood cells. Phenol chloroform was used for the
extraction of genomic DNA.
Biochemical determinations
Assessment of serum levels of total cholesterol,
triglyceride (TG), high-density lipoprotein cholesterol (HDL-C),
platelet count, uric acid and creatine kinase (CK) was performed
using approved and regular procedures at the Central Laboratory of
First Affiliated Hospital of Xinjiang Medical University. An enzyme
linked immunosorbent assay (ELISA) approach using a microplate
reader (Bio-Rad, Hercules, CA, USA) was used for detecting the
level of C-reactive protein (CRP), D-dimer (DD), plasminogen
activator inhibitor-1 (PAI-1) and leptin using a human IL-6 ELISA
kit, human CRP ELISA kit, human DD ELISA kit, human PAI-1 ELISA kit
and human leptin ELISA kit, respectively, in the VTE and control
groups. All ELISA kits were purchased from Groundwork Biotechnology
Diagnosticate (San Diego, CA, USA).
A total of 401 VTE cases and 379 controls were
investigated for the -597G/C genotyping and -572G/C single
nucleotide polymorphisms (SNPs) in the promoter of the IL-6 gene
were analyzed using polymerase chain reaction (PCR) restriction
fragment length polymorphism (Fig.
1). PCR was administered utilizing the following primers
purchased from TIB Molbiol (Berlin, Germany): IL-6 597G/A, forward
5′-AAAGGAGTCACACACTCC-3′ and reverse 5′-GCGTTCCAGTTAATTTGTATTTG-3′
and IL-6 572C/G, forward 5′-AAAGGAGTCACACACTCC-3′ and reverse
5′-GCGTTCCAGTTAATTTGTATTTG-3′. The reaction was performed in a 10
μl sample volume using 0.25 μg/ml DNA as a template,
50 mmol/l MgCl2, 10 mmol/l dNTPs (Boehringer Ingelheim,
Ingelheim, Germany), 5 U/μl Taq DNA polymerase (CinnaGen,
Tehran, Iran) and 10 pM of primers.
The amplification parameters for -597G/C and -572C/G
were 95°C for 5 min and then five cycles of 95°C for 30 sec, 55°C
for 30 sec and 72°C for 30 sec followed by 30 cycles of 95°C for 30
sec, 58°C for 30 sec and 72°C for 30 sec. The samples were
incubated at 72°C for an additional 5 min to complete the final
extension step following the final cycle. The PCR products were
digested with restriction endonucleases obtained from Fermantes
(Vilnius, Lithuania) to determine the genotype of every subject.
FokI was used for digestion of the PCR products containing
position -597G/C and BrsBI was used for position -572C/G.
Subsequently, the DNA fragments were separated using a 3% agarose
gel for the two samples and were detected by ethidium bromide
staining. The PCR product size was 198 bp for the -597G/C
polymorphism. The PCR product containing the G allele was digested
into two fragments of 198 and 175 bp (Fig. 2), while the PCR product containing
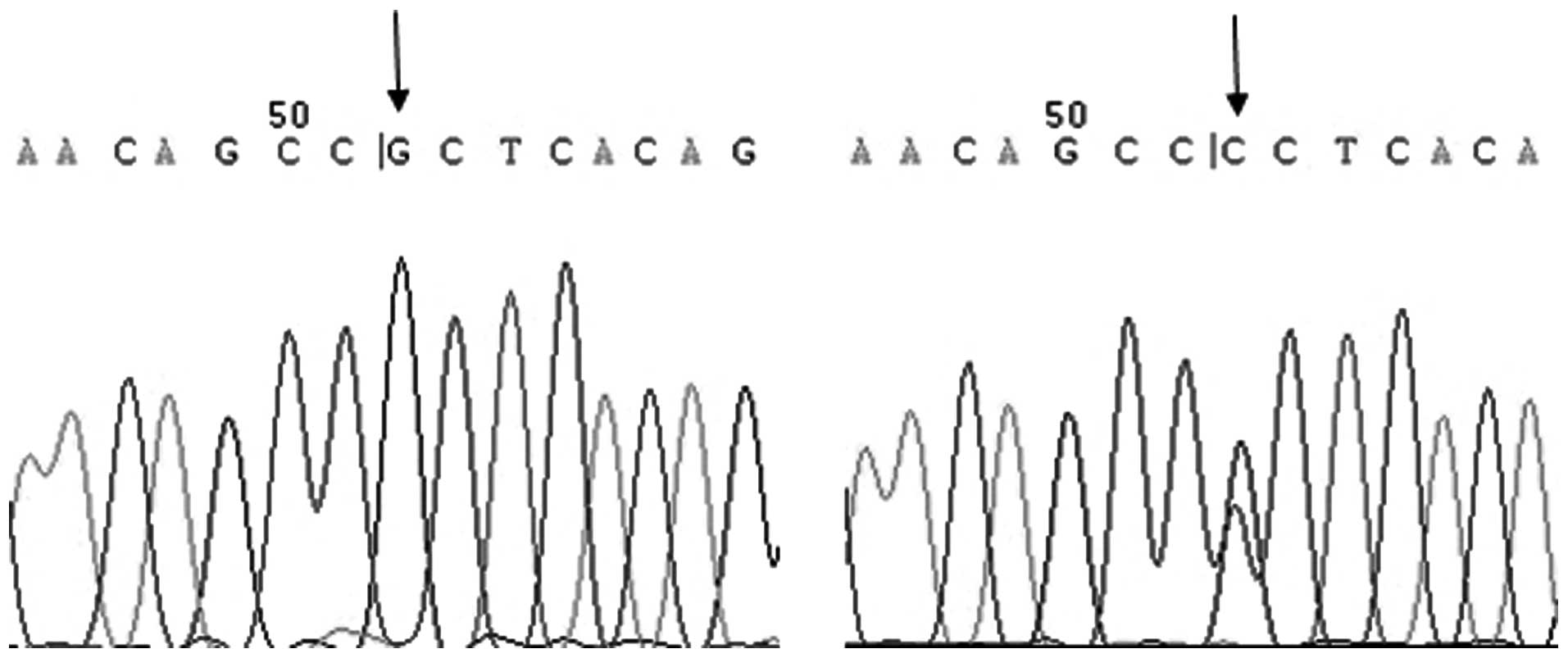
the C allele was not able to be cut by the enzyme. For -572 G/C,
the PCR product size was 232 bp (Fig.
3). The PCR product containing the C allele was digested into
two fragments of 149 and 83 bp. The PCR product containing the G
allele was unaltered.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software for Windows (SPSS, Inc., Chicago, IL, USA). Complete
genotype frequencies were assessed in the two groups separately by
using the Hardy-Weinberg equilibrium. While IL-6 and CRP values did
not conform to a normal distribution, the Mann-Whitney U test and
the Kruskal-Wallis test were applied for comparison between groups.
Continuous variables were correlated applying an unpaired t-test
and discrete variables with the χ2 test. The IL-6 and
CRP values are expressed as the median and range and the remaining
variables are expressed as the mean ± standard deviation or a ratio
(%). Genotype and allele frequency differences were distinguished
among the patient and control groups using the χ2 test,
where appropriate. A logistic regression model was used to
calculate odds ratios (ORs) and their 95% confidence intervals (95%
CI) to assess the strength of the association between individual
genetic polymorphisms and the risk of VTE. All regression analyses
were adjusted for potential confounders. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical characteristics of the
patients
The main characteristics of the patients and
controls are summarized in Table
I. No significant differences were identified between the two
groups with regards to the distribution of age, gender, systolic
blood pressure, diastolic blood pressure or the levels of CK, CK-MB
and HDL-C (all P>0.05). However, smoking and levels of IL-6,
CRP, leptin, TG, low-density lipoprotein cholesterol (LDL-C) and
body mass index (BMI) were significantly higher in the case group
compared with the control group (all P<0.05).
Genotypes and allele distribution of VTE
patients and control participants in the two populations
The genotype and allele frequencies of IL-6 in
controls and patients with VTE are shown in Table II. The distribution of genotypes
was not observed to be significantly deviated from the
Hardy-Weinberg equilibrium in the VTE and control groups (all
P>0.05). The -572C/G promoter polymorphisms of the IL-6 gene
contained CC, CG and GG genotypes. The gene frequencies of the
-572C/G promoter polymorphisms in the Han population for CC, CG and
GG were 41.25, 46.25 and 12.50%, respectively in the VTE group,
while it was 26.47, 50.00% and 23.53%, respectively in the control
group. The frequencies of C and G alleles of IL-6 -572C/G were
64.37 and 35.63%, respectively, in the VTE group and 51.47 and
48.53%, respectively, in the control group in the Han population.
Significant differences were identified in the -572C/G promoter
polymorphism genotypes and allele distribution between the VTE
group and control group in the Han population (P<0.05). The gene
frequencies of the -572C/G promoter polymorphism in the Uyghur
population for CC, CG and GG were 40.70, 45.35 and 13.95%,
respectively in the VTE group, while it was 25.41, 48.36 and
26.23%, respectively in the control group. The frequencies of C and
G alleles of IL-6 -572C/G were 63.37 and 36.63% in the VTE group
and 49.59 and 50.41% in the control group in the Uyghur population.
There was a significant difference in the -572C/G promoter
polymorphisms between the VTE and control group in the Uyghur
population (P<0.05). However, no significant difference was
identified in the -572C/G promoter polymorphism allele distribution
between the VTE group and the control group in the Uyghur
population (P<0.05). In patients with the -597G/A polymorphism,
individuals all carried the GG and GA type; AA genotypes were not
detected (Fig. 2).
 | Table IIGenotypes and allele distribution of
the VTE patients and control participants. |
Table II
Genotypes and allele distribution of
the VTE patients and control participants.
| Risk factors | VTE | Control | P-value | OR (95% CI) |
|---|
| IL-6 gene variants
in the Han population | 160 | 170 | | |
| -572C/G Alleles (n,
%) |
| C | 206 (64.37) | 175 (51.47) | | |
| G | 114 (35.63) | 165 (48.53) | 0.001a | 1.704
(1.247–2.328) |
| Genotypes (n,
%) | | | | |
| CC | 66 (41.25) | 45 (26.47) | | |
| CG | 74 (46.25) | 85 (50.00) | 0.047b | 1.685
(1.031–2.752) |
| GG | 20 (12.50) | 40 (23.53) | 0.001c | 2.933
(1.521–5.659) |
| IL-6 gene variants
in the Uyghur population | 86 | 122 | | |
| -572C/G Alleles (n,
%) |
| C | 109 (63.37) | 121 (49.59) | | |
| G | 63 (36.63) | 123 (50.41) | 0.343 | 1.347
(0.785–2.312) |
| Genotypes (n,
%) |
| CC | 35 (40.70) | 31 (25.41) | | |
| CG | 39 (45.35) | 59 (48.36) | 0.111 | |
| GG | 12 (13.95) | 32 (26.23) | 0.010d | 3.011
(1.325–6.842) |
Identification of independent risk
factors for VTE
Following adjustment for the following confounding
factors of age, gender, hypertension, family history,
hyperlipidemia and smoking, a multiple logistic regression model
revealed four independent factors (Tables III and IV), including CRP (OR=1.079; 95%
CI=1.048–1.110; P<0.05), IL-6 (OR=1.005; 95% CI=1.033–1.010;
P<0.05), obesity (OR=0.381; 95% CI=0.187–0.796; P<0.05) and
IL-6 -572CC (OR=2.853; 95% CI=1.248–1.110; P<0.05) for VTE in
the Uyghur population. Regarding the VTE group in the Han
population the data revealed: BMI (OR=1.110; 95% CI=1.033–1.193;
P<0.05), CRP (OR=1.052; 95% CI=1.044–1.124; P<0.05), IL-6
(OR=1.079; 95% CI=1.041–2.115; P<0.05), IL-6 -572CC (OR=1.762;
95% CI=1.376–5.312; P<0.05), HDL-C (OR=0.381; 95%
CI=0.187–0.796; P<0.05). In addition, the CC homozygosity of the
IL-6 -572C/G gene was also an independent risk factor for VTE with
a 2.85-fold higher relative risk of developing VTE compared with GG
homozygotes in the Uyghur population (OR=2.853; 95% CI=1.248–6.241;
P<0.05). Multiple logistic regression analysis (Tables III and IV) revealed that in the CC homozygotes
of the IL-6 -572G/C polymorphism, CRP, IL-6, HDL-C and obesity were
independent risk factors for VTE in the Uyghur and Han populations
(P<0.05).
 | Table IIILogistic regression for the risk
factors of venous thromboembolism in the Uyghur population. |
Table III
Logistic regression for the risk
factors of venous thromboembolism in the Uyghur population.
| Risk factors | B | S.E. | Wald | P-value | OR | OR (95% CI) |
|---|
| IL-6 (-572CC) | 1.048 | 0.422 | 6.177 | 0.013 | 2.853 | 1.248–6.241 |
| CRP | 0.052 | 0.043 | 22.564 | 0.002 | 1.079 | 1.048–1.110 |
| IL-6 | 0.005 | 0.002 | 5.249 | 0.022 | 1.005 | 1.033–1.010 |
| BMI | −0.966 | 0.376 | 6.585 | 0.010 | 0.381 | 0.187–0.796 |
 | Table IVLogistic regression for the risk
factors of venous thromboembolism in the Han population. |
Table IV
Logistic regression for the risk
factors of venous thromboembolism in the Han population.
| Risk factors | B | S.E. | Wald | P-value | OR | OR (95% CI) |
|---|
| IL-6 (-572CC) | 1.158 | 0.221 | 6.377 | 0.013 | 1.762 | 1.376–5.312 |
| BMI | 1.511 | 0.507 | 6.731 | 0.001 | 1.110 | 1.033–1.193 |
| CRP | 0.076 | 0.015 | 26.892 | 0.000 | 1.052 | 1.044–1.124 |
| IL-6 | 0.321 | 0.034 | 7.893 | 0.030 | 1.079 | 1.041–2.115 |
Discussion
In the present study, SNPs of IL-6 were identified
to be associated with the occurrence of VTE. The inflammatory
markers CRP, IL-6 and leptin; hemostasis markers, fibrinogen (FIB),
DD and PAI-1 and traditional risk factors TG and LDL-C were
significantly higher in the VTE group in the Uyghur and Han
populations. An SNP was identified (-572G/C) and it was observed
that the minor allele of -572G/C (SNP in IL-6) had a higher
frequency in VTE patients compared with controls. To the best of
our knowledge, the present study is the first to demonstrate an
association between IL-6 and VTE in the Uyghur population.
The -572G/C and -597A/G polymorphisms were genotyped
and the association between IL-6 and VTE was assessed. The
frequency of the GC genotype of -572G/C was significantly increased
in VTE patients compared with in control subjects, not only in the
Han population but also in the Uyghur population. This indicated
that the risk of VTE was increased in the Uyghur and Han
populations with the G allele. Terry et al (30) was the first study to describe the
IL-6 -572 polymorphism, which is a substitution in the 5′ region of
the IL-6 promoter. This G to C substitution is close to a potential
glucocorticoid receptor element at position -557 to -552. The
polymorphism -572G/C has been revealed to be essential for IL-6
production, causing inflamed vascular walls and increasing the
production of cytokines, including IL-6, IL-1β and tumor necrosis
factor-α (TNF-α), which have major regulatory roles in the hepatic
synthesis of acute phase proteins, including FIB and the
development of thrombosis (9,11–13,19,31–34).
Several studies have demonstrated that patients with VTE are more
likely to have elevated levels of plasma IL-8, IL-6, monocyte
chemotactic protein-1 and TNF-α (10), that inflammation affects clotting
factor levels (35), that an
inflammatory gene is associated with VTE (36) and that acute inflammation does
contribute to VTE (37). The GG
and CC genotypes for IL-6-174G/C and matrix metallopeptidase-9
(MMP-9)-1562C/T polymorphisms, respectively, are associated with a
risk of DVT in cancer patients by inducing the release of IL-6 with
subsequent increases in MMP-9 (31). Inflammation is possibly necessary
for the initiation of venous thrombosis formation. IL-6 controls
CRP gene expression (32) and the
minor allele at position -572 (G allele) has been revealed to be
associated with increased IL-6 levels in inflammation (11). Circulating levels of CRP are under
regulation of secretion of IL-6. Therefore, the ability to produce
CRP and successive inflammation depends at least partially on the
capacity to produce IL-6. Together, these results provided evidence
for the varied effects of the IL-6 gene on plasma IL-6 levels in
VTE patients. In the present study, the mean CRP and IL-6 levels
were significantly higher in patients with VTE compared with the
controls in the Han and Uyghur populations, which suggested that
inflammation was more prominent among the VTE cases than among the
controls corresponding to the data of Kamphuisen et al
(33). As stated in the present
study, inflammation may affect VTE risk as a procoagulant state may
be affected by pro-inflammatory cytokines and chemokines,
stimulating monocytes to produce tissue factor (31). Therefore, the present findings
indicate that the levels of CRP and IL-6 may be valuable in
mediating activation of the coagulation and fibrinolytic process in
thrombosis.
Factors, including obesity, hypertension, diabetes,
smoking, hypercholesterolemia, cancer, surgery, immobilization,
pregnancy and the use of estrogens have been reported to affect the
pathogenesis of VTE (9–11,13,28,31).
In the present study, it was found that, following adjustment for
other risk factors, the CC genotype remained significant between
VTE patients and control subjects of the Han and Uyghur populations
with an OR of 1.762 (95% CI=1.376–5.312) for the Han population and
an OR of 2.853 (95% CI=1.248–6.241) for the Uyghur population,
respectively. In the significant but rather low OR in the Han
population in comparison with that of the Uyghur population, an
association was identified between IL-6 levels and VTE, which may
represent the residual part, independent of other confounding risk
factors. Gene polymorphisms in the IL-6 gene are known to affect
IL-6 production. Whether the existence of a G allele or the C
allele leads to enhanced IL-6 production remains to be elucidated.
A number of studies have identified that increased IL-6 production
is associated with the G allele, while others observed an
association with the C allele (10,34).
A further study demonstrated that the high concentration of IL-6 in
VTE patients was likely to be due to the presence of VTE rather
than a cause of VTE (12). The
results of the current study were in accordance with those
published by Mahemuti et al (13), which demonstrated an association
between IL-6 and CRP levels, as well as VTE and the CC homozygote
of the IL-6 -572C/G gene, which was an independent risk factor for
VTE. Only a weak statistical significance was identified, which may
be due to the small sample size of the Han population. The present
study reported for the first time, to the best of our knowledge, an
association between the IL-6 gene and VTE in the Uyghur population
in Xinjiang.
Leptin has also been demonstrated in vitro to
trigger coagulation by the upregulation of tissue factor (38). Obesity is also linked with higher
levels of leptin, an adipokine independently associated with a
raised risk for cardiovascular disease (38). Various causative factors and
pathological mechanisms have been suggested for the high occurrence
of thromboembolism in obesity. Obesity is known as a chronic,
low-grade inflammatory state, as evidenced by increased levels of
the pro-inflammatory cytokines IL-6 and TNF-α and acute phase
proteins, including CRP. This pro-inflammatory condition is
attenuated by weight loss. Besides its direct effects, inflammation
can indirectly lead to thrombosis by causing oxidative stress and
endothelial dysfunction. In a previous study, it was established
that leptin had effects on platelets and endothelial cells through
its functional receptor (38).
These effects were associated with a prothrombotic tendency. Leptin
concentrations used in the present experiments corresponded to that
of leptin in the circulation of obese individuals. Thus, it is
hypothesized that increased leptin may be a risk factor for
thrombosis in obese individuals (39) in accordance with the present
study.
In conclusion, the current study presented evidence
to suggest that the -572G/C SNP in the promoter region of IL-6 may
predict the risk of VTE in the Uyghur and Han populations in
Xinjiang. In the present sample of patients from Northern China,
VTE was associated with the GC genotype of the -572G/C SNP in the
IL-6 gene. The present study may have clarified the mechanism of
VTE and improved understanding of genetic variants and
disease-association studies. Undertaking genome wide association
studies in different populations certainly merits investigation.
The early identification, treatment and prevention of VTE,
including lifestyle changes and management for controlling VTE, are
major challenges.
Acknowledgments
This study was supported by the State Key Laboratory
Incubation Base of Xinjiang Major Diseases Research Fund (grant no.
SKLIB-XJMDR-2012-7).
References
|
1
|
Margaglione M and Grandone E: Population
genetics of venous thromboembolism, A narrative review. J Thromb
Haemost. 105:221–231. 2011. View Article : Google Scholar
|
|
2
|
Raskob GE, Silverstein R, Bratzler DW,
Heit JA, Heit JA and White RH: Surveillance for deep vein
thrombosis and pulmonary embolism: recommendations from a national
workshop. Am J Prev Med. 38:S502–S509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White RH, Zhou H and Romano PS: Incidence
of idiopathic deep venous thrombosis and secondary thromboembolism
among ethnic groups in California. Ann Intern Med. 128:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prandoni P and ten Cate JW: Epidemiology,
risk factors, and natural history of venous thromboembolism.
Pulmonary Embolism. Oudkerk M, van Beek EJR and ten Cate JW:
Blackwell Science; Berlin, Germany: pp. 2–32. 1999
|
|
5
|
Kearon C, Salzman EW and Hirsh J:
Epidemiology, pathogenesis, and natural history of venous
thrombosis. Hemostasis and Thrombosis: Basic Principles &
Clinical Practice. Colman RW, Hirsh J, Marder VJ, Clowes AW and
George JN: 4th. Lippincott Williams & Wilkins; Philidelphia,
PA: pp. 1153–78. 2000
|
|
6
|
Heit J: Venous thromboembolism
epidemiology: implications for prevention and management. Semin
Thromb Hemost. 28(Supp 2): 3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bauer KA: The thrombophilias: well defined
risk factors with uncertain therapeutic implications. Ann Intern
Med. 135:367–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lensing AWA, Prandoni P, Prins MH and
Büller HR: Deep-vein thrombosis. Lancet. 353:479–85. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia XW, Tian YP, Wang Y, Den XX and Dong
ZN: Correlation of polymorphism in IL-6 gene promoter with BMI,
inflammatory factors and pathogenesis and progression of CHD.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 15:1270–1275. 2007.In Chinese.
PubMed/NCBI
|
|
10
|
Fox EA and Kahn SR: The relationship
between inflammation and venous thrombosis. A systematic review of
clinical studies. Thromb Haemost. 94:362–365. 2005.PubMed/NCBI
|
|
11
|
Vickers MA, Green FR and Terry C: Genotype
at a promoter polymorphism of the interleukin-6 gene is associated
with baseline levels of plasma C-reactive protein. Cardiovasc Res.
53:1029–1034. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christiansen SC, Naess IA, Cannegieter SC,
Hammerstrøm J, Rosendaal FR and Reitsma PH: Inflammatory cytokines
as risk factors for a first venous thrombosis: a prospective
population-based study. PLoS Med. 3:e3342006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahemuti A, Abudureheman K, Aihemaiti X,
Hu XM, Xia YN, Tang BP and Upur H: Association of interleukin-6 and
C-reactive protein genetic polymorphisms levels with venous
thromboem-bolism. Chin Med J (Engl). 125:3997–4002. 2012.
|
|
14
|
Mälarstig A, Wallentin L and Siegbahn A:
Genetic variation in the interleukin-6 gene in relation to risk and
outcomes in acute coronary syndrome. Thromb Res. 119:467–473. 2007.
View Article : Google Scholar
|
|
15
|
Reitsma PH and Rosendaal FR: Activation of
innate immunity in patients with venous thrombosis: the Leiden
Thrombophilia Study. J Thromb Haemost. 2:619–622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan WH, Liu DL, Xiao LM, Xie CJ, Sun SY
and Zhang JC: Coronary heart disease and chronic periodontitis: Is
polymorphism of interleukin-6 gene the common risk factor in a
Chinese population? Oral Dis. 17:270–276. 2011. View Article : Google Scholar
|
|
17
|
Basso F, Lowe GD, Rumley A, McMahon AD and
Humphries SE: Interleukin-6 -174G>C polymorphism and risk of
coronary heart disease in West of Scotland coronary prevention
study (WOSCOPS). Arterioscler Thromb Vasc Biol. 22:599–604. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maitra A, Shanker J, Dash D, John S,
Sannappa PR, Rao VS, Ramanna JK and Kakkar VV: Polymorphisms in the
IL6 gene in Asian Indian families with premature coronary artery
disease - the Indian Atherosclerosis Research Study. Thromb
Haemost. 99:944–950. 2008.PubMed/NCBI
|
|
19
|
Zheng GH, Chen HY and Xiong SQ:
Polymorphisms of -174G>C and -572G>C in the interleukin 6
(IL-6) gene and coronary heart disease risk: a meta-analysis of 27
research studies. PLoS One. 7:e348392012. View Article : Google Scholar
|
|
20
|
Pan S, Yu ZX, Ma YT, et al: Appropriate
body mass index and waist circumference cutoffs for categorization
of overweight and centraladiposity among Uighur adults in Xinjiang.
PLoS One. 8:e801852013. View Article : Google Scholar
|
|
21
|
Li SS, Pan S, Ma YT, et al: Optimal cutoff
of the waist-to-hip ratio for detecting cardiovascular risk factors
among Han adults in Xinjiang. BMC Cardiovasc Disord. 14:932014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayinu and Chen X: Evaluation of LOXL1
polymorphisms in exfoliation syndrome in the Uygur population. Mol
Vis. 17:1734–1744. 2011.PubMed/NCBI
|
|
23
|
Xu S, Huang W, Qian J and Jin L: Analysis
of genomic admixture in uyghur and its implication in mapping
strategy. Am J Hum Genet. 82:883–894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou R, An L, Wang X, et al: Testing the
hypothesis of an ancient Roman soldier origin of the Liqian people
in northwest China: a Y-chromosome perspective. J Hum Genet.
52:584–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou R, Yang D, Zhang H, et al: Origin and
evolution of two Yugur sub-clans in Northwest China: a case study
in paternal genetic landscape. Ann Hum Biol. 35:198–211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao FX, Yang JF, Cassiman JJ and Decorte
R: Diversity at eight polymorphic Alu insertion loci in Chinese
populations shows evidence for European admixture in an ethnic
minority population from northwest China. Hum Biol. 74:555–568.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang SL, He BX, Liu HL, He ZY, Zhang H,
Luo JP, Hong XF and Zou YC: Apolipoprotein E gene polymorphisms and
risk for coronary artery disease in Chinese Xinjiang Uygur and Han
population. Chin Med Sci J. 19:150–154. 2004.PubMed/NCBI
|
|
28
|
Wang K, Ao Y, Zhao L, et al: Analysis on
obesity and its risk factors among inhabitants of Bortala
prefecture of Xinjiang autonomous region. Chinese Journal of Public
Health. 22:1128–1130. 2006.In Chinese.
|
|
29
|
Yao J, Ma YT, Xie X, Liu F, Chen BD and An
Y: Association of rs1805127 polymorphism of KCNE1 gene with atrial
fibrillation in Uigur population of Xinjiang. Zhonghua Yi Xue Yi
Chuan Xue Za Zhi. 28:436–440. 2011.In Chinese. PubMed/NCBI
|
|
30
|
Terry CF, Loukaci V and Green FR:
Cooperative influence of genetic polymorphisms on interleukin 6
transcriptional regulation. J Biol Chem. 24:18138–18144. 2000.
View Article : Google Scholar
|
|
31
|
Malaponte G, Polesel J, Candido S,
Sambataro D, Bevelacqua V, Anzaldi M, Vella N, Fiore V, Militello
L, Mazzarino MC, Libra M and Signorelli SS: IL-6-174 G > C and
MMP-9 1562 C > T polymorphisms are associated with increased
risk of deep vein thrombosis in cancer patients. Cytokine.
62:64–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jordanides N, Eskdale J, Stuart R and
Gallagher G: Allele associations reveal four prominent haplotypes
at the human interleukin- 6 (IL-6) locus. Genes Immun. 1:451–455.
2000. View Article : Google Scholar
|
|
33
|
Kamphuisen PW, Eikenboom JC, Vos HL, Pablo
R, Sturk A, Bertina RM and Rosendaal FR: Increased levels of factor
VIII and fibrinogen in patients with venous thrombosis are not
caused by acute phase reactions. Thromb Haemost. 81:680–683.
1999.PubMed/NCBI
|
|
34
|
Vormittag R, Hsieh K, Kaider A, Minar E,
Bialonczyk C, Hirschl M, Mannhalter C and Pabinger I: Interleukin-
6 and interleukin-6 promoter polymorphism (-174) G > C in
patients with spontaneous venous thromboembolism. Thromb Haemost.
95:802–806. 2006.PubMed/NCBI
|
|
35
|
Levi M, van der Poll T and Büller HR:
Bidirectional relation between inflammation and coagulation.
Circulation. 109:2698–2704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morange PE, Bezemer I, Saut N, et al: A
follow-up study of a genome-wide association scan identifies a
susceptibility locus for venous thrombosis on chromosome 6p24.1. Am
J Hum Genet. 86:592–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smeeth L, Cook C, Thomas S, Hall AJ,
Hubbard R and Vallance P: Risk of deep vein thrombosis and
pulmonary embolism after acute infection in a community setting.
Lancet. 367:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh P, Peterson TE, Barber KR, Kuniyoshi
FS, Jensen A, Hoffmann M, Shamsuzzaman AS and Somers VK: Leptin
upregulates the expression of plasminogen activator inhibitor-1 in
human vascular endothelial cells. Biochem Biophys Res Commun.
392:47–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maruyama I, Nakata M and Yamaji K: Effect
of leptin in platelet and endothelial cells. Obesity and arterial
thrombosis. Ann NY Acad Sci. 902:315–319. 2000. View Article : Google Scholar : PubMed/NCBI
|