Introduction
Prostate cancer (PC) is one of the most common types
of cancer amongst males worldwide (1). In the United States of America, PC is
the most common form of male malignancy. The occurrence and
development of tumors are associated with gene mutation and
disorders of signal transduction pathways; therefore, treatments
aimed at targeting these abnormal genes and pathways may provide a
novel focus for the development of cancer therapeutics (2). Patients with PC may also benefit from
the development of such therapeutics. Recently, PC suppressor genes
and oncogenes have been identified and have emerged as a
significant area of study among researchers.
Metadherin (MTDH) is also known as
astrocyte-elevated gene-1 (3,4), and
was first cloned in 2002 (4).
MTDH has been confirmed as an oncogene by multiple studies
(4–12). Results taken from in vitro
data and findings from the analysis of tissue specimens have
confirmed that MTDH expression is significantly higher in
cancerous tissue than in peritumoral tissue or normal cells, this
comparison includes hepatocellular carcinoma (5,6),
malignant glioma (7), breast
cancer (8), renal cell carcinoma
(9), neuroblastoma cell lines
(10) and PC (11–13).
MTDH is not only overexpressed in numerous types of cancer,
but is also involved in tumor metastasis. Since 2004, MTDH
has been considered a potential mediator of cancer metastasis
involving lung metastases from breast cancer (3). In vivo and in vitro, it
has been demonstrated that the 8q22 genomic gain increases
expression of the dual-function metastasis gene MTDH
(14). In addition, a further
study indicated that MTDH promotes angiogenesis (15). MTDH overexpression enhances
human umbilical vein endothelial cell formation, while MTDH
knockout has opposing effects (15). These previous studies have
confirmed that MTDH may activate signaling transduction
pathways associated with tumor development, which may influence the
biological features of the tumors. These features are characterized
as transformation, tumor escape, apoptosis, proliferation,
invasion, metastasis, angiogenesis and chemotherapy resistance
(5–15).
To the best of our knowledge, to date, only few
studies have been conducted investigating the association between
MTDH and PC. However, there is evidence demonstrating that
MTDH is expressed at higher levels in PC samples, compared
with those of benign prostatic hyperplasia (12). Previous in vitro studies
have revealed that MTDH regulates FOXO3a protein activity
(11) and BCCIPα expression
(13) using PC cells.
Cisplatin is a platinum compound that has been
available since 1978, and is currently recommended for the
treatment of few types of cancer (16), including PC. A previous study
demonstrated the addition of a low dose of cisplatin enhanced the
effects of a standard dose of 89Sr, without significant side
effects, and produced a significant improvement in pain palliation
and a cytostatic effect on bone disease from PC (17). Recently, targeted delivery of
cisplatin has been shown to markedly improve its tolerability and
efficacy in prostate cancer therapy in vivo (18). The present study aimed to elucidate
the effects of MTDH as an oncogene in the biological
behavior of PC and chemotherapy sensitivity to cisplatin in
vitro.
Materials and methods
Cell culture and transfection
The human PC cell lines PC3, DU145 and LNCap were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Beijing, China). Cells were cultured in RPMI-1640 (Gibco Life
Technologies, Carlsbad, CA, USA), in a humidified incubator at 37°C
containing 5% CO2. Three small interfering RNAs (siRNAs)
for MTDH intevention were all purchased from Shanghai
GenePharma Technology Co., Ltd. (Shanghai, China). Their sequences
are as follows: MTDH-744 sense, 5′-GCUGUUCGAACACCUCAAATT-3′,
antisense, 5′-UUUGAGGUGUUCGAACAGCTT-3′; MTDH-1432 sense,
5′-GCCGUAAUCAACCCUAUAUTT-3′, antisense,
5′-AUAUAGGGUUGAUUACGGCTT-3′; and MTDH-1883 sense,
5′-GCCAUCUGUAAUCUUAUCATT-3′, and antisense,
5′-UGAUAAGAUUACAGAUGGCTT-3′. The LNCap cells were divided into five
groups: Two control groups of conventional cultured LNCap cells and
LNCap cells transfected with an empty vector (Shanghai GenePharma
Technology Co., Ltd.), and three interventional groups of LNCap
cells transfected with MTDH-744, MTDH-1432 and
MTDH-1883. MTDH intervention sequences were
transfected at working concentrations, according to manufacturer’s
insructions, using Lipofectamine® 2000 reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). Briefly, 250
μl Opti-MEM® I (Invitrogen Life Technologies) was
added to dilute siRNA (2 μM) and Lipofectamine®
2000 (0.02 mg/ml), respectively. After 5 min, the two dilutions
were mixed together, in order to prepare the
siRNA-Lipofectamine® 2000 complex. The cells
(5×105 per well in six-well plates) were then
transfected with the siRNA-Lipofectamine® 2000 complex
and cultured for 48h at 37°C, in an atmosphere containing 5%
CO2. The transfection efficiency was then assessed by
measuring the percentage of transfected cells via microscopy, and
MTDH protein expression levels were determined in each
group; MTDH-1432 was selected for further studies.
Untransfected cells were the control cells.
Optimum concentration of cisplatin
Based on the levels of MTDH expression, the
LNCap cell line was selected for use in the present experiment.
Cisplatin was purchased from a subsidiary of Selleck Chemicals
(Houston, TX, USA); Shanghai Blue Wood Chemical Co. (Shanghai,
China). Various concentrations of cisplatin (0, 0.1, 0.5, 1.0, 5.0,
10.0, 20.0 and 50.0 μg/ml) were selected and added to the
culture medium, ensuring that the cell viability of the LNCap cell
culture remained at ~80% following 24 h of treatment. An MTT assay
(Sigma-Aldrich, St. Louis, MO, USA) was conducted to assess cell
viability. A curve was constructed to select the optimum
concentration of cisplatin, with cell viability and cisplatin
concentration on the y- and x-axes, respectively.
Experimental groups
The experimental groups were designated as follows:
Control group A, untreated LNCap cells; intervention group B, LNCap
+ MTDH intervention sequence; control group C, LNCap +
cisplatin and intervention group D, LNCap + MTDH
intervention sequence + cisplatin. All cells were harvested
following 24 h (37°C) of treatment with cisplatin and/or the
MTDH intervention sequence.
MTT assay
Cells were plated in 96-well plates at
1×104 cells/well in a final volume of 100 μl, and
treated with MTDH intervention sequences and/or cisplatin.
MTT was added following incubation for 24 h in a humidified
incubator at 37°C with 5% CO2. Dilution buffer (25 ml;
Sigma-Aldrich) was subsequently added and the plates were incubated
for a further 4 h. Following removal of the culture medium,
dimethyl sulfoxide (Sigma-Aldrich) was administered to the cells at
37°C for 10 min. The absorbance was measured at 570 nm using a
microplate reader (SpectraMax® 340PC384; Molecular
Devices, Sunnyvale, CA, USA).
Apoptosis assay
Cell apoptosis was detected using an Annexin
V-fluorescein isothiocyanate (FITC)-labeling kit purchased from
Nanjing Kaiji Biotech Company (Nanjing, China) and was performed
according to the manufacturer’s instructions. FITC-labeled cells
were counted and analyzed using the FACS Aria™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA)
Transwell chamber invasion assay
Matrigel (BD Biosciences) was used according to the
manufacturer’s instructions. Following dilution with fetal bovine
serum (FBS)-free RPMI-1640 (Sigma-Aldrich) at a ratio of 1:8, the
Matrigel was added to the bottom chamber of the Transwell. LNCap
cells in the exponential growth stage were treated with 0.25%
tryptase and added to RPMI-1640 to produce a 1×106/ml
single-cell suspension. A Transwell chamber was placed into a
24-well plate. A total of 600 μl of RPMI-1640 containing 10%
FBS (Gibco Life Technologies) and 200 μl of the prepared
single-cell suspension were added. The cells were cultured at 37°C
with 5% CO2 for 24 h. Subsequently, the liquid was
removed from the Transwell chamber and the bottom chamber. The
membrane was then washed three times with phosphate buffered
saline, immersed in methanol (Sigma-Aldrich) and maintained for 20
min at room temperature, followed by hematoxylin staining for 10
min. The cells that had migrated through the pores to the lower
surface of the membrane were counted under a microscope
(magnification, ×400; TS100; Nikon, Tokyo, Japan).
Western blot analysis
Protein lysates were separated using a 10% SDS-PAGE
(Sigma-Aldrich) and transferred onto nitrocellulose blotting
membranes (Pierce Biotechnology, Inc., Rockford, IL, USA). The
blots were incubated with rabbit monoclonal immunoglobulin G (IgG)
MTDH antibody which was purchased from Abcam (1:1,000; cat.
no. ab124789; Abcam, Cambridge, MA, USA). The membranes were
visualized using horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:40,000; cat. no. 14-13-06; KPL, Inc., Gaithersburg, MD,
USA). GAPDH was used as a control.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using a
TRIzol RNA extraction kit (Qiagen, Valencia, CA, USA). RT-qPCR was
performed using an All-in-One™ qPCR mix (GeneCopoeia, Rockville,
MD, USA) on an ABI Prism 7900HT sequence detection system (Applied
Biosystems, Foster City, CA, USA). MTDH primers were
purchased from Invitrogen Life Technologies, the sequences were as
follows: sense, 5′-CCATGATGGAAAGGAAGTTG-3′, antisense
5′-GAACCAACAGGAAATGATGC-3′ (189 bp); and β-actin sense,
5′-CATTAAGGAGAAGCTGTGCT-3′, and antisense
5′-GTTGAAGGTAGTTTCGTGGA-3′ (208 bp). The RT-qPCR amplification
conditions were: 95°C for 5 min, 40 cycles at 94°C for 10 sec, 61°C
for 20 sec and 72°C for 20 sec, followed by a final extension step
at 72°C for 5 mins. The qPCR experiments were repeated at least
three times. All samples were normalized to internal controls. The
fold change in expression was then determined using the ΔΔCT method
(19).
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. All data are expressed as the
mean ± standard deviation of three independent experiments. A
two-tailed Student’s t-test was used for comparisons between two
independent groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
MTDH is differentially expressed in
the PC3, DU145 and LNCap cell lines
MTDH expression was evaluated in the PC3,
DU145 and LNCap cell lines using RT-qPCR and western blot analyses.
Among the three cell lines, the relative expression levels of
MTDH mRNA and protein in the DU145 (4.3±0.12; 0.72±0.04) and
LNCap (4.13±0.03; 0.73±0.035) cells were significantly higher, as
compared with those in the PC3 cells (0.97±0.08; 0.35±0.026)
(P<0.01). However, no significant difference was observed
between the DU145 and LNCap cells (P>0.05).
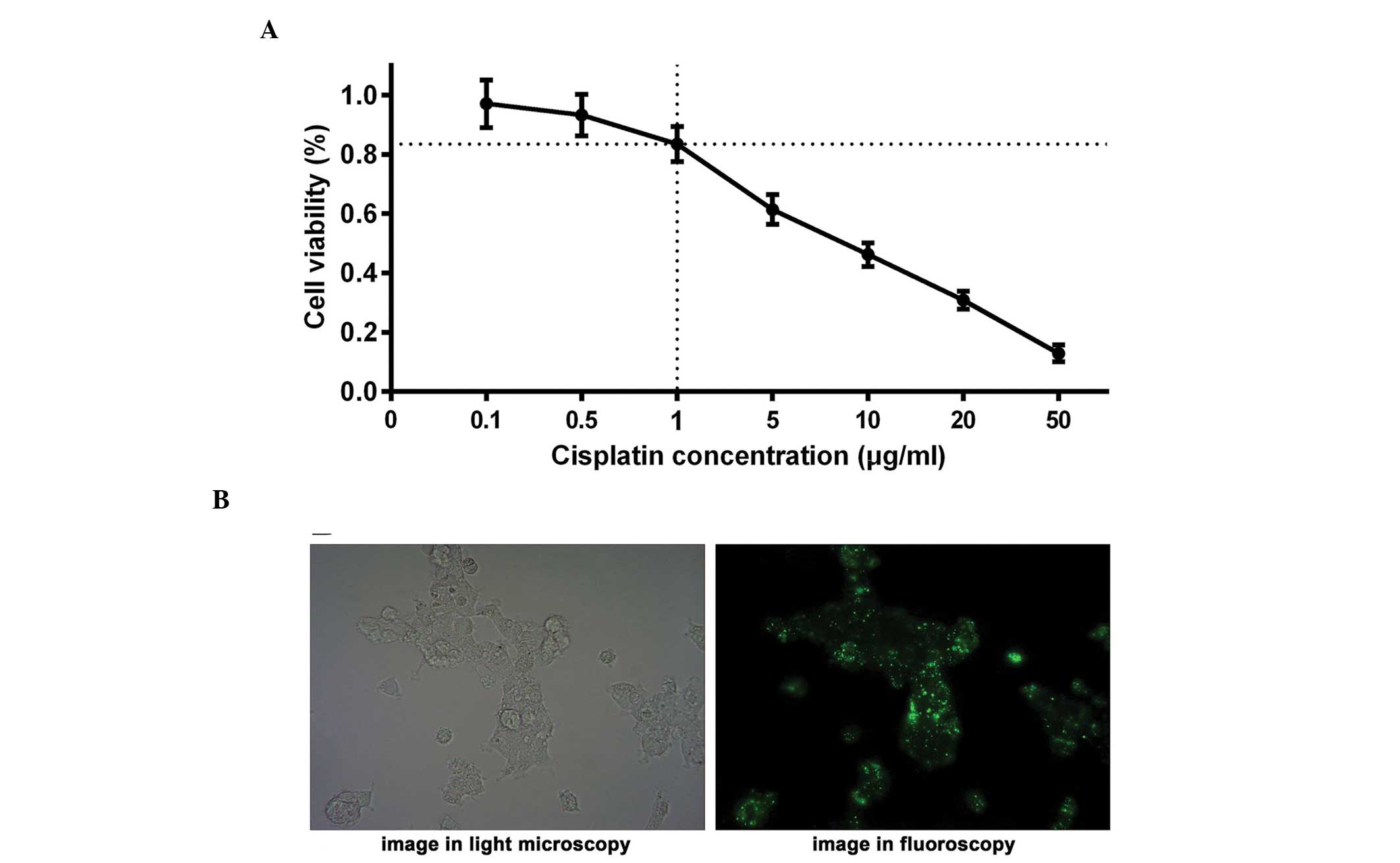
Optimum concentration of cisplatin
The LNCap cell line was treated with various
concentrations of cisplatin (0, 0.1, 0.5, 1.0, 5.0, 10.0, 20.0 and
50.0 μg/ml). An MTT assay was performed and a curve was
constructed to identify the optimum concentration of cisplatin,
with cell viability on the y-axis and cisplatin concentration on
the x-axis (Fig. 1A). To avoid
experimental errors due to excessive cell death induced by
cisplatin, an appropriate concentration of cisplatin was selected
to ensure that the viability of the LNCap cells remained at ~80%
following treatment with cisplatin for 24 h. The results suggested
that the cell viability of the LNCap cells was ~83% when treated
with 1.0 μg/ml cisplatin for 24 h, demonstrating that 1.0
μg/ml cisplatin was the optimal concentration for further
investigation. The half maximal inhibitory concentration
(IC50) of cisplatin was also measured, indicating an
IC50 of 7.1 μg/ml in the LNCap cell line.
MTDH intervention sequence effectively
inhibits MTDH expression
Prior to conduction of the present study, LNCap
cells were divided into five groups: Two control groups Two control
groups of conventional cultured LNCap cells and LNCap cells
transfected with an empty vector, and three interventional groups
of LNCap cells transfected with MTDH-744, MTDH-1432
and MTDH-1883. After a 48 h culture, the transfection
efficiency of the MTDH intervention sequences in LNCap cells
were assessed. It was observed that >80% of cells were
transfected as elucidated via light microscopy and fluoroscopy
(Fig. 1B), indicating that the
transfection was successful and the subsequent experiments could be
performed. In addition, MTDH protein expression levels were
determined in each group, and the results indicated that
MTDH protein expression levels were lowest in the LNCap
cells transfected with MTDH-1432 (0.07±0.01), as compared
with the other groups (P<0.01; conventional cultured LNCap
0.58±0.04, LNCap transfected with empty vector 0.55±0.04, LNCap
transfected with MTDH-744 0.40±0.05 and with
MTDH-1883 0.27±0.03). Therefore, MTDH-1432 was
selected to perform further studies. The expression levels of
MTDH mRNA and MTDH protein were significantly lower
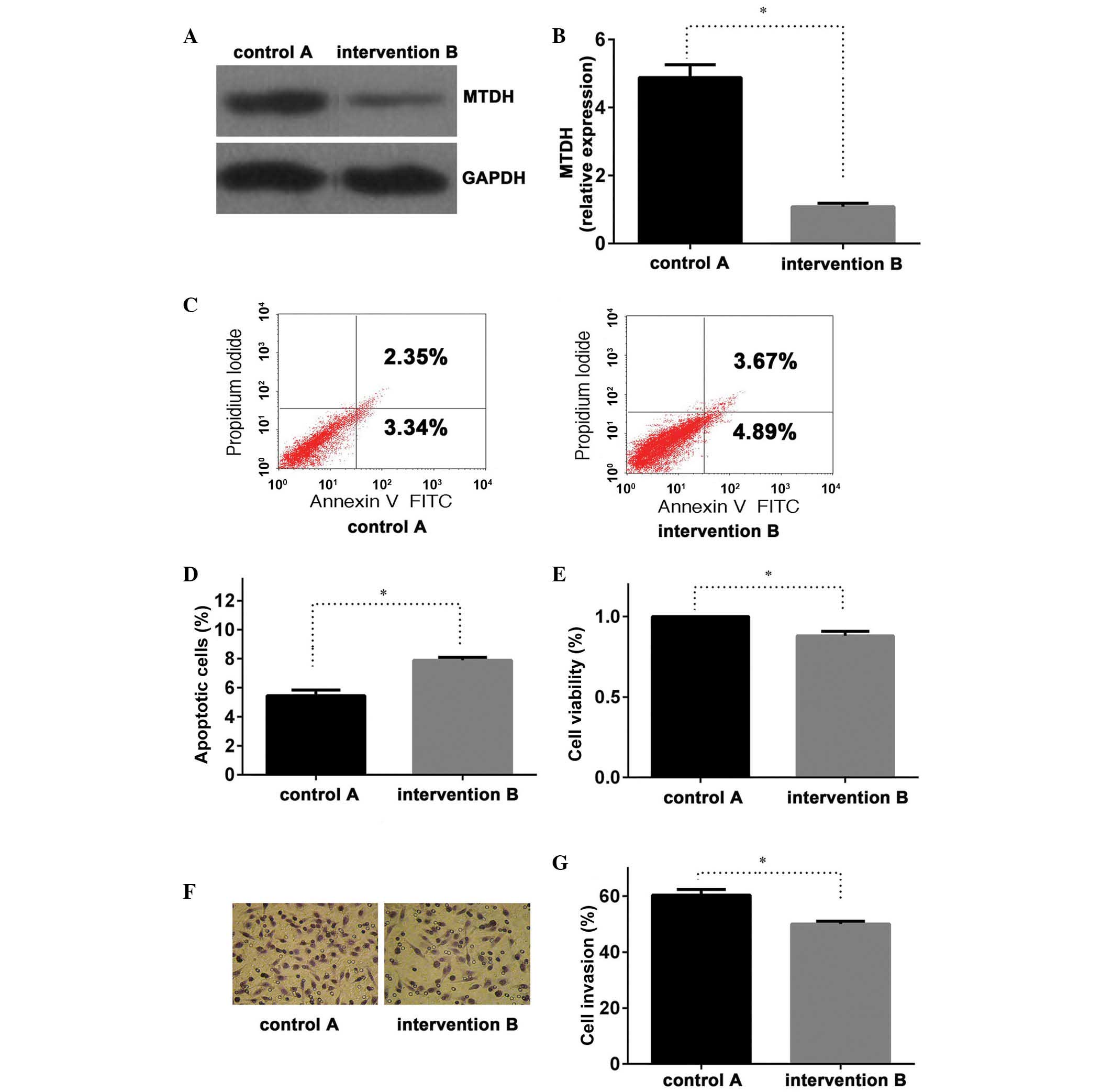
in group B compared with those of group A (P<0.01; Fig. 2A and B), with similar results
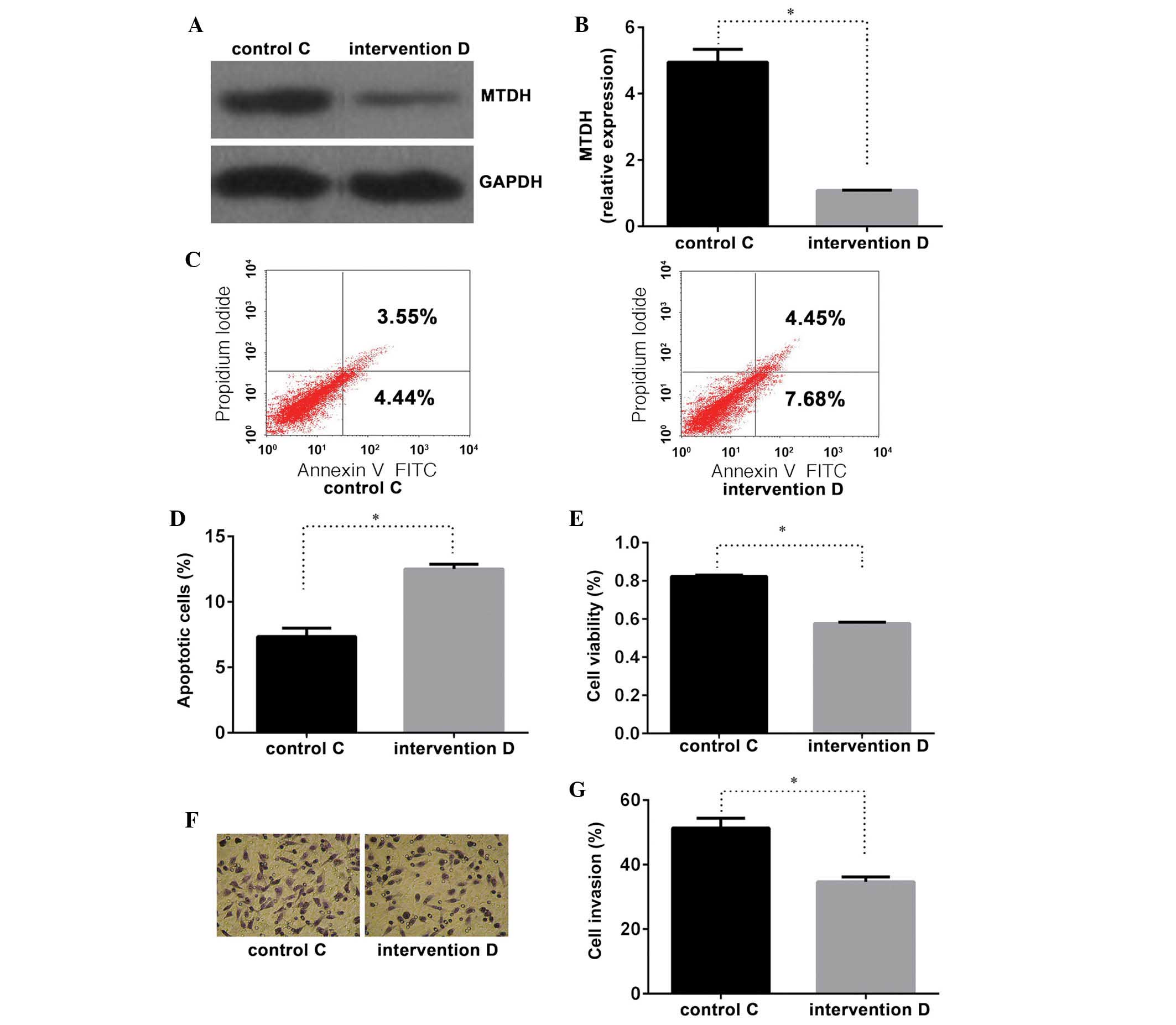
observed in groups C and D (Fig. 3A
and B), indicating that the MTDH intervention sequence
was able to effectively inhibit MTDH expression.
Suppression of MTDH expression
promotes cell apoptosis, reducing cell viability and invasion of
PC
The present study demonstrated an effective
transfection of the MTDH intervention sequence (Figs. 1B, 2A and 2B). Once the LNCap cell line had been
treated for 24 h, an apoptosis assay was performed. The results of
the assay suggested that group B had a higher apoptotic rate than
that of control group A (P<0.01; Fig. 2C and D), combined with the above
results indicating that, compared with group A, MTDH mRNA
and protein expression were significantly lower in group B, these
findings may indicate that the repression of MTDH expression
promoted PC cell apoptosis. Similar results were also observed in
the MTT assay for cell viability (Fig.
2E) and Transwell chamber invasion assay, between groups A and
B (Fig. 2F and G; P<0.05 and
P<0.01, respectively). These results suggested that the
inhibition of MTDH expression in group B may lead to a
reduction in LNCap cell viability and invasive potential.
Suppression of MTDH expression enhances
PC cell sensitivity to cisplatin
Following the evaluation of various concentrations
of cisplatin, cisplatin was administered at 1.0 μg/ml for 24
h in order to assess PC cell sensitivity to cisplatin (Fig. 1A). Compared with control group C,
cells transfected with the MTDH intervention sequence as
well as cisplatin (group D) exhibited a higher apoptotic rate
(Fig. 3C and D), lower cell
viability (Fig. 3E) and decreased
cellular invasiveness (Fig. 3F and
G). These differences were confirmed to be statistically
significant (P<0.01). Significant differences were identified in
the MTDH mRNA and protein expression levels between the two
groups (Fig. 3A and B), supporting
the hypothesis that the repression of MTDH expression may
enhance PC cell sensitivity to cisplatin.
Suppression of MTDH expression inhibits
the phosphoinositide 3-kinase (PI3K)/Akt signal transduction
pathway
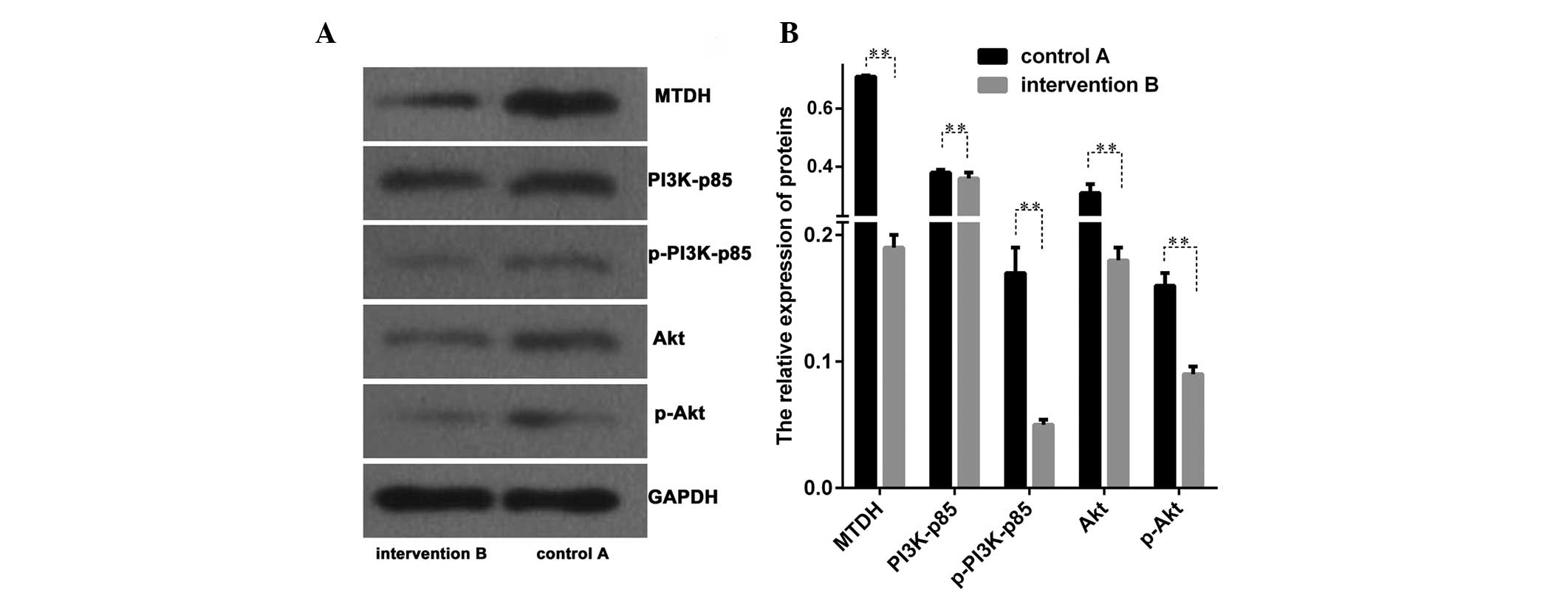
The protein levels associated with the PI3K/Akt
signal transduction pathway of MTDH between groups A and B
were evaluated. Proteins of PI3K, phosphorylated PI3K (p-PI3K), Akt
and phosphorylated Akt (p-Akt) were analyzed and significant
differences were identified between these two groups (P<0.01;
Fig. 4A and B). The present
results demonstrated that the PI3K/Akt signal transduction pathway
may be inhibited following the transfection of LNCap cells with the
MTDH intervention sequence.
Discussion
Previous studies have revealed that MTDH
functions as an oncogene (5–13).
Overexpression of MTDH has been observed in multiple types
of cancer, including breast cancer (3,8),
hepatocellular carcinoma (5),
human glioma (20), neuroblastoma
(7,21), esophageal cancer (22), non-small-cell lung cancer (23), cervical cancer (24) and PC (11–12).
In the present study, three common PC cell lines LNCap, DU145 and
PC3 were used and the level of MTDH expression was detected
using RT-qPCR and western blot analyses. Varying levels of
expression were observed in the three cell lines, and it was
observed that MTDH expression in the LNCap and DU145 cell
lines was higher than that in the PC3 cell line. These results
contradicted a previous study, in which MTDH expression in
the DU145 and PC3 cell lines was observed to be markedly higher
than that in the LNCap cell line (11). This may be due to differences in
experimental conditions. It may be useful to adjust for these
differences in future studies. The LNCap cell line was selected for
the experiment as MTDH was detected at a relatively high
expression level. The results of microscopy and fluoroscopy
analyses suggested that transfection of the MTDH
intervention sequence had occurred successfully. When comparing the
experimental group subjected to transfection with the MTDH
intervention sequence (group B) with the control group (group A),
the repression of MTDH expression was observed to promote
cell apoptosis, reduce cell viability and reduce the invasive
potential of PC cells. These results were in agreement with those
of previous studies (11,12).
MTDH has been observed to be important in
conferring drug resistance in cancer treatment. Previous studies
have demonstrated that knockdown of the MTDH gene led to an
increase in breast cancer cell sensitivity to paclitaxel,
doxorubicin and cisplatin (25,26).
In hepatocellular carcinoma cells, MTDH is able to induce
late SV40 factor leading to fluorouracil resistance as well as
inducing the expression of multidrug resistance gene 1 and
resulting in the development of doxorubicin resistance (15,26).
In the present study, the inhibition of MTDH expression
reduced tumor drug resistance in PC cells. The optimum
concentration of cisplatin for use in the present experiments was
evaluated and the administration of 1.0 μg/ml cisplatin for
24 h was considered appropriate. The results indicated that the
cisplatin-treated intervention group D exhibited a higher apoptotic
rate, lower cell viability and decreased cellular invasiveness. All
differences were significant compared with control group C.
As a classic signal transduction pathway, excessive
activation of PI3K/Akt is closely associated with tumor development
(27,28). A previous study observed that the
MTDH gene is able to regulate the PI3K/Akt signal
transduction pathway (29). The
study demonstrated that MTDH gene activation may be
suppressed by the PI3K/Akt inhibitors LY294002 and PTEN and in
addition, the anti-apoptotic ability of MTDH may be reduced.
In the present study, an examination was conducted in order to
identify MTDH-mediated signaling pathways in PC cells.
Following transfection with an MTDH interference fragment,
the expression of proteins p-PI3K-p85 and p-Akt were significantly
reduced compared with that of the control group. It was
demonstrated that MTDH expression, associated with the
PI3K-Akt signaling pathway may be involved in the biological
behavior of PC.
In conclusion, the results of the present study
demonstrated that the inhibition of MTDH expression may
reduce the carcinogenic behavior of PC cells and increase their
sensitivity to cisplatin. In addition, MTDH associated
PI3K-Akt signaling pathways may be involved in PC development.
References
|
1
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Gasparini G, Longo R, Torino F and
Morabito A: Therapy of breast cancer with molecular targeting
agents. Ann Oncol. 16:iv28–iv36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown DM and Ruoslahti E: Metadherin, a
cell surface protein in breast tumors that mediates lung
metastasis. Cancer Cell. 5:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su ZZ, Kang DC, Chen Y, et al:
Identification and cloning of human astrocyte genes displaying
elevated expression after infection with HIV-1 or exposure to HIV-1
envelope glycoprotein by rapid subtraction hybridization. RaSH
Oncogene. 21:3592–3602. 2002. View Article : Google Scholar
|
|
5
|
Yoo BK, Emdad L, Su ZZ, et al: Astrocyte
elevated gene-1 regulates hepatocellular carcinoma development and
progression. J Clin Invest. 119:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen D, Yoo BK, Santhekadur PK, et al:
Insulin-like growth factor-binding protein-7 functions as a
potential tumor suppressor in hepatocellular carcinoma. Clin Cancer
Res. 17:6693–6701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu H, Song X, Liu C, Xie L, Wei L and Sun
R: Knockdown of astrocyte elevated gene-1 inhibits proliferation
and enhancing chemo-sensitivity to cisplatin or doxorubicin in
neuroblastoma cells. J Exp Clin Cancer Res. 28:192009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Zhang N, Song LB, et al: Astrocyte
elevated gene-1 is a novel prognostic marker for breast cancer
progression and overall patient survival. Clin Cancer Res.
14:3319–3326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Ke Z, Shi H, Yang S and Wang L:
Overexpression of AEG-1 in renal cell carcinoma and its correlation
with tumor nuclear grade and progression. Neoplasma. 57:522–529.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yaari S, Jacob-Hirsch J, Amariglio N,
Haklai R, Rechavi G and Kloog Y: Disruption of cooperation between
Ras and MycN in human neuroblastoma cells promotes growth arrest.
Clin Cancer Res. 11:4321–4330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kikuno N, Shiina H, Urakami S, et al:
Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thirkettle HJ, Girling J, Warren AY, et
al: LYRIC/AEG-1 is targeted to different subcellular compartments
by ubiquiti-nylation and intrinsic nuclear localization signals.
Clin Cancer Res. 15:3003–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ash SC, Yang DQ and Britt DE: LYRIC/AEG-1
overexpression modulates BCCIPalpha protein levels in prostate
tumor cells. Biochem Biophys Res Commun. 371:333–338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu G, Chong RA, Yang Q, et al: MTDH
activation by 8q22 genomic gain promotes chemoresistance and
metastasis of poor-prognosis breast cancer. Cancer Cell. 15:9–20.
2009. View Article : Google Scholar :
|
|
15
|
Yoo BK, Gredler R, Vozhilla N, et al:
Identification of genes conferring resistance to 5-fluorouracil.
Proc Natl Acad Sci USA. 106:12938–12943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Desoize B and Madoulet C: Particular
aspects of platinum compounds used at present in cancer treatment.
Crit Rev Oncol Hematol. 42:317–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sciuto R, Festa A, Rea S, et al: Effects
of low-dose cisplatin on 89Sr therapy for painful bone metastases
from prostate cancer: A randomized clinical trial. J Nucl Med.
43:79–86. 2002.PubMed/NCBI
|
|
18
|
Dhar S, Kolishetti N, Lippard SJ and
Farokhzad OC: Targeted delivery of a cisplatin prodrug for safer
and more effective prostate cancer therapy in vivo. Proc Natl Acad
Sci USA. 108:1850–1855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Liu L, Wu J, Ying Z, et al: Astrocyte
elevated gene-1 upregulates matrix metalloproteinase-9 and induces
human glioma invasion. Cancer Res. 70:3750–3759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SG, Jeon HY, Su ZZ, et al: Astrocyte
elevated gene-1 contributes to the pathogenesis of neuroblastoma.
Oncogene. 28:2476–2484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu C, Chen K, Zheng H, et al:
Overexpression of astrocyte elevated gene-1 (AEG-1) is associated
with esophageal squamous cell carcinoma (ESCC) progression and
pathogenesis. Carcinogenesis. 30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song L, Li W, Zhang H, et al:
Over-expression of AEG-1 significantly associates with tumour
aggressiveness and poor prognosis in human non-small cell lung
cancer. J Pathol. 219:317–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Emdad L, Sarkar D, Su ZZ, et al:
Activation of the nuclear factor kappaB pathway by astrocyte
elevated gene-1: implications for tumor progression and metastasis.
Cancer Res. 66:1509–1516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harris T, Jimenez L, Kawachi N, et al:
Low-level expression of miR-375 correlates with poor outcome and
metastasis while altering the invasive properties of head and neck
squamous cell carcinomas. Am J Pathol. 180:917–928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoo BK, Chen D, Su ZZ, et al: Molecular
mechanism of chemoresistance by astrocyte elevated gene-1. Cancer
Res. 70:3249–3258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fresno VJ, Casado E, de Castro J, Cejas P,
Belda-Iniesta C and González-Barón M: PI3K/Akt signalling pathway
and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar
|
|
28
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke
TF and Fisher PB: Astrocyte elevated gene-1 activates cell survival
pathways through PI3K-Akt signaling. Oncogene. 27:1114–1121. 2008.
View Article : Google Scholar
|