Introduction
Hypoxia inducible factor-1α (HIF-1α) may regulate
the expression of numerous cytokines, such as vascular endothelial
growth factor-A (VEGF-A), and promote the proliferation (1) and the angiogenic potential of small
cell lung cancer (SCLC) (2). The
results of previous studies indicated that HIF-1α may be an
effective molecular therapeutic target for SCLC (3,4).
Several different approaches were used to effectively inhibit
HIF-1α expression in tumors, including anti-sense oligonucleotides
against HIF-1α (5), a
dominant-negative form of HIF-1α, and a peptide, which inhibits the
binding between HIF-1α and the coactivator p300/CBP (6). Inhibiting HIF-1α expression by
increasing the expression of certain anti-tumor factors acts as a
therapeutic tool for the molecular treatment of tumors (7).
Suppressor of cytokine signaling 3 (SOCS3) is a
member of the SOCS family (8) and
is structurally composed of distinct functional domains, which
include an N-terminal kinase inhibitory region, a central SH2
domain and a C-terminal homologous region termed the SOCS-box
(9). SOCS3 has been reported to be
silenced in certain tumors (10).
SOCS3 expression is suppressed due to aberrant methylation in its
promoter region, which frequently occurs in a variety of human
tumors (11,12). Restoring the genetic function of
the SOCS3 gene by demethylation or SOCS3 gene transfection was
shown to suppress tumor cell growth by attenuating activation of
signal transducer and activator of transcription 3 (STAT3) in
several human cancer cell lines (13). However, apart from STAT3, little is
known about the mechanism by which SOCS3 modulates other
intracellular signaling cascades, such as PI3K/Akt, which also has
a critical role in tumorigenesis and in turn affects the biological
characteristics of malignant tumors, including angiogenesis,
migration and proliferation (14).
The PI3K/AKT pathway has a critical role in multiple cellular
functions, including metabolism, proliferation, growth and survival
(15). There is growing evidence
that this pathway is frequently deregulated during tumorigenesis,
which in turn affects certain biological phenotypes (16).
The present study explored the effect of SOCS3
restoration by gene transfection or demethylation on HIF-1α
expression and proliferation of SCLC cells, and assessed the
involvement of Akt and STAT3 signaling in these processes. The
results indicated that SOCS3 may be considered as a novel
therapeutic for treating SCLC.
Materials and methods
Ethics
The present study was approved by the Institutional
Review Board of Biomedicine of Anhui Medical School (Hefei,
China).
Cell culture and experimental
treatment
The human SCLC cell line NCI-H446 (Cell Bank of
Shanghai Institutes for Biological Sciences, China Academy of
Sciences, Shanghai, China) was cultured as in a previous study
(1). Once passaged to the fifth
generation, the cells were grown in 75 cm2 culture
flasks and harvested in a solution of trypsin-EDTA at the
logarithmic growth phase. A cytometry assay was used for cell
counting (2). For the
demethylation treatment, NCI-H446 cells (60–70% confluence) were
treated with 5 μm 5-aza-2′-deoxy-cytidine for six days and
the medium was replaced every other day. DNA, RNA and protein were
extracted for analysis using kits obtained from Sangon Biotech Co.,
Ltd. (Shanghai, China). For blocking Akt expression, wortmannin
(Sigma-Aldrich, St. Louis, MO, USA) was used as an inhibitor of the
PI3K/Akt signaling pathway. Wortmannin was dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich) and final concentrations of 20
μM were used to treat the cells. Equal volumes of DMSO were
used for the vehicle control (17). CPA-7, which is a specific inhibitor
of the PI3K/Akt signaling pathway, was used for blocking STAT3
expression and dissolved in DMSO to achieve a final concentration
of 10 μM (18). For plasmid
transfection, pcDNA3-STAT3 and pcDNA3-Akt were received from the
Department of Viral-Gene Therapy, Shanghai Eastern Hepatobiliary
Surgery Hospital (Shanghai, China).
Adenovirus vector construction and cell
transfection
Tumor-specific replication-defective adenovirus type
5 (Ad5) was used as the vector. Ad5-SOCS3-green fluorescence
protein (GFP) construct was received from the Department of
Viral-Gene Therapy, Shanghai Eastern Hepatobiliary Surgery Hospital
(Shanghai, China). SOCS3 cDNA was amplified using gene-specific
primers: Sense, 5′-GCGGTCGACATG
TACCCATACGACGTCCCGATTACGCTATGGTCACCCA-CAGCAAG-3′ and antisense,
5′-GATGCGGCCGCTTA AAGCGGGGC-3′ (Sangon Biotech Co., Ltd.). This was
designed to generate an N-terminal hemagglutinin (HA) tag and
restriction sites SalI and NotI at the 5′- and
3′-cDNA ends. A recombinant adenovirus (pAd5-SOCS3-HIF-1α) encoding
human HIF-1a gene and human SOCS3 gene was contructed and then
NCI-H446 cells were transfected. The HIF-1α gene which was
amplified by polymerase chain reaction and the SOCS3 gene from the
plasmid pIRES2-SOCS3 were both subcloned into the shuttle vector
pShuttle-CMV. A shuttle vector pShuttle-SOCS3-HIF-1α was obtained
and then it was co-transformed into adenoviral backbone plasmid
pAd5 to make homologous recombination with the HIF-1α and SOCS3
genes. For transfection, cells were cultured in six-well plates and
exposed to viral supernatant in the absence of cytokines and serum
with different multiplicities of infection (MOI). A constructed
recombinant adenovirus vector was used for NCI-H446 cell
transfection.
Methylation-specific polymerase chain
reaction (PCR)
Methylation-specific PCR (MSP) was performed based
on results of a previous study (9). The methylation-specific primer was
designed using the Methyl Primer Express software v1.0 (Applied
Biosystems, Foster City, CA, USA). Primer sequences are shown in
Table I. The PCR reaction
conditions were as follows: Initial denaturation at 95°C for 10
min; 40 cycles of denaturation at 95°C for 40 sec; annealing at
65°C for 30 sec and elongation at 75°C for 40 sec. Finally, cycling
was completed with an elongation step at 75°C for 10 min. Sequences
of MSP primers in exon 1 of SOCS3 were: Sense,
5′-TTCGAGGTGTTCGATTAGAC-3′ and antisense,
5′-AAAATGCTTCCGACATAGAT-3′. Sequences of MSP primers in intron 1 of
SOCS3 were: Sense, 5′-GCCTCCGGGTAAGCGTGGATTAG-3′ and antisense,
5′-AATACATAGAGGCTGCGCGAAGC-3′.
 | Table IMethylation-polymerase chain reaction
conditions and primer sequences. |
Table I
Methylation-polymerase chain reaction
conditions and primer sequences.
| Primer | Sequences | Tm
(°C) | Length
(bp) |
|---|
| Methylation
primers | Sense:
5′-TTCGAGGTGTTCGAGTAGTC-3′
Antisense: 5′-AACGATCTTC CGACA AAAAT-3′ | 65 | 310 |
| Unmethylation
primers | Sense:
5′-TTTTTTGAGGTGTTTGAGTAGTT-3′
Antisense: 5′-AACAAT CTTCCAACAAAAATACT-3′ | | |
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
RT-qPCR was performed using a SYBR ExScript RTPCR
kit (Takara Biotechnology Co. Ltd., Dalian, China) according to the
manufacturer’s instructions and using the iCycler Real-Time PCR
detection system (Bio-Rad, Hercules, CA, USA). All RNA samples were
run in duplicate on 96-well optical PCR plates. The PCR conditions
were set as follows: Initial incubation for 2 min at 50°C, denature
at 95°C for 10 min, 32 cycles of 94°C for 40 sec, 60°C for 40 sec
and 60°C for 40 sec, followed by 72°C for 1 min. PCR reaction
conditions and primer sequences are shown in Table II. Relative changes in gene
expression were calculated using the equation: Relative changes in
gene expression =2−ΔΔCT where ΔCt = Ct target - Ct
β-actin and ΔΔCt = ΔCt Unmethylated - ΔCt control.
 | Table IIPolymerase chain reaction conditions
and primer sequences |
Table II
Polymerase chain reaction conditions
and primer sequences
| Gene | Primer | Tm
(°C) | Length
(bp) |
|---|
| SOCS3 | Sense:
5′-GCTGGCGAAGGAAATGGT-3′
Antisense: 5′-GGAGCCTAGGGTGAAAGATG-3′ | 62 | 346 |
| β-actin | Sense:
5′-CCAAGGCCAACCGCGAGAAGATGAC-3′
Antisense: 5′-AGGGTACATGGTGGTGGCGCCAGAC-3′ | 65 | 587 |
Western blot analysis
Cells were harvested and analyzed for the protein
levels of SOCS3, HIF-1α, STAT3, phosphorylated (p)STAT3, Akt and
pAkt. Briefly, all proteins were extracted by disrupting cells in
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), separated on a 10%
SDS-polyacrylamide gel (Beyotime Institute of Biotechnology) and
then transferred onto a polyvinylidene difluoride (PVDF) membrane.
The membranes were then blocked at room temperature for 1 h with 5%
non-fat milk in Tris-buffered saline containing Tween 20 (TBST).
Subsequently, the membranes were incubated with rabbit monoclonal
anti-SOCS3 (1:400 dilution); rabbit monoclonal anti-HIF-1α (1:500
dilution); rabbit monoclonal anti-STAT3 (1:1,000 dilution), rabbit
monoclonal anti-β-actin (1:1,000 dilution) and rabbbit monoclonal
anti-Akt (1:1,000 dilution; Wuhan Boster Biological Engineering
Technology Limited Company, Wuhan, China), rabbit monoclonal
anti-phospho-Akt (1:1,000 dilution; Cell Signaling Technology,
Beverly, MA, USA) or rabbit monoclonal anti-phospho-STAT3 (1:800
dilution; Santa Cruz Biotechnology, Austin, TX, USA) at 37°C for 2
h. The membranes were subsequently incubated with goat anti-rabbit
horseradish peroxidase (HRP)-conjugated immunoglobulin G (Wuhan
Boster Biological Engineering Technology Limited Company) at room
temperature for 1 h. Immunoreactivity was detected using an
enhanced chemiluminescence kit (EZ-ECL kit for HRP; Beyotime
Institute of Biotechnology) and images of gels were captured on
X-ray film (Shanghai Baiyun Sanhe Sensitive Materials Co., Ltd.,
Shanghai, China). β-actin was used as an internal control.
Immunofluorescence
Once the cells were passaged to the fifth
generation, a cell suspension (1×105 cells/ml) was
prepared, transferred to cover slips and incubated at 37°C in
humidified atmosphere containing 5% CO2 and 20%
O2 for 24 h. The cells were then fixed with 4%
paraformaldehyde (Beyotime Institute of Biotechnology) for 20 min,
permeabilized with 0.5% Triton X-100 (Beyotime Institute of
Biotechnology) and incubated with anti-CD34 primary antibody (Wuhan
Boster Biological Engineering Technology Limited Company) overnight
at 4°C. Subsequently, cells were washed and incubated with
Rhodamine Red-X secondary antibodies (1:1,000 dilution) (Wuhan
Boster Biological Engineering Technology Limited Company) for 1 h
at 37°C. Images were observed using a laser confocal microscope
(LSM710; Carl Zeiss, Jena, Germany).
In vivo tumor experiments and angiogenic
measurements
A total of 36 male congenital athymic BALB/c nude
mice were obtained from the Experimental Animal Center of the
Shanghai Jiao Tong University School of Medicine (Shanghai, China).
They were maintained under pathogen-free conditions in accordance
with established institutional guidance and approved protocols. The
mice were divided into three groups (n=12/group): Ad5 group,
Ad5-SOCS3 group and Ad5-SOCS3+HIF-1α group. All experiments were
performed using 6–8 week-old mice weighing 16–22 g. The mice were
maintained under controlled light (12 h light/12 h dark) and
temperature (22°C) conditions, were housed in individual cages and
were given ad libitum access to standard mouse chow. Animal
care and experimental procedures were performed with the approval
of the Animal Care and Experimental Center of Anhui Medical
University, under estanlished guidelines. The mice were sacrificed
by cervical dislocation. In vitro cultured NCI-H446 cells
(1×107) transfected with Ad5-SOCS3 or empty Ad5 vector
suspended in 100 ml PBS were subcutaneously injected into the flank
area of mice. NCI-H446 cells (1×107) without
transfection were injected when tumors reached 3–5 mm in diameter.
Mice were injected with either vehicle (10% DMSO/PBS), 4 mg/kg
wortmannin or 5 mg/kg CPA-7 twice weekly. The tumor size was
measured with calipers every three days and the tumor volume was
calculated according to the formula: Volume = width2 ×
length × 0.5. Subsequently, tumors were removed and weighed 30 days
following inoculation.
According to the method of QingXu (19), angiogenesis quantification was
performed in a double-blinded manner and imaging software was used
to capture bright-field images from two fields for each sample.
Data for these image files were collected using Image-Pro Plus
software (v. 5.0; National Institutes of Health, Bethesda, MD,
USA). CD34-positive areas (blood vessels) were selected using the
histogram and dropper tools. The Count function was then used to
total the number of CD34-positive areas. Measurement was selected
within the Count/Size function and the Area tool was utilized to
calculate the area of CD34-positive blood vessels in each entire
field. Every field in each of the entire tumor sections was
examined and analyzed.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data processing. An independent-samples t-test was
used to evaluate the differences in optical density (OD) values or
counting of tumor cells between groups with various treatments. All
values are expressed as the mean ± standard deviation of three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
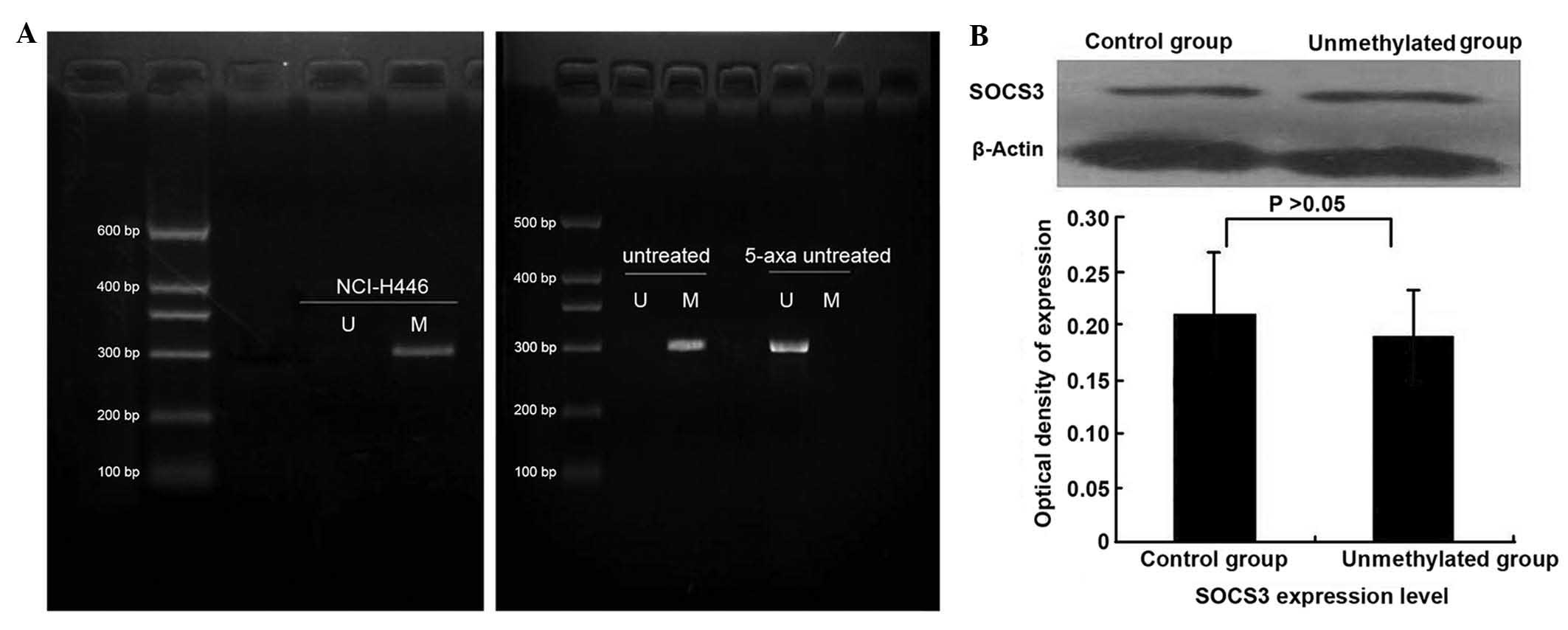
Demethylation does not markedly affect
protein expression of SOCS3
SOCS3 mRNA expression was weak in NCI-H446 cells,
but was markedly increased demethylation by treatment with
5-aza-2′-deoxycytidine (Fig. 1A).
At the protein level, it was found that following treatment with
5-aza-2′-deoxycytidine, the expression of SOCS3 was not upregulated
(Fig. 1B). This finding differed
from results of previous studies, in which demethylation-associated
upregulation of SOCS3 in A549 cells and squamous cell carcinoma
cells of the head and neck was observed following treatment with
5-aza-2′-deoxycytidine (20).
Transfection efficiency of SOCS3
In the present study, cells were divided into 2
groups: Ad5-SOCS3-GFP transfection group and Ad5-GFP transfection
group. The efficiency of transfection was estimated by determining
the percentage of fluorescent cells in cells infected with
Ad-SOCS3-GFP. The appropriate MOI was selected using the following
formula: MOI = titer (pfu) × viral fluid (L)/cell number. Using
fluorescence microscopy, it was observed that the transfection
efficiency of the adenoviral vectors into cells was high and
reached >90% at a MOI of 50. mRNA and protein levels of SOCS3
were significantly upregu-lated following transfection with
Ad-SOCS3-GFP (Fig. 2A and B).
 | Figure 2Transfection with Ad-SOCS3 upregulates
the expression levels of SOCS3 in NCI-H446 cells and inhibits their
proliferative and angiogenic potential. (A) Determination of
transduction conditions and evaluation of SOCS3 induction
efficiency. Five different MOIs (20, 30, 40, 50 and 70) were
assessed in the transduction experiment (60 h). The transduction
efficiency was the highest when the MOI was 50 (MOI 50 vs. MOI 40;
P<0.05; MOI 50 vs. MOI 70; P<0.05). Transduction efficiency
of NCI-H446 cells with Ad5-GFP after 60 h is shown in the
fluorescent image (MOI 50; magnification, ×200). After the cells
were transduced with Ad5 and Ad5-SOCS3 (MOI, 50), the mRNA
expression levels of SOCS3 were measured using quantitative
polymerase chain reaction (NCI-H446/Ad5-SOCS3 group vs. control
group, P<0.01; NCI-H446/Ad5 group vs. NCI-H446/Ad5-SOCS3 group,
P<0.01) (B) Western blot analysis of SOCS3 and VEGF-A protein
expression. Representative images of three independent experiments
(lane 1, SOCS3 and VEGF-A protein expression in the control group;
lane 2, SOCS3 and VEGF-A protein expression in Ad5-SOCS3
transfection group; lane 3, SOCS3 and VEGF-A protein expression in
Ad5 transfection group) and relative expression of SOCS3 and VEGF-A
protein normalized to β-actin (SOCS3 expression in Ad5-SOCS3
transfection group vs. control group, P<0.05; SOCS3 expression
in Ad5-SOCS3 transfection group vs. Ad5 transfection group,
P<0.05; VEGF-A expression in Ad5-SOCS3 transfection group vs.
control group, P<0.01; VEGF-A expression in Ad5-SOCS3
transfection group vs. Ad5 transfection group, P<0.01). (C)
Growth curve of the cells in two groups. In the control group,
NCI-H446 cells entered the period of logarithmic growth after the
fourth day. Following transfection with Ad5-SOCS3, cell growth was
significantly decreased and the logarithmic growth phase was not
entered (NCI-H446/SOCS3 group vs. NCI-H446, P<0.05). Ad5,
tumor-specific replication-defective adenovirus type 5; SOCS3,
suppressor of cytokine signaling 3; VEGF, vascular endothelial
growth factor; MOI, multiplicity of infection; GFP,
green-fluorescent protein. |
Upregulation of SOCS3 inhibits the
proliferative and angiogenic potential of SCLC
Following transfection with Ad-SOCS3, mRNA and
protein expression of SOCS3 were upregulated in NCI-H446 cells
(Fig. 2A and B). In addition the
cell growth curve demonstrated that following transfection with
Ad5-SOCS3-GFP, the cell proliferation rate was significantly
inhibited, particularly from the fifth day (Fig. 2C). The expression of the angiogenic
factor VEGF-A was found to be significantly inhibited following
upregulation of SOCS3 (Fig.
2B).
HIF-1α is required for SOCS3-mediated
inhibition of proliferation and angiogenesis of SCLC cells
Previous studies by our group demonstrated that
HIF-1α enhances the proliferative and angiogenic potential of SCLC
cells by regulating functional genes, including VEGF-A and
interleukin-6 (1,2). From a previous microarray analysis,
it was identified that the two other members of the SOCS family,
SOCS1 and SOCS2, were also regulated by HIF-1α (1). However, the mutual regulation between
SOCS3 and HIF-1α has not been previously reported. In the present
study, it was found that following transfection with Ad5-SOCS3,
SOCS3 expression was upregulated, but HIF-1α expression was
downregulated; cell proliferation as well as VEGF-A and CD34
expression were also inhibited (Fig.
3A–C). Following co-transfection with SOCS3 and HIF-1α, HIF-1α
expression, cell proliferation, VEGF-A and CD34 expression were all
significantly upregulated compared with those following
transfection with Ad5-SOCS3 only (Fig.
3A–C).
 | Figure 3HIF-1α is required for proliferation
of SOCS3-transduced SCLC cells and their angiogenic potential. (A)
Western blot analysis of HIF-1α and VEGF-A protein expression.
Representative images of three independent experiments (lane 1,
HIF-1α and VEGF-A protein expression in the non-transfection group;
lane 2, VEGF-A and HIF-1α protein expression in SOCS3 transfection
group; lane 3, HIF-1α and VEGF-A protein expression in SOCS3 and
HIF-1α co-transfection group) and quantified expression of HIF-1α
and VEGF-A protein normalized to β-actin (HIF-1α expression in
SOCS3 transfection group vs. non-transfection group, P<0.05;
HIF-1α expression in SOCS3 transfection group vs. SOCS3+HIF-1α
co-transfection group, P<0.05; VEGF-A expression in SOCS3
transfection group vs. non-transfection group, P<0.05; VEGF-A
expression in SOCS3 transfection group vs. SOCS3+HIF-1α
co-transfection group, P<0.05) (B) Growth curves of cells in
SOCS3 transfection and SOCS3+HIF-1α co-transfection groups.
Co-transfection with SOCS3 and HIF-1α significantly promoted the
growth rate of the cells (NCI-H446/SOCS3 group vs.
NCI-H446/SOCS3+HIF-1α group, P<0.05). (C) CD34 expression in the
three groups (magnification, ×200). Immunofluorescence staining
showed that following transfection with SOCS3, CD34 expression
intensity was significantly decreased, while it was significantly
intensified following upregulation of SOCS3 and HIF-1α. SOCS3,
suppressor of cytokine signaling 3; VEGF, vascular endothelial
growth factor; SCLC, small cell lung cancer; HIF-1α,
hypoxia-inducible factor-1α. |
SOCS3 inhibits HIF-1α expression through
the Akt pathway, but not the STAT3 pathway
As it is well-established that SOCS3 negatively
regulates the JAK/STAT signaling pathway, SOCS3 was suggested to
have a role in the Akt-mediated signaling pathway. To examine the
underlying molecular mechanism by which SOCS3 inhibited HIF-1α
expression in SCLC cells, the effect of SOCS3 on Akt and STAT3
phosphorylation as well as HIF-1α expression was assessed in
NCI-H446 cells using western blot analysis. When SOCS3 was
exogenously expressed in NCI-H446 cells, HIF-1α expression was
inhibited and tyrosine phosphorylation of Akt and STAT3 as well as
the protein levels of Akt and STAT3 were decreased (Fig. 4A). In addition, pcDNA3-STAT3 or
pcDNA3-Akt was transfected into cells modified by Ad-SOCS3. It was
found that along with the increased expression of Akt, rather than
STAT3, HIF-1α expression was upregulated (Fig. 4B). Treatment of cells with
wortmannin inhibited the Akt protein, including pAkt, and the
expression of HIF-1α was also inhibited. However, treatment with
CPA-7 decreased levels of STAT3 protein, including pSTAT3, but the
expression of HIF-1α was not markedly inhibited (Fig. 4C). This suggested that SOCS3
inhibited HIF-1α expression in HCI-H446 cells through the Akt
pathway but not the STAT3 pathway.
 | Figure 4SOCS3 inhibits HIF-1α expression
through the Akt pathway but not the STAT3 pathway. Protein levels
of HIF-1α, STAT3, Akt and the phosphorylated, activated forms
pSTAT3 and pAkt were assessed using western blot analysis.
Representative images of three independent experiments and
quantified levels normalized to β-actin are shown. (A) Following
transfection of NCI-H446 cells with Ad5-SOCS3, the expression
levels of HIF-1α, STAT3, Akt as well as pSTAT3 and pAkt decreased
significantly (P<0.05, Ad5-SOCS3 group vs, control group). (B)
In NCI-H446 co-transfected with Ad5-SOCS3 and pcDNA3-STAT3, STAT3
and pSTAT3 expression were upregulated (*P<0.05),
while HIF-1α expression levels exhibited no significant change
(▲P>0.05). However, when co-transfected with
pcDNA3-Akt not only Akt and pAkt, but also HIF-1α expression levels
were upregulated (*P<0.05). (C) In NCI-H446 cells
treated with the STAT3 inhibitor CPA-7, HIF-1α expression levels
exhibited no significant change (▲P>0.05). By
contrast, when treated with Akt inhibitor wortmannin, HIF-1α
expression levels were markedly inhibited (*P<0.05).
SOCS3, suppressor of cytokine signaling 3; HIF-1α,
hypoxia-inducible factor-1α; STAT3, signal transducer and
transcription activator 3; p, phosphorylated. |
Targeting Akt may inhibit the
proliferative and angiogenic potential of SCLC cells
A previous study confirmed that JAK/STAT3 and
PI3K/Akt pathways were involved in inhibiting the proliferative and
angiogenic potential of tumor cells (21). The present study found that
targeting Akt but not STAT3 inhibited SCLC cell growth and
angiogenesis. Wortmannin-treatment of NCI-H446 cells significantly
inhibited the growth rate and also CD34 and VEGF expression were
inhibited. However, following the treatment with CPA-7, it was
found that growth rate, CD34 and VEGF expression were not
significantly inhibited (Fig.
5A–C).
 | Figure 5Targeting Akt inhibits the
proliferation and angiogenic potential of SCLC cells. (A) Growth
curves of cells in the control, CPA-7 and wortmannin groups.
Following treatment with the Akt inhibitor wortmannin, the growth
rate was significantly decreased; however, when treated with STAT3
inhibitor CPA-7, the growth curve was vimilar to that of the
control group (NCI-H446/wortmannin group vs. NCI-H446/control
group, P<0.05; NCI-H446/CPA-7 group vs. NCI-H446/control group,
P>0.05). (B) Western blot analysis of VEGF-A protein expression.
Representative images of three independent experiments and
quantified results normalized to β-actin are shown. Following
treatment with wortmannin, VEGF-A expression was significantly
inhibited, but following treatment with CPA-7, VEGF-A expression
exhibited no significant change (NCI-H446/wortmannin group vs.
NCI-H446/control group, P<0.05; NCI-H446/CPA-7 group vs.
NCI-H446/control group, P>0.05). (C) CD34 expression in three
groups (magnification, x200). Immunofluorescence staining showed
that following treatment with wortmannin, the CD34 expression was
significantly decreased, while following treatment with CPA-7, CD34
expression was not significantly change. VEGF, vascular endothelial
growth factor; SCLC, small cell lung cancer. |
Upregulation of SOCS3 inhibits tumor
growth in vivo
As it was demonstrated that transfection with
Ad5-SOCS3 produced a stably expressing cell line, these cells were
subcutaneously injected into nude mice to form tumors. Compared
with the tumor formed by cells transfected with the empty vector
Ad5, tumors overexpressing SOCS3 exhibited a lower growth rate and
lower rate of angiogenesis (Fig.
6A–C). These results demonstrated that SOCS3 is a tumor
suppressor and inhibits the proliferative and angiogenic potential
of SCLC cells. In a further in vivo experiment, the tumor
formed by cells co-transfected with SOCS3 and HIF-1α exhibited a
higher growth rate and level of angiogenesis compared with that of
cells transfected with Ad5-SOCS3 only (Fig. 6A–C). This demonstrated that the
inhibition of the proliferative and angiogenic potential of SCLC
cells may be realized through the inhibition of HIF-1α expression.
In another experiment, wortmannin and CPA-7 were injected for
intervention of the subcutaneous transplantation tumor. In
accordance with the results of the in vitro experiment,
following wortmannin injection, the growth rate and angio-genesis
of tumors were significantly inhibited, whilst CPA-7 injection had
no significant effect (Fig. 7A and
B). These results demonstrated that Akt is the key signaling
pathway involved not only in HIF-1α expression, but also the
proliferative and angiogenic potential of SCLC cells.
 | Figure 6SOCS3 causes growth suppression and
angiogenesis of NCI-H446 cells in vivo and HIF-1α is
required in this process. (A) NCI-H446 cells were transfected with
empty Ad5 vector, Ad5-SOCS3 or Ad5-SOCS3+HIF-1α. Subsequently,
transfected cells (1×107) of the three groups were
subcutaneously injected into mice to form transplantation tumors.
The growth curve shows that compared with that of the Ad5 group,
the growth rate of Ad5-SOCS3 cells was decreased, particularly from
21 days after inoculation (Ad5-SOCS3 group vs. Ad5 group,
*P<0.05; n=11). Compared with that of the Ad5-SOCS3
group, the growth rate in the Ad5-SOCS3+HIF-1α co-transfection
group was increased, particularly from 18 days after inoculation
(Ad5-SOCS3+HIF-1α group vs. Ad5-SOCS3 group, ▲P<0.05,
n=11). (B) Following sacrification of the mice 30 days after
inoculation, tumor weight was significantly decreased in the
Ad5-SOCS3 group (Ad5-SOCS3 group vs. Ad5 group,
*P<0.05, n=11) as compared with that in the Ad5
group, but following co-transfection with HIF-1α, the tumor weight
increased (Ad5-SOCS3+HIF-1α group vs. Ad5-SOCS3 group,
▲P<0.05, n=11). (C) Representative microscopic images
of CD34 antibody-stained tumor sections (magnification, ×10). The
bar graph shows the mean neovascular densities ± standard
deviation. Images of the entire area of each tumor were captured
and analyzed. Results of immunohistochemical semiquantitative
analysis showed that the number of CD34-positive areas was
decreased following transfection with SOCS3 as compared with that
in the empty vector-transfected group (*P<0.05),
while this effect was significantly reduced following
co-transfection with HIF-1α (Ad5-SOCS3+HIF-1α group vs. Ad5-SOCS3
group, ▲P<0.05). Ad5, tumor-specific
replication-defective adenovirus type 5; SOCS3, suppressor of
cytokine signaling, 3; HIF-1α, hypoxia-inducible factor-1α. |
Discussion
The tumor microenvironment is characterized by
hypoxia and nutrient deprivation, which leads to genetic and
epigenetic adaptation of cell clones, therefore enabling its
invasive and metastatic nature. The adaptation of tumor cells to
hypoxia makes them more difficult to treat and highly resistant to
therapy (22). An important part
of this process is the adaptation of gene products as a response to
hypoxia and evidence suggested that a number of these
hypoxia-regulated genes are mediated by HIF-1α (23). HIF-1α is involved in the response
to hypoxia through oxygen homeostasis and also in myocardial, brain
and retinal ischemia, pulmonary hypertension, preeclampsia,
intrauterine growth, retardation and cancer (24). It has a crucial role in
physiological homeostatic and etiopathological mechanisms. HIF-1α
acts as a target factor for cancer therapeutics, as its function is
regulated by growth factors and genetic abnormalities involved in
tumor progression (25). It was
identified that HIF-1α levels were adapted in SCLC cells to
maintain a high rate of proliferation; however, the increased cell
proliferation may induce an increased expression of HIF-1α
(26). HIF-1α may be an effective
therapeutic target for SCLC and inhibiting the expression of HIF-1α
may be a suitable approach for developing molecular targeted
therapies.
A previous study has confirmed that SOCS3 may
significantly inhibit the proliferation of lung cancer cells in
vitro and indicated that SOCS3 may act as an anti-oncogene
involved in the development of tumors (10). In addition, SOCS3 may regulate the
movement and migration of tumor cells (27). Hypermethylation-mediated silencing
of SOCS3 has been identified in multiple types of cancer (20). Restoration of SOCS3 expression may
be realized by demethylation or ectogenic SOCS3 gene transfer. He
et al (28) reported that
promoter hypermethylation-mediated silencing was found in human
non-small cell lung cancer samples and several other tumor cell
lines. Restoring SOCS3 expression through demethylation in such
cancer cells may successfully suppress tumorigenesis. Shouda et
al (29) reported that
exogenous SOCS3 may suppress malignant fibrous histiocytoma tumor
progression. In the present study, the methylation levels of SOCS3
were high in SCLC cells and SOCS3 expression was only moderately
detected in NCI-H446 cells. When treated with the demethylation
agent 5-aza-2′-deoxycytidine, SOCS3 mRNA levels were significantly
upregulated but no marked changed in protein levels were observed.
Therefore, it was hypothesized that the material cause was protein
degradation and the avoidance of SOCS3 protein degradation during
the experimental process would be a key priority in future
investigation. However, transfection with ectogenic genes through
adenovirus vectors enabled stable SCOS3 expression at the protein
level, and therefore, through in vivo and in vitro
study, it was found that SOCS3 inhibited the proliferation and
angiogenesis of SCLC cells and these biological effects were
achieved through the regulation of HIF-1α expression.
Previous studies have confirmed that expression of
SOCS3 may be induced by a variety of cytokines and growth factors
and directly antagonizes the STAT3-mediated signaling pathway as a
classic negative feedback loop (30,31).
A number of studies have demonstrated that other intracellular
signaling cascades, including Ras/Erk1/2 (13) and PI3K/Akt (32), are also regulated by SOCS3, and
their marked and persistent activation has been implicated in
tumorigenesis. For the signaling pathway, the Jak/STAT and PI3K/Akt
are two parallel pathways mediating functions of numerous receptor
and non-receptor tyrosine kinases, including EGFR, Her-2 and c-Src
(33). Yamasaki et al
(34) found that Jak/STAT3 and
PI3K/Akt were the main signaling pathways to regulate the
expression of HIF-1α in numerous types of cancer. Xu et al
(19) found that targeting STAT3
with STAT3 small interfering (si)RNA inhibited MCF-7 breast cancer
cell proliferation induced by Jak/STAT and PI3K/Akt pathways, as
transfection with STAT3 siRNA blocked not only STAT3 expression but
also Akt expression. Yang et al (35) also hypothesized that curcumin
blocked the proliferation of SCLC cells through the Jak/STAT
signalling pathway to achieve its therapeutic effects. By contrast,
the results of the present study differed from those of previous
studies; it was found that although SOCS3 inhibited STAT3
expression, this regulation had no effect on the HIF-1α levels in
SCLC cells, which was achieved through inhibition of Akt by SOCS3.
In addition, the results of the present study demonstrated that
targeting Akt, not STAT3, may inhibit the proliferative and
angiogenic potential of SCLC cells. Therefore, it was hypothesized
that SOCS3 as a therapeutic factor may inhibit the proliferation
and HIF-1α expression in SCLC cells, which proceeded via the
PI3K/Akt signaling pathway instead of the Jak/STAT signaling
pathway, which has been the focus of investigation in previous
years.
SCLC represents a malignant and particularly
aggressive form of cancer with marked proliferation and a poor
prognosis. Previous studies have mainly focused on radiotherapy and
chemotherapy, but recently, molecular-targeted therapy has aroused
increasing interest. For this, an effective therapeutic factor and
a significant therapeutic target are important. SOCS3 was initially
introduced as the therapeutic factor for SCLC and it was confirmed
that SOCS3 may downregulate HIF-1α expression to inhibit the
proliferation of SCLC cells. To date, there is no data delineating
the signaling pathway involved in the SOCS3 regulation of HIF-1α
expression in SCLC cells; however, it is hypothesized that the
PI3K/AKT pathway may be involved. Therefore, the present study
examined the regulation of biological activity by SOCS3, including
migration, invasion, angiogenesis and the involved molecular
mechanism.
In conclusion, the present study showed that SOCS3
inhibited the proliferative and angiogenic potential of NCI-H446
cells and HIF-1α was necessary in this process. A negative role of
SOCS3 in Akt signaling, rather than STAT3 signaling to block HIF-1α
expression as well as a previously unidentified regulatory
mechanism for Akt function were observed. These results provided a
theoretical basis for targeting SOCS3 for the treatment of
SCLC.
Acknowledgments
The present study was supported by the Nature
Science Foundation of Anhui Province (grant no. 1208085QH159).
References
|
1
|
Wan J, Ma J, Mei J and Shan G: The effects
of HIF-1alpha on gene expression profiles of NCI-H446 human small
cell lung cancer cells. J Exp Clin Cancer Res. 28:1502009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wan J, Chai H, Yu Z, et al: HIF-1alpha
effects on angiogenic potential in human small cell lung carcinoma.
J Exp Clin Cancer Res. 30:772011. View Article : Google Scholar
|
|
3
|
Lee GW, Go SI, Cho YJ, et al:
Hypoxia-inducible factor-1alpha and excision repair
cross-complementing 1 in patients with small cell lung cancer who
received front-line platinum-based chemotherapy: a retrospective
study. J Thorac Oncol. 7:528–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ioannou M, Papamichali R, Kouvaras E, et
al: Hypoxia inducible factor-1 alpha and vascular endothelial
growth factor in biopsies of small cell lung carcinoma. Lung.
187:321–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang W, Zhang H and Xing L: Antisense
oligonucleotide of hypoxia-inducible factor-1alpha suppresses
growth and tumorigenicity of lung cancer cells A549. J Huazhong
Univ Sci Technolog Med Sci. 26:448–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kappler M, Taubert H, Schubert J,
Vordermark D and Eckert AW: The real face of HIF1alpha in the tumor
process. Cell Cycle. 11:3932–3936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park KW, Nozell SE and Benveniste EN:
Protective role of STAT3 in NMDA and glutamate-induced neuronal
death: negative regulatory effect of SOCS3. PLoS One. 7:e508742012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Guo D, Jiang L, Zhang Q, Qiu X
and Wang E: SOCS3 inhibiting migration of A549 cells correlates
with PYK2 signaling in vitro. BMC Cancer. 8:1502008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baltayiannis G, Baltayiannis N and Tsianos
EV: Suppressors of cytokine signaling as tumor repressors.
Silencing of SOCS3 facilitates tumor formation and growth in lung
and liver. J BUON. 13:263–265. 2008.PubMed/NCBI
|
|
11
|
Tokita T, Maesawa C, Kimura T, et al:
Methylation status of the SOCS3 gene in human malignant melanomas.
Int J Oncol. 30:689–694. 2007.PubMed/NCBI
|
|
12
|
Tischoff I, Hengge UR, Vieth M, et al:
Methylation of SOCS-3 and SOCS-1 in the carcinogenesis of Barrett’s
adenocarcinoma. Gut. 56:1047–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu ZB, Bai L, Qian P, et al: Restoration
of SOCS3 suppresses human lung adenocarcinoma cell growth by
downregulating activation of Erk1/2, Akt apart from STAT3. Cell
Biol Int. 33:995–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokunaga E, Oki E, Egashira A, et al:
Deregulation of the Akt pathway in human cancer. Curr Cancer Drug
Targets. 8:27–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dillon RL, White DE and Muller WJ: The
phosphatidyl inositol 3-kinase signaling network: implications for
human breast cancer. Oncogene. 26:1338–1345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell IG, Russell SE, Choong DY, et al:
Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer
Res. 64:7678–7681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Cui GB, Zhang J, et al:
Inhibition of PI3 kinases enhances the sensitivity of non-small
cell lung cancer cells to ionizing radiation. Oncol Rep.
24:1683–1689. 2010.PubMed/NCBI
|
|
18
|
Littlefield SL, Baird MC, Anagnostopoulou
A and Raptis L: Synthesis, characterization and Stat3 inhibitory
properties of the prototypical platinum(IV) anticancer drug,
[PtCl3(NO2) (NH3)2] (CPA-7). Inorg Chem. 47:2798–2804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Q, Briggs J, Park S, et al: Targeting
Stat3 blocks both HIF-1 and VEGF expression induced by multiple
oncogenic growth signaling pathways. Oncogene. 24:5552–5560. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weber A, Hengge UR, Bardenheuer W, et al:
SOCS-3 is frequently methylated in head and neck squamous cell
carcinoma and its precursor lesions and causes growth inhibition.
Oncogene. 24:6699–6708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Li G, Li R, et al: IL-6 promotion
of glioblastoma cell invasion and angiogenesis in U251 and T98G
cell lines. J Neurooncol. 100:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar K, Wigfield S, Gee HE, et al:
Dichloroacetate reverses the hypoxic adaptation to bevacizumab and
enhances its antitumor effects in mouse zenografts. J Mol Med
(Berl). 91:749–758. 2013. View Article : Google Scholar
|
|
23
|
Kimbro KS and Simons JW: Hypoxia-inducible
factor-1 in human breast and prostate cancer. Endocr Relat Cancer.
13:739–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fraga A, Ribeiro R and Medeiros R: Tumor
hypoxia: the role of HIF. Actas Urol Esp. 33:941–951. 2009.In
Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi YH and Fang WG: Hypoxia-inducible
factor-1 in tumour angiogenesis. World J Gastroenterol.
10:1082–1087. 2004.PubMed/NCBI
|
|
26
|
Fan LF, Diao LM, Chen DJ, et al:
Expression of HIF-1 alpha and its relationship to apoptosis and
proliferation in lung cancer. Ai Zheng. 21:254–258. 2002.In
Chinese. PubMed/NCBI
|
|
27
|
Zhang S, Wang W, Wang E and Qiu X: SOCS3
expression is inversely correlated with Pyk2 in non-small cell lung
cancer and exogenous SOCS3 inhibits proliferation and invasion of
A549 cells. Pathology. 44:434–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He B, You L, Uematsu K, et al: SOCS-3 is
frequently silenced by hypermethylation and suppresses cell growth
in human lung cancer. Proc Natl Acad Sci USA. 100:14133–14138.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shouda T, Hiraoka K, Komiya S, et al:
Suppression of IL-6 production and proliferation by blocking STAT3
activation in malignant soft tissue tumor cells. Cancer Lett.
231:176–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokogami K: Hypoxia-induced decreases in
SOCS3 increase STAT3 activation and upregulate VEGF gene
expression. Brain Tumor Pathol. 30:135–143. 2013. View Article : Google Scholar
|
|
31
|
Shen A, Chen Y, Hong F, et al: Pien Tze
Huang suppresses IL-6-inducible STAT3 activation in human colon
carcinoma cells through induction of SOCS3. Oncol Rep.
28:2125–2130. 2012.PubMed/NCBI
|
|
32
|
Francipane MG, Eterno V, Spina V, et al:
Suppressor of cytokine signaling 3 sensitizes anaplastic thyroid
cancer to standard chemotherapy. Cancer Res. 69:6141–6148. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamasaki M, Nomura T, Sato F and Mimata H:
Chronic hypoxia induces androgen-independent and invasive behavior
in LNCaP human prostate cancer cells. Urol Oncol. 31:1124–1131.
2013. View Article : Google Scholar
|
|
35
|
Yang CL, Liu YY, Ma YG, et al: Curcumin
blocks small cell lung cancer cells migration, invasion,
angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3
signalling pathway. PLoS One. 7:e379602012. View Article : Google Scholar : PubMed/NCBI
|