Introduction
Lung cancer is the most common cause of
cancer-associated mortality and its morbidity is increasing
worldwide, and ~85–90% of lung cancer cases are non-small cell lung
cancer (NSCLC) (1). Surgery,
chemotherapy and radiation are the three major therapeutic
approaches for the treatment of lung cancer. Radiotherapy is
commonly used for the treatment of NSCLC, and >50% of newly
diagnosed patients with lung cancer worldwide receive radiotherapy,
either alone or in combination with surgery or chemotherapy, during
their treatment (2). However, the
rates of complete recovery are low and long-term survival rates
remain poor, since the curative potential of radiotherapy is often
limited due to intrinsic radio-resistance of cancer cells and
systemic dose-limiting toxicity to normal tissues (3–6).
Therefore, there is increasing interest in enhancing the
radiosensitivity of lung cancer cells for the development of more
effective and less toxic treatments.
Proteins of the B-cell lymphoma (Bcl)-2 family have
been identified as key regulators of apoptosis (7). The Bcl-2 family of proteins is
comprised of anti-apoptotic proteins, including Bcl-2, Bcl-extra
large (xL) and myeloid leukemia cell differentiation protein;
pro-apoptotic proteins, including Bcl-2-associated X protein (Bax);
and Bcl-2-antagonist/killer and Bcl-2 homology domain 3-only
proteins, including Bim, Bcl-2-associated death promoter (Bad),
Phorbol-12-myristate-13-acetate-induced protein 1, p53 upregulated
modulator of apoptosis and hara-kiri (8). The overexpression of anti-apoptotic
Bcl-2 family proteins contributes to the development of cancer and
to cancer cell resistance against a wide variety of anticancer
agents (9–11). Bcl-xL, a major member of the
anti-apoptotic Bcl-2 family, is overexpressed in NSCLC (12,13).
The over-expression of Bcl-xL has been observed to counteract the
pro-apoptotic functions of Bax and Bad by preventing their
translocation between the cytosol and the mitochondria (14). Several studies have revealed that
inhibiting the expression of Bcl-xL, using antisense
oligonucleotides or small interfering (si)RNA, suppresses the
proliferation of and sensitizes tumor cells to chemotherapeutic
agents (15–17). Varin et al demonstrated that
knockdown of Bcl-xL using siRNA sensitizes two highly
chemoresistant mesothelioma cell lines to treatment with cisplatin
(18). Guichard et al found
that short hairpin (sh) RNAs targeting Bcl-xL modulated senescence
and apoptosis following exposure to SN-38 and irinotecan in a model
of colon cancer (15). Lei et
al demonstrated that Bcl-xL siRNA contributed to an increase in
diamminedichloroplatinum (DDP)-induced cell death in NSCLC and
sensitized cells to DDP, leading to an increase the effectiveness
of the drug in treating NSCLC (19). Notably, a previous study revealed
that downregulation of Bcl-xL using siRNA increased the in
vitro and in vivo radiosensitivity of colorectal cancer
cells by increasing caspase-dependent apoptosis (20). These studies suggest that the
protein expression of Bcl-xL is critical for tumor development,
progression and resistance to therapy, including chemotherapy and
radiation. However, whether the inhibition of Bcl-xL is an
effective approach to overcome the radioresistance exhibited by
NSCLC remains to be elucidated. The present study aimed to examine
Bcl-xL as a therapeutic target for the treatment of human
NSCLC.
The successful use of siRNA in downregulating gene
expression in a number of model systems has led to several attempts
to examine this methodology as a potentially therapeutic approach
(21). DNA vector-based shRNA
technology can achieve persistent silencing of endogenous gene
expression (22). In the present
study, the expression of Bcl-xL was downregulated using RNA
interference (RNAi) to investigate the role of Bcl-xL in
radioresistance, and to determine the feasibility and efficacy of
combination therapy, involving siRNA targeting Bcl-xL and
radiotherapy, on NSCLC cells.
Materials and methods
shRNA design and plasmid
construction
The cDNA sequence of Bcl-xL was obtained from
GenBank (accession no. Z23115). Bcl-xL-shRNA was designed using
siRNA target design finder (Ambion Inc., Austin, TX, USA) and the
sequences were as follows: Bcl-xL-shRNA, sense
5′-CAGGGACAGCATATCAGAG-3′ and antisense 5′-GTCCCTGTCGTATAGTCTC-3′.
The oligo-nucleotides were annealed and inserted into the
BamHI and HindIII sites on the pSilencer4.1-CMVneo
vector (Ambion Inc.), according to the manufacturer’s instructions
(Ambion Inc.). The pSilencer4.1-CMVneo vector contains the SV40
early promoter to provide G418 resistance in mammalian cells. The
recombinant vectors were confirmed by digestion analysis using
restriction endonucleases BamHI and HindIII (Takara
Bio, Inc., Dalian, China) at 37°C for 2 h and the inserted
sequences were verified by DNA sequencing. A negative control
vector, expressing a hairpin siRNA with limited homology to any
known sequences of the human genome, was commercially available
(Ambion Inc.). The shRNA vector containing the oligonucleotides
encoding Bcl-xL was termed Bcl-xL-shRNA and the negative control
(NC) vector was termed NC-shRNA. The purified DNA was diluted to 1
mg/ml and stored at -20°C until use.
Cell culture and transfection
The A549 human NSCLC cell line was purchased from
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). The A549 cells were cultured in RPMI-1640 medium
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
heat-inactivated 10% fetal bovine serum (Biochrom AG, Berlin,
Germany), 100 U/ml penicillin and 100 mg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified
atmosphere containing 5% CO2.
The A549 cells were then seeded into 6-well plates
at a density of 2×104 cells/well and cultured overnight
to 80–90% confluence prior to transfection. Transfection was
performed using Lipofectamine Plus (Invitrogen Life Technologies),
and the ratio of the plasmids to transfection reagent was 1 mg:2
ml. The cells were transfected with either the Bcl-xL-shRNA or
NC-shRNA plasmids, according to the manufacturer’s instructions.
G418 (800 μg/ml; Sigma-Aldrich) was used to screen for
stably transfected clones. The stable transfectants were termed
A549/Bcl-xL-shRNA and A549/NC-shRNA.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The transfected and non-tranfected cells were
collected and washed with phosphate-buffered saline (PBS;
Sigma-Aldrich). The total RNA was extracted from the cells using
TRIzol reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. Moloney Murine Leukemia Virus reverse
transcriptase (Fermentas, Waltham, MA, USA) was used to amplify the
cDNA, according to the manufacturer’s instructions. The RT-qPCR
assays were performed using SYBR TAQ real-time kits (Takara Bio,
Inc., Otsu, Japan) and RT-PCR amplification equipment (ABI PRISM
7900HT; Applied Biosystems, Foster City, CA, USA). The primer
sequences used for qPCR were as follows: Bcl-xL, sense
5′-CGTGGAAAGCGTAGACAAGGA-3′ and antisense
5′-ATTCAGGTAAGTGGCCATCCAA-3′ and GAPDH, sense
5′-TGTGGGCATCAATGGATTTGG-3′ and antisense
5′-ACACCATGTATTCCGGGTCAAT-3′. The PCR conditions were as follows:
Predenaturation at 94°C for 5 min, followed by 40 cycles of
denaturation at 94°C for 10 sec, annealing/extension at 60°C for 15
sec and final extension at 72°C for 10 min. The specificity of the
amplification was confirmed using melting curve analysis. The
expression of target genes were normalized against the expression
of GAPDH. The fold-change was calculated, as described previously
(23), and the data are presented
as the fold-change in expression relative to the untransfected
controls.
Western blotting
The cells were homogenized in lysis buffer
(Sigma-Aldrich) containing 50 mmol/l Tris-HCl, 5 mmol/l EDTA, 150
mmol/l NaCl, 1% sodium deoxycholate, 500 μmol/l
Na3VO4, 0.5% Triton X-100, 10 μmol/l
4-(2-amino-ethyl) benzenesulfonyl fluoride hydrochloride (AEBSF)
and 10 mmol/l NaF, on ice for 30 min. The homogenates were
subsequently centrifuged at 12,000 × g at 4°C for 15 min, the
supernatants, containing the total cellular protein, were collected
and the protein concentration was determined using a Bicinchoninic
Acid Assay kit (Sigma-Aldrich). Equal quantities of protein lysate
(50 μg) were electrophoretically separated on 10 or 8%
sodium dodecyl sulfatepolyacrylamide gels, transferred onto
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA)
and were blocked in 3% bovine serum albumin for 2 h. Following
blocking, the membranes were incubated with the following
antibodies: Mouse anti-Bcl-xL monoclonal antibody (1:1,500;
sc-271121), mouse anti-caspase-3 polyclonal antibody (1:2,000;
sc-7272) and mouse anti-caspase-8 polyclonal antibody (1:3,000;
sc-56070), which were all purchased from Santa Cruz Biotechnology,
Inc (Santa Cruz, CA, USA), as well as mouse anti-poly(ADP-ribose)
polymerase (PARP) polyclonal antibody (1:1,000; #9544; Cell
Signaling Technology, Inc., Beverly, MA, USA) and mouse monoclonal
anti-β-actin (1:5,000; A2228; Sigma-Aldrich), overnight at 4°C. The
membranes were subsequently incubated for 2 h at 37°C with
horseradish peroxidase-conjugated anti-mouse secondary antibody
(Santa Cruz Biotechnology, Inc.). β-actin was used as a loading
control. The bound antibodies were detected using an enhanced
chemilluminescence kit (Santa Cruz Biotechnology, Inc.).
Densitometric analysis was performed using Quantity One image
analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
Cell proliferation assay
The cell viabilities of the untransfected, stably
transfected A549/Bcl-xL-shRNA and A549/NC-shRNA A549 cells were
measured using a 3-(4, 5-dimethylthazol-2-yl)-2, 5- diphenyl
tetrazolium bromide (MTT) assay (Sigma-Aldrich). Briefly, the A549
cells were seeded into seven 96-well plates at a density of
4×103 cells/well, with eight wells per group/subgroup.
Following 48 h culture, 200 μl MTT (5 mg/ml) was added to
each well, followed by incubation at 37°C for 4 h. The supernatant
was then removed and 200 μl dimethyl sulfoxide was added to
each well, followed by agitation for 10 min. The optical densities
were determined using a Versamax microplate reader (Molecular
Devices, LLC., Sunnyvale, CA, USA) at 490 nm, and the growth
inhibition was calculated as follows: Inhibition rate
(%)=[1-(average absorbance of experimental group/average absorbance
of blank control group)]×100%.
Analysis of apoptosis
Cell apoptosis was identified by fluorescence
staining using acridine orange (AO) and ethidium bromide (EB) from
Molecular Probes (Eugene, OR, USA). For the morphological
examination of apoptosis, the untransfected, stably transfected
A549/Bcl-xL-shRNA and A549/NC-shRNA A549 cells
(5×103/well) were seeded into a separate 24-well
microplate for 48 h, washed three times with PBS and mixed with an
identical volume of dual AO/EB solution (100 μg/ml). The
final volume (200 μl) was observed using a CKX41
fluorescence microscope at ×20 magnification (Olympus, Tokyo,
Japan). For quantification, five random fields were selected and at
least 300 cells were quantified in each field. All experiments were
performed in triplicate. At the molecular level, the protein
expression levels of PARP, caspase-3 and caspase-8 were assessed by
western blotting, as described above, an additional indicator of
apoptosis.
Clonogenic cell survival assay
The untransfected or stably transfected
A549/Bcl-xL-shRNA and A549/NC-shRNA A549 cells were seeded
seperately at a density of 5×103 cells/well into each
well of 96-well plates in triplicate. Following culture for 72 h,
the cells were trypsinized and cells (1×104/well) were
seeded into six-well plates and allowed to attach for 6 h at 37°C
in a humidified atmosphere containing 5% CO2. The cells
were then irradiated with different doses (0, 2, 4, 6, and 8 Gy) of
6 MV X-ray radiation using a 23EX accelerator (Varian Medical
Systems,. Inc., Palo Alto, CA, USA) at room temperature, and were
subsequently incubated for 14 days. The colonies were stained with
crystal violet and the number of colonies containing >50 cells
were quantified. The plating efficiency was calculated as follows:
Plating efficiency (%) = (colony number / total cells seeded) ×
100%. All experiments were performed in triplicate. The cell
survival fraction was determined and a cell survival curve was
produced.
Statistical analysis
All experiments were performed in triplicate as
independent experiments. The data are expressed as the mean ±
standard deviation. Comparisons between two samples were calculated
using Student’s t-test and comparisons of >2 groups were
calculated using one-way analysis of variance followed by a Tukey’s
post hoc test using Graphpad Prism 6.0 software (San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Specific downregulation of the expression
of Bcl-xL by Bcl-xL-shRNA
The mRNA and protein expression levels of Bcl-xL in
the NSCLC cells were analyzed by RT-qPCR and western blotting. As
shown in Fig. 1A, the mRNA
expression of Bcl-xL in the Bcl-xL-shRNA group was significantly
decreased compared with the untransfected control group and the
NC-shRNA group (P<0.05). No significant difference was observed
between the NC-shRNA group and the control group. Additionally, the
protein expression level was significantly reduced in the
Bcl-xL-shRNA group compared with the control and NC-shRNA groups
(P<0.05; Fig. 1B). No
significant change in the protein expression levels of Bcl-xL was
observed between the NC-shRNA group and control group (P>0.05).
These results demonstrated that the expression levels of Bcl-xL in
the A549 cells were downregulated, specifically and effectively, by
Bcl-xL-shRNA.
Effect of Bcl-xL-shRNA on A549 cell
proliferation
Using Bcl-xL-shRNA, the effects of downregulation of
Bcl-xL on tumor cell proliferation were examined in vitro
using an MTT assay (Fig. 2). The
results demonstrated that transfection of the A549 cells with
Bcl-xL-shRNA significantly inhibited cell proliferation compared
with the control and NC-shRNA groups (P<0.01).
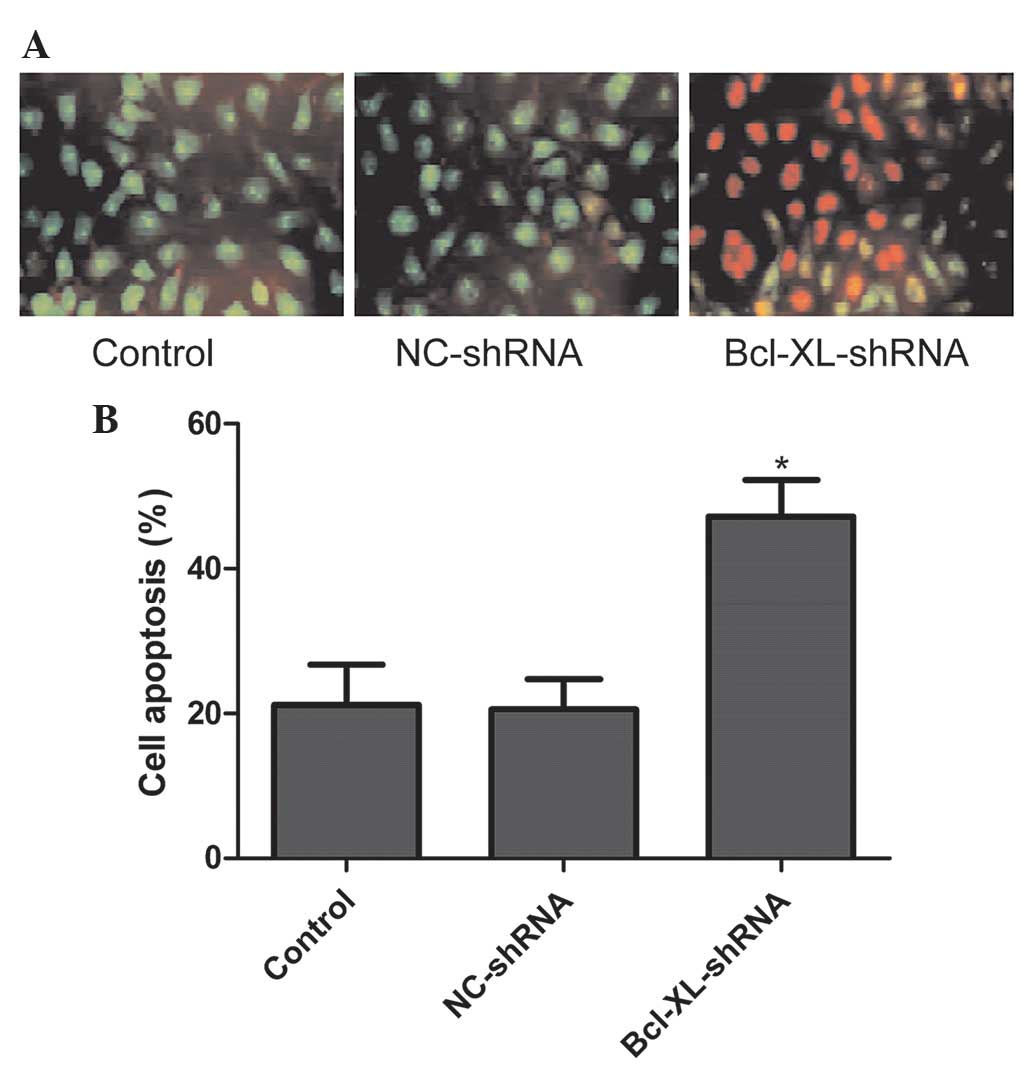
Effect of XIAP-shRNA on the apoptosis of
A549 cells
To further investigate the effect of the
shRNA-mediated down-regulation of XIAP, on cell apoptosis in the
A549 cells the, A549/Bcl-xL-shRNA and A549/NC-shRNA stably
transfected cells were collected and stained with AO/EB. The
results demonstrated that cells transfected with Bcl-xL siRNA
underwent typical apoptotic morphological changes of nuclear and
cytoplasmic condensation, loss of cell volume and nuclear
fragmentation. By contrast, the untransfected and NC-shRNA
transfected cells exhibited no apoptotic characteristics (Fig. 3A). Statistical analysis revealed
that the A549 cells transfected with Bcl-xL-shRNA significantly
induced cell apoptosis compared with the untransfected and the
NC-shRNA cells (P<0.01; Fig.
3B). Therefore, Bcl-xL-shRNA significantly accelerated the
apoptosis of A549 cells.
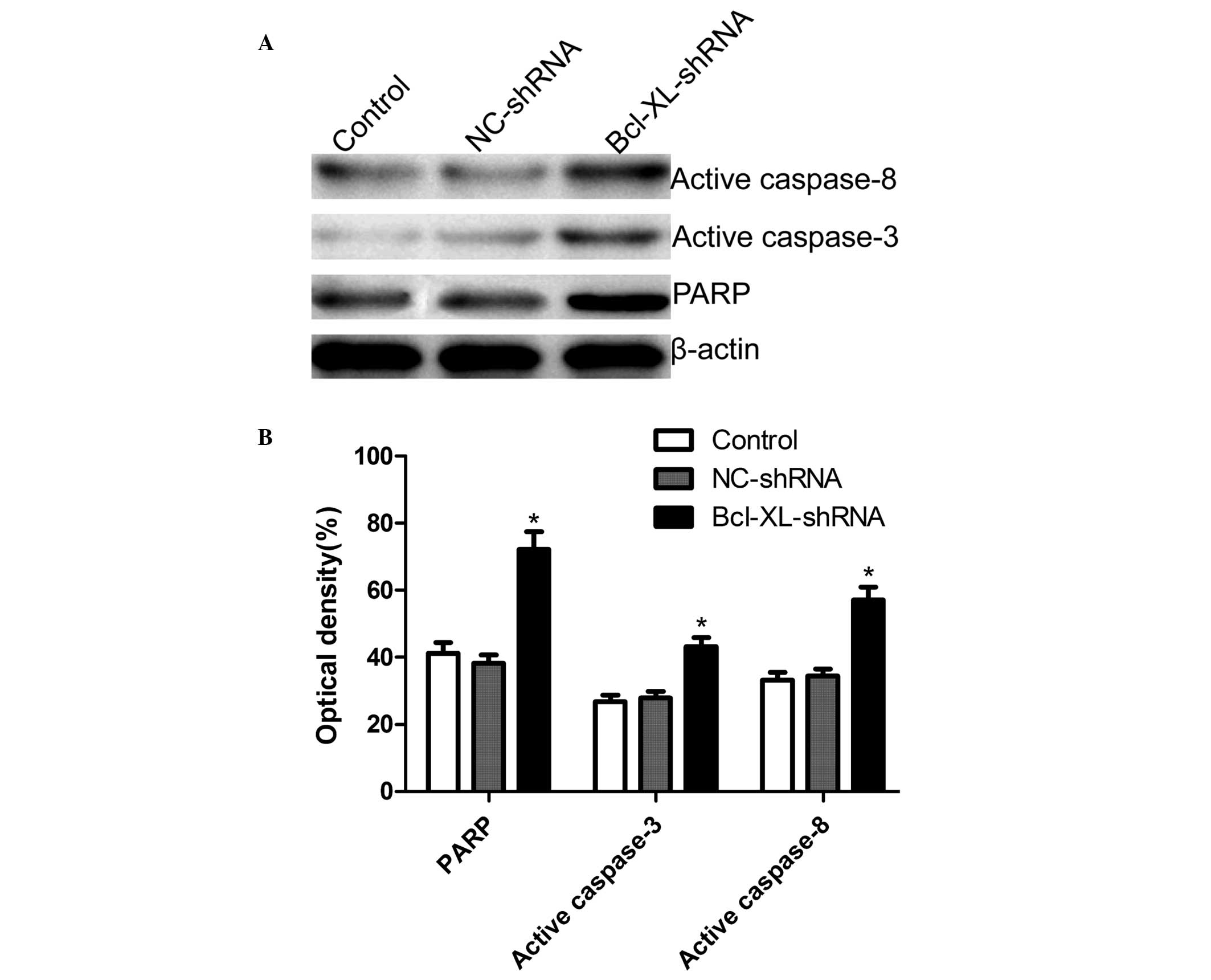
Preliminary mechanisms underlying
Bcl-xL-regulated cell apoptosis
To examine the mechanism underlying the induction of
cell apoptosis, the expression levels of PARP, caspase-3 and
caspase-8 in the A549 cells were determined by western blotting,
following treatment with Bcl-xL-shRNA or NC-shRNA. As shown in
Fig. 4, the expression levels of
caspase-3, caspase-8 and PARP were markedly increased in the cells
transfected with Bcl-xL-shRNA compared with those in the
untransfected and the NC-shRNA-transfected cells.
Effect of Bcl-xL-shRNA on the
radiosensitivity of A549 cells
To investigate the effect of Bcl-xL-shRNA on the
radiosensitivity of A549 cells, clonogenic cell survival assays
were performed. As shown in Table
I, the plating efficiencies of the A549 cells transfected with
Bcl-xL-shRNA cells at the same dose of radiation were significantly
decreased compared with the control cells and the cells transfected
with NC-shRNA (P<0.05). The cell survival curve revealed a
marked decreased in the survival of the cells in the Bcl-xL-shRNA
group compared with that observed in the untransfected A549 cells
and the NC-shRNA group (P<0.05; Fig. 5). No significant difference was
observed in the radiosensitivity of the untransfected cells and
NC-shRNA cells. These results demonstrated that downregulatiion in
the expression of Bcl-xL led to enhanced radiosensitivity in the
NSCLC cells.
 | Table IPlating efficiency at different
radiation doses. |
Table I
Plating efficiency at different
radiation doses.
| Cell group | Plating efficiency
(%)
|
|---|
| 0 Gy | 2 Gy | 4 Gy | 6 Gy | 8 Gy |
|---|
| A549 cell | 96.45±2.12 | 82.45±2.08 | 66.24±1.48 | 44.34±1.67 | 21.12±1.07 |
| A549/NC-shRNA | 97.67±2.41 | 80.45±1.33 | 64.38±0.78 | 43.45±1.88 | 19.89±0.84 |
|
A549/Bcl-xL-shRNA | 89.89±1.35 |
54.23±0.89a |
31.23±0.56a |
9.89±0.45a |
3.35±0.38a |
Discussion
NSCLC patients with non-resectable stage III or
medically inoperable disease account for ~40% of all patients
diagnosed with NSCLC (24).
Radiation therapy is important in achieving local control of the
tumor and in the relief of symptoms resulting from metastatic
disease, therefore, radiotherapy is important in the management of
NSCLC (25). However, partial lung
cancer cell resistance to radiotherapy affects the therapeutic
effects, and the 5-year survival rate of patients receiving
radiotherapy alone is 5–10% (26).
Local recurrence occurs in 80% of patients and metastasis occurs in
60% of patients (27), therefore,
overcoming the resistance of NSCLC to radiotherapy remains a major
challenge and requires further investigation to identify an
effective radiosensitizer, which enhances tumor radiosensitivity
with minimal negative effects on normal tissues.
The Bcl-2 family comprises a group of structurally
associated proteins, which are fundamental in the regulation of the
intrinsic pathway by controlling mitochondrial membrane
permeability and the release of the the pro-apoptotic factor,
cytochrome c (28).
Therefore, the Bcl-2 family are key regulators of apoptosis and are
important in regulating cell apoptosis (7). Bcl-xL is an important member of the
anti-apoptotic Bcl-2 family, which has been reported to be
important in tumor progression, development and chemoresistance
(15–19). Several studies have demonstrated
that Bcl-xL is involved in tumor apoptosis and is important in
radioresistance in several types of tumor (20,29–31).
Yang et al demonstrated that the downregulation of Bcl-xL by
adenovirus-mediated shRNA increases the in vitro and in
vivo radiosensitivity of colorectal cancer cells by increasing
caspase-dependent apoptosis (20).
Streffer et al revealed that the Bcl-xL and BAX proteins
modulate radiosensitivity in human glioma cells, and that targeting
alterations in Bcl-2 family proteins, including the expression of
Bcl-2, may be a promising therapeutic approach to improve the
efficacy of radiotherapy for gliomas (29). Masui et al demonstrated that
the antisense oligonucleotide against Bcl-xL inhibits cell
proliferation and increases the radiosensitivity of pancreatic
cancer (30). Wang et al
revealed that downregulation of Bcl-xL by siRNA increases the
sensitivity of prostate cancer cells to radiation (31). However, there are no reports, to
the best of our knowledge, on the association between the
expression of Bcl-xL and radiosensitivity of human NSCLC cells. The
present study examined changes in the radiosensitivity of A549
cells with downregulated expression of Bcl-xL using clonogenic
survival assays. The results revealed that downregulation of the
expression of Bcl-xL increased cell radiosensitization, which was
consistent with previous studies (20,29–31).
Caspases are important in apoptosis triggered by
various pro-apoptotic signals (32). A previous study demonstrated that
Bcl-xL siRNA triggers a decrease in the protein expression of
Bcl-xL and the activation of procaspase-3, followed by the cleavage
of PARP, in lung cancer (19). In
addition, Yang et al reported that silencing of Bcl-xL
increases the activities of caspase-3 and caspase-8 in colorectal
cancer cells (20). Consistent
with this study, the present study demonstrated that downregulation
Bcl-xL using shRNA activated PARP, caspase-3 and caspase-8 and
induced cell apoptosis in the NSCLC cells. These results suggested
that the downregulation of Bcl-xL induced apoptosis in the tumor
cells by increasing caspase activity.
RNAi using double stranded siRNA molecules of ~20–25
nucleotides is a powerful method for preventing the expression of a
particular gene, with high efficiency, high specificity and low
toxicity (33). This technology is
widely used to investigate gene function, cancer and viral disease
therapy (33,34). To examine the possibility of Bcl-xL
as an effective therapeutic target, the present study used an RNAi
method to silence the endogenous expression of Bcl-xL in the A549
NSCLC cell line and analyzed the phenotypic changes in the stable
transfectants. Stable downregulation in the expression of Bcl-xL by
DNA vector-based shRNA was observed in the A549 cells, which
inhibited proliferation, induced apoptosis and reduced the
radioresistancse of the NSCLC cells. These results suggested that
Bcl-xL may be a potential therapeutic target for the treatment of
human NSCLC.
Acknowledgments
This study was supported by the Science and
Technology Research and Innovation Team, funded by Jilin province
(no. JL2011538).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nygren P and Glimelius B; SBU-group:
Swedish Council on Technology Assessment in Health Care: The
Swedish Council on Technology Assessment in Health Care (SBU)
report on Cancer Chemotherapy - Project objectives, the working
process, key definitions and general aspects on cancer trial
methodology and interpretation. Acta Oncol. 40:155–165. 2001.
View Article : Google Scholar
|
|
3
|
Impicciatore G, Sancilio S, Miscia S and
Di Pietro R: Nutlins and ionizing radiation in cancer therapy. Curr
Pharm Des. 16:1427–1442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Wang L, Liu H, Zhao G and Ming L:
Enhancement of recombinant myricetin on the radiosensitivity of
lung cancer A549 and H1299 cells. Diagn Pathol. 9:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumont F, Altmeyer A and Bischoff P:
Radiosensitising agents for the radiotherapy of cancer: novel
molecularly targeted approaches. Expert Opin Ther Pat. 19:775–799.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bischoff P, Altmeyer A and Dumont F:
Radiosensitising agents for the radiotherapy of cancer: advances in
traditional and hypoxia targeted radiosensitisers. Expert Opin Ther
Pat. 19:643–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walensky LD: BCL-2 in the crosshairs:
tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang TM, Barbone D, Fennell DA and
Broaddus VC: Bcl-2 family proteins contribute to apoptotic
resistance in lung cancer multi-cellular spheroids. Am J Respir
Cell Mol Biol. 41:14–23. 2009. View Article : Google Scholar :
|
|
10
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirkin V, Joos S and Zörnig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soini Y, Kinnula V, Kaarteenaho-Wiik R,
Kurttila E, Linnainmaa K and Pääkko P: Apoptosis and expression of
apoptosis regulating proteins bcl-2, mcl-1, bcl-X, and bax in
malignant mesothelioma. Clin Cancer Res. 5:3508–3515.
1999.PubMed/NCBI
|
|
13
|
Karczmarek-Borowska B, Filip A,
Wojcierowski J, et al: Estimation of prognostic value of Bcl-xL
gene expression in non-small cell lung cancer. Lung Cancer.
51:61–69. 2006. View Article : Google Scholar
|
|
14
|
Gottlieb E, Vander Heiden MG and Thompson
CB: Bcl-x(L) prevents the initial decrease in mitochondrial
membrane potential and subsequent reactive oxygen species
production during tumor necrosis factor alpha-induced apoptosis.
Mol Cell Biol. 20:5680–5689. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guichard SM, Hua ML, Kang P, Macpherson JS
and Jodrell DI: Short hairpin RNAs targeting Bcl-xL modulate
senescence and apoptosis following SN-38 and irinotecan exposure in
a colon cancer model. Cancer Chemother Pharmacol. 60:651–660. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu H, Guo W, Zhang L, et al: Bcl-xL small
interfering RNA suppresses the proliferation of
5-fluorouracil-resistant human colon cancer cells. Mol Cancer Ther.
4:451–456. 2005.PubMed/NCBI
|
|
17
|
Nita ME, Ono-Nita SK, Tsuno N, et al:
Bcl-X(L) antisense sensitizes human colon cancer cell line to
5-fluorouracil. Jpn J Cancer Res. 91:825–832. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varin E, Denoyelle C, Brotin E, et al:
Downregulation of Bcl-xL and Mcl-1 is sufficient to induce cell
death in mesothelioma cells highly refractory to conventional
chemotherapy. Carcinogenesis. 31:984–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lei X, Huang Z, Zhong M, Zhu B, Tang S and
Liao D: Bcl-xL small interfering RNA sensitizes cisplatin-resistant
human lung adenocarcinoma cells. Acta Biochim Biophys Sin
(Shanghai). 39:344–350. 2007. View Article : Google Scholar
|
|
20
|
Yang J, Sun M, Zhang A, Lv C, De W and
Wang Z: Adenovirus-mediated siRNA targeting Bcl-xL inhibits
proliferation, reduces invasion and enhances radiosensitivity of
human colorectal cancer cells. World J Surg Oncol. 9:1172011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uprichard SL: The therapeutic potential of
RNA interference. FEBS Lett. 579:5996–6007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sui G, Soohoo C, Affar el B, et al: A DNA
vector-based RNAi technology to suppress gene expression in
mammalian cells. Proc Natl Acad Sci USA. 99:5515–5520. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chetty C, Bhoopathi P, Rao JS and Lakka
SS: Inhibition of matrix metalloproteinase-2 enhances
radiosensitivity by abrogating radiation-induced FoxM1-mediated
G2/M arrest in A549 lung cancer cells. Int J Cancer. 124:2468–2477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baumann M, Stamatis G and Thomas M:
Therapy of localized non-small cell lung cancer (take home
messages). Lung Cancer. 33(Suppl 1): S47–S49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duchesne GM: Fundamental bases of combined
therapy in lung cancer: cell resistance to chemotherapy and
radiotherapy. Lung Cancer. 10(Suppl 1): S67–S72. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gressen EL and Curran WJ:
Hyperfractionated radiotherapy for lung cancer. Curr Oncol Rep.
2:71–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Llambi F and Green DR: Apoptosis and
oncogenesis: give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Streffer JR, Rimner A, Rieger J, Naumann
U, Rodemann HP and Weller M: BCL-2 family proteins modulate
radiosensitivity in human malignant glioma cells. J Neurooncol.
56:43–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Masui T, Hosotani R, Ito D, et al: Bcl-xL
antisense oligonucleotides coupled with antennapedia enhances
radiation-induced apoptosis in pancreatic cancer. Surgery.
140:149–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang R, Lin F, Wang X, et al: Suppression
of Bcl-xL expression by a novel tumor-specific RNA interference
system inhibits proliferation and enhances radiosensitivity in
prostatic carcinoma cells. Cancer Chemother Pharmacol. 61:943–952.
2008. View Article : Google Scholar
|
|
32
|
Lei XY, Zhong M, Feng LF, Zhu BY, Tang SS
and Liao DF: Bcl-xL small interfering RNA enhances sensitivity of
Hepg2 hepatocellular carcinoma cells to 5-fluorouracil and
hydroxycamptothecin. Acta Biochim Biophys Sin (Shanghai).
38:704–710. 2006. View Article : Google Scholar
|
|
33
|
Zhang J and Hua ZC: Targeted gene
silencing by small interfering RNA-based knock-down technology.
Curr Pharm Biotechnol. 5:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsuyama Y, Yamayoshi A, Kobori A and
Murakami A: Functional regulation of RNA-induced silencing complex
by photoreactive oligonucleotides. Bioorg Med Chem. 22:1003–1007.
2014. View Article : Google Scholar : PubMed/NCBI
|