Introduction
Hepatitis C is a disease of global epidemic status,
the prevalence of which is >2%, with >120 million individuals
infected with the hepatitis C virus (HCV) (1). Chronic HCV infection may eventually
progress to severe fibrosis or cirrhosis, which necessitates
surveillance for hepatocellular carcinoma and screening for varices
(2). A natural history study
revealed that a quarter of patients with chronic hepatitis C (CHC)
with severe liver fibrosis succumbed after a median interval of 3.5
years, although this poor prognosis was improved following
combination antiviral treatment (3). Therefore, the evaluation of severe
hepatic fibrosis involves assessing the prognosis of, and
developing a treatment strategy for, patients with CHC. Although a
liver biopsy is the gold standard for assessing the stage of
hepatic fibrosis, its clinical application is usually limited due
to the invasiveness of this procedure. In addition, factors such as
the sampling and observational methods also impact the veracity and
the reliability of the results (4–5).
Serological markers with easy accessibility, are the primary factor
used to assist in evaluating liver fibrosis. However, the
insufficient validation of their function means they are inadequate
for accurately monitoring changes in the stages of fibrosis in CHC
(4). Therefore, it is necessary to
identify novel and effective noninvasive markers for the diagnosis
and treatment of hepatic fibrosis, particularly for severe fibrosis
in CHC.
Sphingolipids, consisting of a sphingosine backbone,
are fundamental structural components of cell membranes and
incorporate other constituents in order to form lipid rafts
(6). Certain receptors or kinases
are associated with lipid rafts, which form platforms that function
in signaling and trafficking on the plasma membrane, and
sphingolipids are involved in the regulation of signaling pathways
in cell growth, differentiation and apoptosis (7,8).
During HCV infection, the HCV RNA level may be significantly
reduced if the gathering of non-structural proteins of HCV on lipid
rafts is disrupted via the inhibition of
serine-palmitoyltransferase, a key enzyme in the de novo
synthesis of sphingolipids (9,10).
Thus, host sphingolipids may impact upon the infection process of
HCV and may reflect the disease status of the HCV infection. In
addition, sphingolipids also have the potential to affect the
pathogenesis of tissue fibrosis (11). Sphingosine 1-phosphate (S1P), as a
bioactive lipid mediator, is involved in numerous signaling
pathways and regulates a wide variety of cellular functions
(12). It has been shown that the
signaling axis with which S1P is involved, exerts a powerful
migratory effect on hepatic myofibroblasts and is involved in the
development of hepatic fibrosis (13). Therefore, it is hypothesized that
sphingolipids may be associated with hepatic fibrogenesis. Although
a clinical study looking at a single sphingolipid in CHC, in the
case of S1P declining in CHC patients has been previously reported
(14), the study of the full
plasma sphingolipid profile in hepatic fibrosis induced by HCV has
not been previously investigated, to the best of our knowledge, and
little is currently known regarding the diagnostic value of plasma
sphingolipids.
A mature high-performance liquid
chromatography-tandem mass spectrometry (HPLC-MS/MS) method was
previously established (15), and
has been employed to identify an association between plasma
sphingolipids and hepatic inflammation in CHC, using improved
quantitative high-throughput lipidomic platform (16). The present study, based on liver
biopsies, analyzed alterations in the plasma profile of
sphingolipids of a cohort of untreated patients with CHC, with and
without severe fibrosis, using HPLC-MS/MS. This approach was
intended to identify the plasma sphingolipids that are associated
with the development of severe fibrosis, in particular, severe
fibrosis in patients with CHC who have developed significant
fibrosis (Metavir F ≥2).
Patients and methods
Patients
A cohort of 122 patients from Dingxi (Gansu, China)
were enrolled in the present study at Beijing YouAn Hospital,
Capital Medical University (Beijing, China) between July 2010 and
June 2011. All patients had a history of paid plasma donation
between 1992 and 1995. The diagnosis of CHC was made in accordance
with previously described criteria (2,17).
Other viral co-infections, including the hepatitis B virus, or
other liver diseases were excluded. No patients had received
antiviral therapy prior to enrolment in the present study. Two
patients were excluded from the study due to the collection of an
invalid specimen from the liver biopsy or due to the presence of
ascites unsuitable for puncture, as this increase the risk of
intra-abdominal infection during biopsy. Thus, 120 patients were
eligible for the study. Based on the liver biopsy, 64 patients with
significant hepatic fibrosis (F ≥2) were eligible for subgroup
analysis.
Blood from the cubital veins of fasting patients was
collected on the day of the biopsy. Routine serological indicators,
such as liver function, blood cell analysis and serum fibrosis
marker assessment, were measured in all patients. Each patient
provided written informed consent at the beginning of the study.
The study protocol was conducted in accordance with the provisions
of the Declaration of Helsinki and its revision, and was approved
by the ethical committee of Beijing YouAn Hospital, Capital Medical
University (Beijing, China).
Liver biopsy
Ultrasound-guided liver biopsy examination was
employed in the present study. The specimens included at least six
complete portal areas and the length was >1.5 cm. Liver biopsy
specimens were fixed in formalin and embedded in paraffin. Biopsy
specimens were independently evaluated for fibrosis status, using
the Metavir scoring system, by two senior pathologists who were
blinded to the clinical data (18,19).
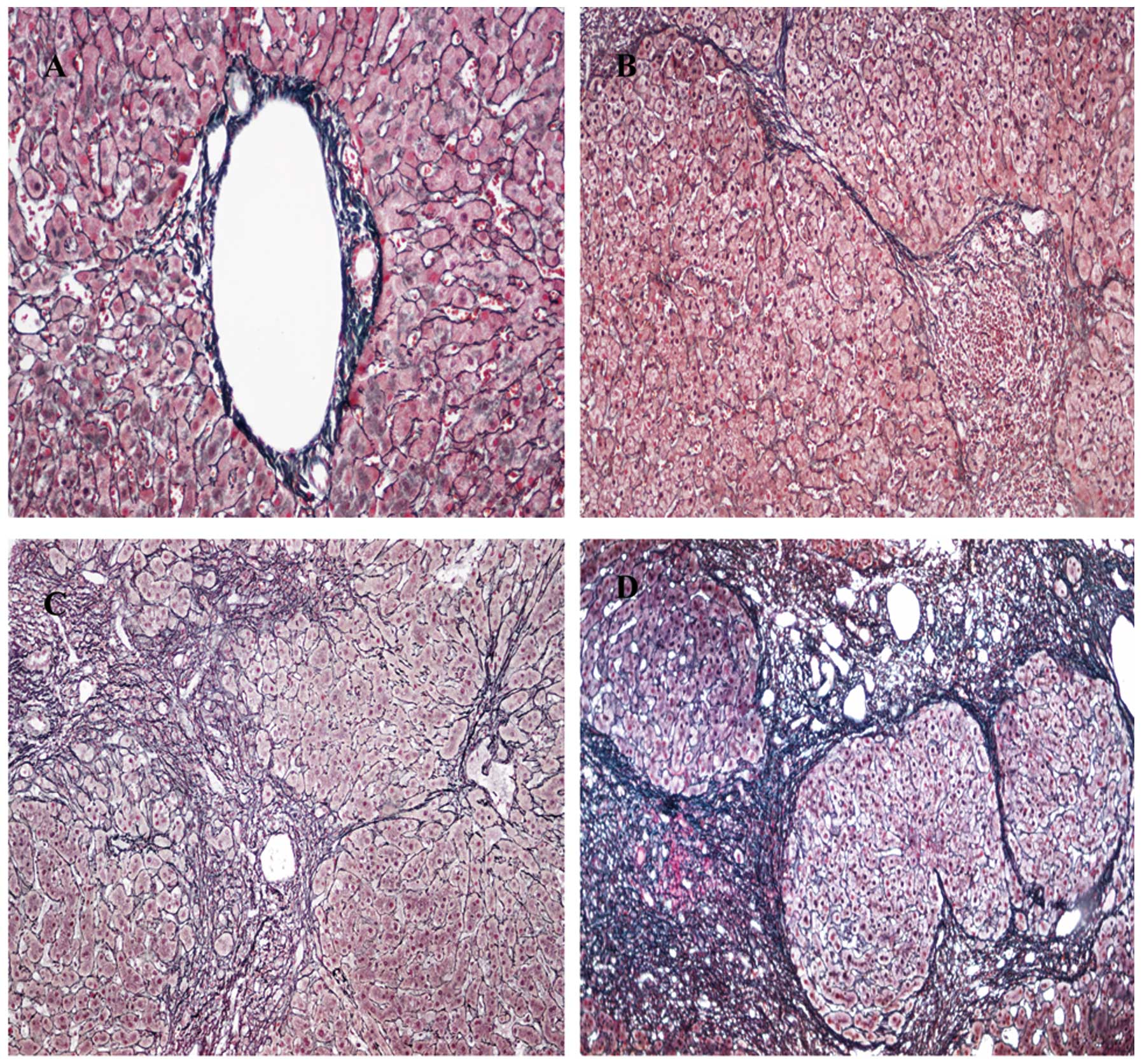
The fibrosis score was assessed on a five point scale (F0, no
fibrosis; F1, portal fibrosis without septa; F2, few septa; F3,
numerous septa without cirrhosis; and F4, cirrhosis; Fig. 1). Significant hepatic fibrosis was
defined as F ≥2, while severe hepatic fibrosis was defined as F
≥3.
HPLC-MS/MS analysis
Blood samples from patients were collected into
sterile tubes using cold lithium heparin as an anticoagulant and
immediately centrifuged at 4°C at 8,000 × g for 10 min. The plasma
samples were stored at −80°C. All lipid standards were purchased
from Avanti Polar Lipids (Alabaster, AL, USA). Ultra Resi-analyzed
grade methanol and HPLC grade methyl-tert-butyl ether were
purchased from Mallinckrodt Baker Inc. (Phillipsburg, NJ, USA).
Formic acid of analytical grade was obtained from Tedia Company
(Fairfield, OH, USA). Ammonium formate (purity, >99.99%) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra-pure water
was prepared using a Milli-Q purification system (Millipore,
Bedford, MA, USA), HPLC-MS/MS was performed on an Agilent 6410B
Triple Quad mass spectrometer (QQQ; Agilent Technologies Inc.,
Santa Clara, CA, USA) comprising a triple quadrupole MS analyzer
with an electrospray ionization interface and an Agilent 1200 RRLC
system. Sphingolipidomic assays were performed at the Institute of
Materia Medica, Peking Union Medical College (Beijing, China) as
previously described (15).
Statistical analysis
Results are expressed as the mean ± standard
deviation unless otherwise stated. Depending on data distribution,
the continuous variables that differed significantly between two
groups were identified by an independent samples t-test or a
Mann-Whitney test. Categorical variables were compared using
Pearson’s χ2 test. The stepwise forward multivariate
logistic regression analysis was performed and the P-values of
entry and removal were respectively set to 0.05 and 0.1. The
diagnostic value of plasma sphingolipids with significant
differences and regression model in multivariate analysis were
assessed using the area under the receiver operating characteristic
(ROC) curve. The negative predictive value (NPV) and positive
predictive value (PPV) were also generated. Statistical analysis
was performed using SPSS version 19.0 (IBM, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the untreated CHC
cohort
Characteristics of the patients with CHC who were
included are summarized in Table
I. A total of 120 CHC patients from the original cohort were
eligible for the present study, with a mean age of 51.33 years. The
average level of serum aminotransferase was mildly elevated
compared with the normal range (<40 U/l). Based on liver
fibrosis staging of the liver biopsy samples (Fig. 1), F1 was assigned to 55 patients,
which accounted for the largest proportion (45.8%) of the cohort;
F2 was assigned to 41.7% (50/120) of the patients; F3 was assigned
to 10.0% (12/120) of the patients; while F0 and F4 were assigned to
1 (0.8%) and 2 (1.7%) patients, respectively.
 | Table ICharacteristics of patients with
chronic hepatitis C virus (n=120). |
Table I
Characteristics of patients with
chronic hepatitis C virus (n=120).
| Indicators | Value |
|---|
| Age (years) | 51.33±7.33 |
| Females | 63 (52.5) |
| Males | 57 (47.5) |
| ALT (U/l) | 60.42±70.88 |
| AST (U/l) | 47.94±44.30 |
| Total bilirubin
(μmol/l) | 16.51±7.25 |
| Direct bilirubin
(μmol/l) | 3.26±1.36 |
| Albumin (g/l) | 43.21±2.36 |
| Prealbumin
(mg/l) | 186.91±176.94 |
| GGT (U/l) | 22.04±16.50 |
| Alkaline phosphatase
(U/l) | 76.29±20.91 |
| White blood cell
(109/l) | 5.08±1.23 |
| Red blood cell
(1012/l) | 4.76±0.67 |
| Hemoglobin (g/l) | 151.09±16.56 |
| Platelets
(109/l) | 171.36±53.20 |
| Type III procollagen
peptide (μg/l) | 33.84±63.73 |
| Type IV collagen
(μg/l) | 38.62±98.14 |
| Hyaluronic acid
(mg/l) | 212.72±730.42 |
| Laminin
(μg/ml) | 37.67±29.51 |
| Fibrosis stage |
| F0 | 1 (0.8) |
| F1 | 55 (45.8) |
| F2 | 50 (41.7) |
| F3 | 12 (10.0) |
| F4 | 2 (1.7) |
Plasma sphingolipid profile in CHC
between F ≤2 and F >2
Using the improved quantitative high-throughput
lipidomic platform, a total of 44 plasma sphingolipids were
detected in patients with CHC through HPLC-MS/MS. A statistically
significant difference was observed between the F ≤2 and F >2
groups in plasma hexosylceramide (HexCer; d18:1/12:0), HexCer
(d18:1/16:0) and HexCer (d18:1/22:0; P<0.05; Fig. 2). No statistical differences were
identified in the remaining plasma sphingolipids (P>0.05).
Indicators associated with severe
fibrosis (F ≥3) in CHC
Using univariate analysis, the routine serological
indicators, alanine aminotransferase (ALT), aspartate
aminotransferase (AST), albumin, prealbumin, γ-glutamyl
transpeptidase (GGT), hyaluronic acid, hemoglobin, platelets, type
III procollagen peptide, and type IV collagen were shown to be
statistically different (P<0.05) between the F ≤2 and F ≥3
groups (Table II). For the
multivariate analysis, HexCer (d18:1/12:0), HexCer (d18:1/16:0),
HexCer (d18:1/22:0), ALT, AST, albumin, GGT, platelets, type III
procollagen peptide, hyaluronic acid and type IV collagen were
included in the forward stepwise logistical regression. The results
demonstrated that HexCer (d18:1/12:0), ALT and AST were retained in
the logistical regression equation. HexCer (d18:1/12:0), with an
odds ratio (OR) value of 1.03, was associated with the presence of
severe hepatic fibrosis (Table
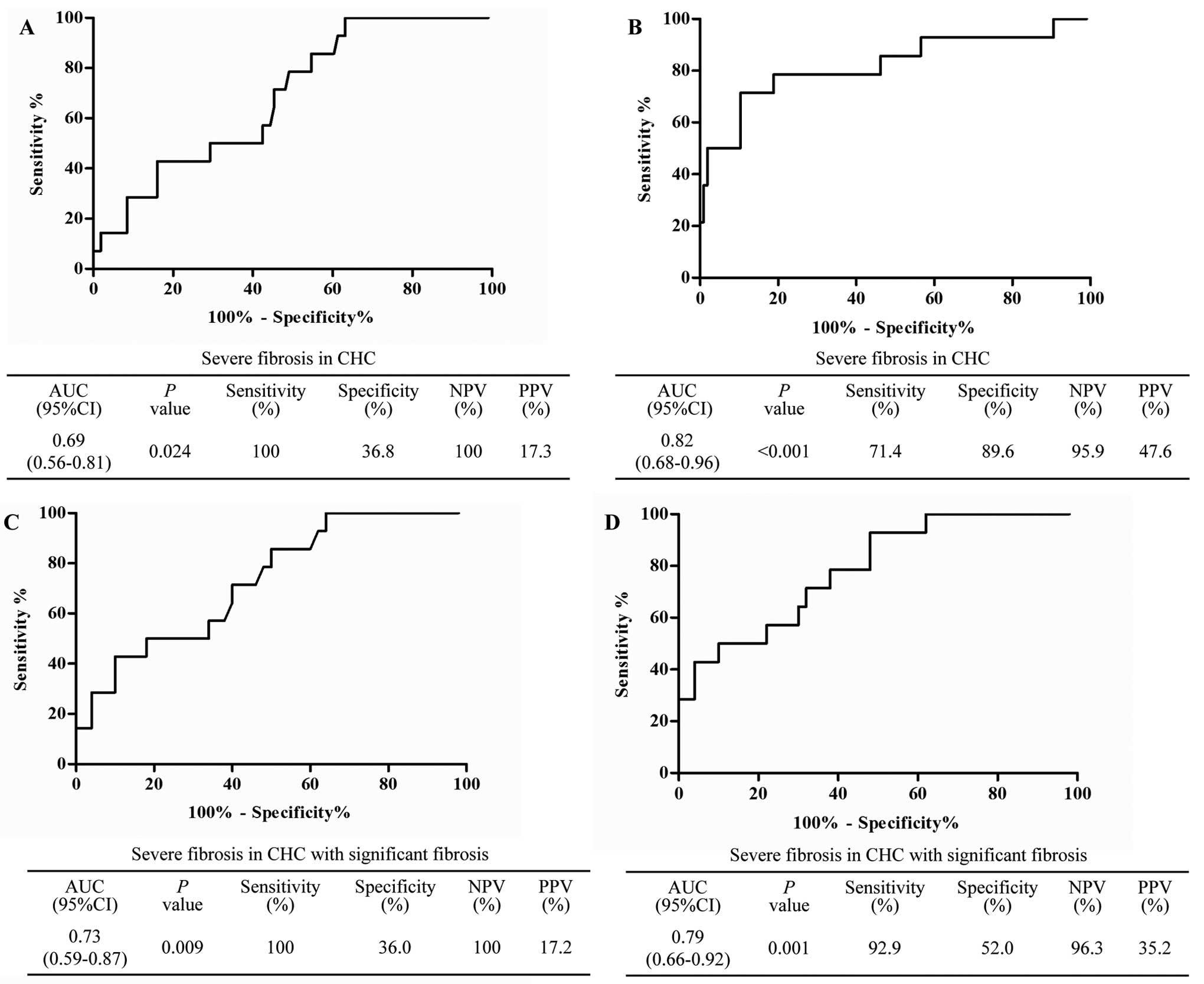
II). In addition, the area under the curve (AUC) of HexCer
(d18:1/12:0), used to identify severe hepatic fibrosis, presented
with 0.69 (P=0.024) via ROC analysis. Its NPV and PPV were 100% and
17.3%, respectively. Additionally, the distinguishing ability of
the three indicators combined logistic regression equation was also
evaluated using ROC analysis. The results revealed that the AUC of
the three indicators in the combined model was 0.82 (P<0.001),
and the sensitivity and specificity were 71.4% and 89.6%,
respectively. The NPV and PPV were 95.9% and 47.6% respectively
(Fig. 3A and B).
 | Table IIIndicators associated with severe
fibrosis (F ≥3) in patients with CHC in univariate and multivariate
analysis. |
Table II
Indicators associated with severe
fibrosis (F ≥3) in patients with CHC in univariate and multivariate
analysis.
| Indicator | F ≤2 (n=106) | F ≥3 (n=14) | P-valuea | OR (95%CI) |
|---|
| Age (years) | 51.08±7.44 | 53.14±6.44 | 0.364 | |
| Females [n (%)] | 57 (53.8) | 6 (42.9) | | |
| Males [n (%)] | 49 (46.2) | 8 (57.1) | 0.442 | |
| ALT (U/l) | 51.85±49.63 | 125.34±144.76 | 0.001 | 0.97 (0.93–1.00) |
| AST (U/l) | 41.27±27.97 | 98.45±92.41 | 0.000 | 1.08 (1.02–1.14) |
| Total bilirubin
(μmol/l) | 16.56±6.92 | 16.12±9.69 | 0.353 | |
| Direct bilirubin
(μmol/l) | 3.23±1.26 | 3.51±2.02 | 0.870 | |
| Albumin (g/l) | 43.37±2.32 | 42.00±2.42 | 0.041 | |
| Prealbumin
(mg/l) | 192.31±187.48 | 146.01±25.94 | 0.008 | |
| GGT (U/l) | 19.86±12.09 | 38.51±31.28 | 0.006 | |
| Alkaline
phosphatase (U/l) | 74.66±19.08 | 88.57±29.60 | 0.095 | |
| White blood cell
(109/l) | 5.15±1.22 | 4.58±1.23 | 0.106 | |
| Red blood cell
(1012/l) | 4.81±0.41 | 4.34±1.60 | 0.919 | |
| Hemoglobin
(g/l) | 149.97±16.21 | 159.57±17.35 | 0.041 | |
| Platelets
(109/l) | 177.25±51.16 | 126.71±48.39 | 0.001 | |
| Type III
procollagen peptide (μg/l) | 28.17±34.27 | 76.81±159.49 | 0.036 | |
| Type IV collagen
(μg/l) | 34.01±87.50 | 73.51±157.65 | 0.026 | |
| Hyaluronic acid
(mg/l) | 188.73±758.21 | 390.93±455.97 | 0.000 | |
| Laminin
(μg/ml) | 38.62±31.02 | 30.52±11.73 | 0.612 | |
| HexCer (d18:1/12:0)
(pmol/ml) | 15.63±11.64 | 26.64±27.64 | 0.024 | 1.03
(1.00–1.06) |
| HexCer (d18:1/16:0)
(pmol/ml) | 1254.68±454.39 | 1620.10±705.52 | 0.016 | |
| HexCer (d18:1/22:0)
(pmol/ml) | 295.10±82.53 | 335.48±82.63 | 0.048 | |
Indicators associated with presence of
severe fibrosis (F ≥3) in CHC with significant fibrosis (F ≥2)
In order to identify potential markers associated
with the presence of severe hepatic fibrosis (F ≥3) in patients
with CHC who had developed significant fibrosis (F ≥2), data were
analyzed using univariate and multivariate analysis (Table III). A total of 64 patients with
CHC who had significant fibrosis were eligible for analysis.
According to the results of the univariate analysis, ALT, AST,
prealbumin, GGT, platelets, hyaluronic acid, HexCer (d18:1/12:0),
HexCer (d18:1/16:0) and HexCer (d18:1/24:0) exhibited a significant
difference (P<0.05) between the F ≥2 and F ≥3 groups. When all
the indicators that exhibited a significant difference were
included in the forward stepwise logistic regression, only HexCer
(d18:1/12:0) and AST were retained in the regression equation.
These variables exhibited a close association with severe fibrosis,
with ORs of 1.08 and 1.01 respectively. The ability to identify
severe fibrosis in patients with CHC who had significant fibrosis
(F ≥2) was also analyzed using the ROC curve. The results
demonstrated that HexCer (d18:1/12:0) had an AUC value of 0.73
(P=0.009), with an NPV and PPV of 100 and 17.2%, respectively.
Additionally, the AUC of this equation, including HexCer
(d18:1/12:0) and AST, reached 0.79 (P=0.001), and the NPV and PPV
reached 96.3% and 35.2%, respectively (Fig. 3C and D).
 | Table IIIIndicators associated with severe
fibrosis (F ≥3) in patients with CHC with significant fibrosis (F
≥2). |
Table III
Indicators associated with severe
fibrosis (F ≥3) in patients with CHC with significant fibrosis (F
≥2).
| Indicator | F ≥2 (n=50) | F ≥3 (n=14) | P-valuea | OR (95%CI) |
|---|
| Age (year) | 51.82±6.32 | 53.14±6.44 | 0.493 | |
| Female [n (%)] | 24 (48.0) | 6 (42.9) | | |
| Male [n (%)] | 26 (52.0) | 8 (57.1) | 0.733 | |
| ALT (U/l) | 64.37±65.11 | 125.34±144.76 | 0.012 | |
| AST (U/l) | 49.50±36.86 | 98.45±92.41 | 0.002 | 1.01
(1.00–1.03) |
| Total bilirubin
(μmol/l) | 17.46±7.63 | 16.12±9.69 | 0.188 | |
| Direct bilirubin
(μmol/l) | 3.43±1.38 | 3.51±2.02 | 0.569 | |
| Albumin (g/l) | 43.12±2.25 | 42.00±2.42 | 0.112 | |
| Prealbumin
(mg/l) | 167.00±32.80 | 146.01±25.94 | 0.031 | |
| GGT (U/l) | 22.88±15.18 | 38.51±31.28 | 0.034 | |
| Alkaline
phosphatase (U/l) | 76.77±20.15 | 88.57±29.61 | 0.158 | |
| White blood cell
(109/l) | 4.96±1.20 | 4.58±1.23 | 0.306 | |
| Red blood cell
(1012/l) | 4.85±0.38 | 4.34±1.60 | 0.981 | |
| Hemoglobin
(g/l) | 149.98±16.00 | 159.57±17.36 | 0.056 | |
| Platelets
(109/l) | 166.14±51.38 | 126.71±48.39 | 0.013 | |
| Type III
procollagen peptide (μg/l) | 30.26±35.97 | 76.81±159.49 | 0.064 | |
| Type IV collagen
(μg/l) | 42.73±120.60 | 73.51±157.65 | 0.129 | |
| Hyaluronic acid
(mg/l) | 305.56±1096.91 | 390.93±455.97 | 0.008 | |
| Laminin
(μg/ml) | 37.74±36.97 | 30.52±11.73 | 0.922 | |
| HexCer (d18:1/12:0)
(pmol/ml) | 13.50±6.56 | 26.64±27.64 | 0.009 | 1.08
(0.99–1.17) |
| HexCer (d18:1/16:0)
(pmol/ml) | 1271.23±485.48 | 1620.10±705.52 | 0.021 | |
| HexCer (d18:1/24:0)
(pmol/ml) | 358.42±89.36 | 422.80±124.25 | 0.033 | |
Discussion
Although a clinical study looking at a single
sphingolipid in CHC, in the case of S1P declining in CHC patients,
has been previously reported, (14), to the best of our knowledge, there
have been no studies that have investigated the full plasma
sphingolipid profile, in order to identify biomarkers associated
with the development of hepatic fibrosis induced by HCV. For the
first time, to the best of our knowledge, the present study used
liver biopsies and the HPLC-MS/MS method to provide a plasma
sphingolipid profile of untreated patients with CHC, with and
without severe hepatic fibrosis, and demonstrated that the plasma
sphingolipid, HexCer (d18:1/12:0), may have a close association
with the formation of severe hepatic fibrosis in CHC, in particular
in patients with CHC who have developed significant fibrosis.
Additionally, plasma HexCer (d18:1/12:0) may be used as a potential
marker for patients with CHC with severe hepatic fibrosis.
In the present study, the plasma sphingolipid
profile was analyzed in a cohort of 120 untreated patients with
CHC, who had chronic HCV infection for ~20 years, which had been
contracted from previous paid plasma donations. This cohort of
patients with CHC were a suitable group to investigate as they
lived in close proximity to each other and reported similar
lifestyles in terms of diet and living environment. Although the
incidence of severe fibrosis in this cohort was not high, it did
reflect the alteration in plasma sphingolipids precisely, according
to the different stages of fibrosis in the context of the
treatment-naïve status. Using the improved quantitative
high-throughput lipidomic methods (16), the results of the plasma
sphingolipid profile showed different levels of plasma
sphingolipids in patients with CHC, with and without severe hepatic
fibrosis. The altered plasma levels of HexCer (d18:1/12:0), HexCer
(d18:1/16:0) and HexCer (d18:1/22:0) were observed to be associated
with the presence of severe fibrosis. Following adjustment for
confounding indicators, HexCer (d18:1/12:0) remained closely
associated with severe fibrosis. This indicated that elevated
plasma HexCer (d18:1/12:0) may be implicated in the pathogenesis of
severe fibrosis in CHC. At present, there is no direct evidence for
the role of this specific glycosphingolipid in the pathogenesis of
severe fibrosis due to CHC. A possible mechanism has been
demonstrated in a previous study using an animal model, which
reported that exogenous administration of α-galactosylceramide
accelerated carbon tetrachloride-induced liver fibrosis in
vivo through the activation of invariant natural killer T cells
(20). This may suggest that
glycosphingolipids contribute to the progression of liver fibrosis.
However, further experimental studies are required to determine the
underlying mechanisms responsible for the association of HexCer
(d18:1/12:0) with severe hepatic fibrosis in CHC.
The primary concern in CHC is the occurrence and
slow evolution of fibrosis over a number of years, culminating in
cirrhosis. A large scale clinical study revealed that severe
fibrosis may go undetected, with few or no clinical symptoms and
signs (21). Additionally, a
previous study confirmed that the rate of progression to cirrhosis
is accelerated in patients whose initial biopsies exhibited septal
fibrosis (F ≥2) and that these individuals are at an increased risk
of developing advanced cirrhosis over the ensuing decade (22,23).
Therefore, it is also important to examine the mechanisms
underlying the evolution of fibrosis, as well as markers of severe
fibrosis, which may provide insight into possible therapeutic
targets to prevent the formation of severe fibrosis in patients
with CHC who have developed septal fibrosis (F ≥2). In the present
study, the plasma sphingolipids associated with severe fibrosis
were evaluated in patients with CHC who had significant fibrosis (F
≥2). It is noteworthy that HexCer (d18:1/12:0), which was closely
associated with severe fibrosis in all patients with CHC, following
adjustment for confounding factors, also exhibited a correlation in
a multivariate regression model, and demonstrated an association
with the presence of severe fibrosis in the population of patients
with CHC who had developed significant fibrosis. Therefore, it is
hypothesized that HexCer (d18:1/12:0) may contribute to the
development of advanced cirrhosis in patients with septal fibrosis
(F ≥2).
ROC analysis was performed in the present study. It
was shown that plasma HexCer (d18:1/12:0), with an AUC of 0.69, had
the diagnostic ability to distinguish severe fibrosis in CHC. In
addition, in multivariate analysis, the AUC of the indicators
retained in the regression model was increased in the combined
model, indicating severe fibrosis. This suggested that HexCer
(d18:1/12:0) has potential as a noninvasive diagnostic indicator of
severe hepatic fibrosis in CHC. This diagnostic ability may be
strengthened when measurement of HexCer (d18:1/12:0) is combined
with that of serum aminotransferase levels. Furthermore, the
present study also identified that HexCer (d18:1/12:0) had
acceptable diagnostic ability (AUC=0.73) with which to identify
severe hepatic fibrosis in patients with CHC who had developed
significant fibrosis. Similarly, the final regression model,
including HexCer (d18:1/12:0), exhibited an improved diagnostic
ability. Therefore, based on the results of the ROC analysis,
HexCer (d18:1/12:0) may be utilized as a noninvasive marker to
detect the presence of severe hepatic fibrosis in CHC, in
particular in patients with CHC who have progressed to a
significant stage of fibrosis.
Issues remain concerning the precise mechanisms
underlying the pathogenesis of CHC, which were not included in the
present study. In addition, CHC plasma donor with the same
background were selected from a cohort that has had a long-term
follow up. The relatively small sample size was a limitation of the
present study. The diagnostic capacity of HexCer (d18:1/12:0)
requires large scale clinical evaluation in the future.
In conclusion, for the first time, to the best of
our knowledge, the present study analyzed the plasma sphingolipid
profile in patients with CHC, with and without severe fibrosis.
Plasma HexCer (d18:1/12:0) exhibited a close association with the
formation of severe fibrosis in CHC, in particular in patients with
CHC who had developed significant fibrosis. The present study also
provided a novel insight into the molecular mechanisms underlying
the development of severe fibrosis in CHC. However, further
experimental studies are required to determine the precise
mechanisms underlying these associations.
Acknowledgments
The present study was supported by the National
Science and Technology Key Project on ‘Major Infectious Diseases
such as HIV/AIDS, Viral Hepatitis Prevention and Treatment’ (grant
nos. 2012ZX10002004-006, 2012ZX10004904-003-001 and
2013ZX10002002-006), the Ministry of Science and Technology of the
People’s Republic of China (grant no. 2012ZX09301002-006), the High
Technical Personnel Training Item in the Beijing Health System
(grant no. 2011-3-083), the Special Scientific Research Fund for
Beijing Health Development (grant no. 2011-2018-04) and YouAn
Scientific Research Fund for Liver Disease and HIV/AIDS (grant no.
BJYAH-2011-045). The authors would like to thank all participants
involved in the present study.
References
|
1
|
Mohd Hanafiah K, Groeger J, Flaxman AD and
Wiersma ST: Global epidemiology of hepatitis C virus infection: new
estimates of age-specific antibody to HCV seroprevalence.
Hepatology. 57:1333–1342. 2013. View Article : Google Scholar
|
|
2
|
Ghany MG, Strader DB, Thomas DL and Seeff
LB: Diagnosis, management, and treatment of hepatitis C: an update.
Hepatology. 49:1335–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lawson A, Hagan S, Rye K, et al: The
natural history of hepatitis C with severe hepatic fibrosis. J
Hepatol. 47:37–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sebastiani G and Alberti A: How far is
noninvasive assessment of liver fibrosis from replacing liver
biopsy in hepatitis C? J Viral Hepat. 19(Suppl 1): 18–32. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Regev A, Berho M, Jeffers LJ, et al:
Sampling error and intrao-bserver variation in liver biopsy in
patients with chronic HCV infection. Am J Gastroenterol.
97:2614–2618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lingwood D and Simons K: Lipid rafts as a
membrane-organizing principle. Science. 327:46–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Milhas D, Clarke CJ and Hannun YA:
Sphingomyelin metabolism at the plasma membrane: implications for
bioactive sphingolipids. FEBS Lett. 584:1887–1894. 2010. View Article : Google Scholar :
|
|
8
|
Bartke N and Hannun YA: Bioactive
sphingolipids: metabolism and function. J Lipid Res. 50(Suppl):
S91–S96. 2009. View Article : Google Scholar :
|
|
9
|
Sakamoto H, Okamoto K, Aoki M, et al: Host
sphingolipid biosynthesis as a target for hepatitis C virus
therapy. Nat Chem Biol. 1:333–337. 2005. View Article : Google Scholar
|
|
10
|
Umehara T, Sudoh M, Yasui F, et al: Serine
palmitoyltransferase inhibitor suppresses HCV replication in a
mouse model. Biochem Biophys Res Commun. 346:67–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shea BS and Tager AM: Sphingolipid
regulation of tissue fibrosis. Open Rheumatol J. 6:123–129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strub GM, Maceyka M, Hait NC, Milstien S
and Spiegel S: Extracellular and intracellular actions of
sphingosine-1-phosphate. Adv Exp Med Biol. 688:141–155.
2010.PubMed/NCBI
|
|
13
|
Li C, Zheng S, You H, et al: Sphingosine
1-phosphate (S1P)/S1P receptors are involved in human liver
fibrosis by action on hepatic myofibroblasts motility. J Hepatol.
54:1205–1213. 2011. View Article : Google Scholar
|
|
14
|
Ikeda H, Ohkawa R, Watanabe N, et al:
Plasma concentration of bioactive lipid mediator sphingosine
1-phosphate is reduced in patients with chronic hepatitis C. Clin
Chim Acta. 411:765–770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu F, Wu CS, Hou JF, Jin Y and Zhang JL:
Sphingolipids as new biomarkers for assessment of delayed-type
hypersensitivity and response to triptolide. PLoS One.
7:e524542012. View Article : Google Scholar
|
|
16
|
Qu F, Zheng SJ, Wu CS, Jia ZX, Zhang JL
and Duan ZP: Lipidomic profiling of plasma in patients with chronic
hepatitis C infection. Anal Bioanal Chem. 406:555–564. 2014.
View Article : Google Scholar
|
|
17
|
Hepatotogy Branch, Infectious and
Parasitology branch, Chinese Medical Association: Guideline of
prevention and treatment of hepatitis C. Zhonghua Yu Fang Yi Xue Za
Zhi. 38:210–215. 2004.
|
|
18
|
Group TFMCS Intraobserver and
interobserver variations in liver biopsy interpretation in patients
with chronic hepatitis C. Hepatology. 20:15–20. 1994. View Article : Google Scholar
|
|
19
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
Cooperative Study Group. Hepatology. 24:289–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park O, Jeong WI, Wang L, et al: Diverse
roles of invariant natural killer T cells in liver injury and
fibrosis induced by carbon tetrachloride. Hepatology. 49:1683–1694.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Bisceglie AM: Natural history of
hepatitis C: its impact on clinical management. Hepatology.
31:1014–1018. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yano M, Kumada H, Kage M, et al: The
long-term pathological evolution of chronic hepatitis C.
Hepatology. 23:1334–1340. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marcellin P, Asselah T and Boyer N:
Fibrosis and disease progression in hepatitis C. Hepatology.
36:S47–S56. 2002. View Article : Google Scholar : PubMed/NCBI
|