Introduction
Accumulating numbers of studies have demonstrated
that myeloid-derived suppressor cells (MDSCs), which were initially
documented as ‘immature myeloid cells (IMCs)’ or ‘myeloid
suppressor cells’, have an important role in immune dysfunction,
including promotion of angiogenesis, tumor cell invasion and
metastases in human cancer (1,2). In
addition, MDSC invasion has been revealed to be critical in
patients with cancer, including head and neck cancer, non-small
cell lung cancer and renal cancer (3–5).
Recent studies have reported that T cell-associated non-response
generated from MDSC accumulation was the protagonist of immune
tolerance (6). Furthermore,
extensive studies have demonstrated that these suppressor cells
were of myeloid origin and the heterogeneous cell population
comprises myeloid progenitor cells and IMCs (7). At present, MDSCs are characterized by
their particular phenotype and functional ability to suppress
T-cell activation.
By contrast to that observed in murine species,
MDSCs in humans are inadequately characterized due to a lack of
uniform markers. In gastrointestinal cancer patients, MDSCs exhibit
diverse phenotypic combinations comprising cluster of
differentiation (CD)11b+CD14− or
CD11b+CD33+ human leukocyte antigen
(HLA)-DR−/low and can be further discriminated by their
expression of CD15 (8). In
addition, identification of CD14+HLA-DR−/low
MDSCs in melanoma and hepatocellular carcinoma patients provided
evidence that different human tumors are likely to induce different
populations of MDSCs (9). Two
major classes of MDSCs have been identified: Granulocytic and
monocytic MDSCs; these two suppressive MDSC subsets may inhibit
T-cell responses through alternative mechanisms. The latter, which
is characterized by their additional expression of CD14 and reduced
expression of CD15, involves a complex network of immune
suppression of CD4+ T cells (10).
Multiple pathways in human cancer have been
demonstrated to be associated with the recruitment, expansion and
activation of MDSCs (7).
Considering the multitude of immune modulatory factors produced by
tumors, it is likely that different subsets of MDSCs may be
generated in the tumor microenvironment, dependent upon the unique
profile of factors secreted by the tumor (11,12).
Accordingly, granulocyte-macrophage colony-stimulating factor
(GM-CSF), interleukin (IL)-6, IL-1β, prostaglandin E2 (PGE2), tumor
necrosis factor (TNF)-α and vascular endothelial growth factor
(VEGF) were considered to have a potent capacity in the generation
of suppressive CD33+ MDSCs (7,11).
In addition, a number of laboratories have investigated the
critical role of vasoactive intestinal polypeptide (VIP) in the
tumorigenesis process (13–15).
VIP and its receptors are present in numerous tissues and have an
important role in the regulation of endocrine and exocrine
secretions, modulation of glucose homeostasis, neuroprotection,
memory, gut function, modulation of the immune system and circadian
function (16,17). Recent evidence has demonstrated the
involvement of VIP in proliferation, adhesion, migration, invasion
and cyclooxygenase-2 expression in prostate cancer cells (13). An additional study was performed to
identify the contribution of VIP to the expression of VEGF and
human epidermal growth factor receptor 2 as well as transactivation
of epidermal growth factor receptor in human breast and prostate
cancer (14,15), suggesting that VIP has a potent
effect in the mechanism of immune tolerance. Furthermore, treatment
with VIP resulted in a substantial reduction in the number of
CD4+ T cells producing effector cytokines IL-2, IL-4,
interferon (IFN)-γ and TNF-α, whereas VIP increased the number of
IL-10- and TGF-β-producing CD4+ T cells (18).
Therefore, the frequency and suppressive function of
CD14+HLA-DR−/low MDSCs in gastric cancer
tissue was evaluated in the present study. In addition, it was
investigated whether the presence of VIP induces the
differentiation of healthy donor CD14+ peripheral blood
mononuclear cells (PBMCs) towards immune suppressive MDSCs with the
capacity to inhibit tumor-specific T-cell activation. Understanding
their mechanism of action is important for developing effective
immunotherapy strategies.
Materials and methods
Patients with cancer and healthy
donors
Tumor and adjacent normal tissue (at least 5 cm
distant from the tumor margin) were obtained from 19 patients with
gastric cancer treated at the Department of Gastrointestinal
Surgery of Union Hospital in Wuhan, China. All patients were
pathologically diagnosed and none of the patients had received
anti-cancer therapy prior to surgical resection. For in
vitro suppression and induction experiments, blood samples of
healthy donors were collected from Wuhan Blood Center (Wuhan,
China). Written and oral consent was obtained prior to blood and
tumor sampling. The Institutional Review Board of Tongji Medical
College of Huazhong University of Science and Technology approved
the study protocol.
Cell isolation
A single-cell suspension was isolated from a freshly
resected tumor sample and adjacent normal tissue using a mechanical
procedure and enzymatic digestion. Subsequently, mononuclear cells
were isolated from resulting cells by Ficoll density gradient
centrifugation (Ficoll-Paque Plus; eBioscience, San Diego, CA, USA)
as described previously (9). PBMCs
were isolated from freshly obtained healthy blood samples by Ficoll
density gradient centrifugation as described above. The cell
viability was assessed using trypan blue dye exclusion. For
isolation of CD14+ cells and CD4+ T cells,
mononuclear cells were purified using the Human CD14 Positive
Selection kit and the Human CD4+ T Cell Enrichment kit
(EasySep™ cat. nos. 18058 and 19052 respectively; STEMCELL,
Vancouver, BC, Canada) according to the manufacturer’s
instructions. The purity of the sorted cells was assessed using
flow cytometry (BD FACSCanto II; Becton-Dickinson, Franklin Lakes,
NJ, USA) and sorted cell populations that were >95% pure target
cells were selected for the experiments.
Antibodies and flow cytometric
analysis
To determine the frequency and phenotype of
CD14+HLA-DR−/low cells in tumor samples and
adjacent normal tissue, mouse monoclonal anti-human CD3 (cat. no.
17-0037; 0.6 μg/ml), mouse monoclonal anti-human CD14 (cat.
no. 45-0149; 5.0 μg/ml) and mouse monoclonal anti-human
HLA-DR (cat. no. 9012-9952; 2.5 μg/ml) antibodies were used
to incubate the tissue for 40 min at 4°C. All monoclonal antibodies
used in the study were purchased from eBioscience. Staining was
performed on mononuclear cells isolated from tumor-infiltrating
tissue or adjacent normal tissue. Fluorescence-activated cell
sorting data were acquired using a flow cytometer (BD FACSCanto II;
Becton-Dickinson) and were analyzed using BD FACSDiva version 6.1.3
(Becton-Dickinson). Results were expressed as the percentage of
CD14+HLA-DR−/low cells.
Suppression assay
To demonstrate the inhibitory capacity of the
CD14+HLA-DR−/low MDSCs in gastric cancer
tissue, CD14+ mononuclear cells were isolated from
tumor-infiltrating tissue and co-cultured with healthy donor
CD4+ T cells in the presence of mouse anti-human CD3
(cat. no. 555336; 2.5 μg/ml; BD Biosciences) and mouse
anti-human CD28 (cat. no. 555725; 1.2 μg/ml; BD Biosciences)
stimulation at 37°C and 5% CO2 for five days. The
co-culture system comprised complete medium consisting of RPMI 1640
supplemented with fetal bovine serum (10%), L-glutamine (200 mM),
sodium pyruvate (1%), nonessential amino acids (1%),
penicillin-streptomycin (1%) and 2-mercaptoethanol (1%). As
controls, CD14+ mononuclear cells were isolated from
adjacent normal tissue and co-cultured with healthy donor
CD4+ T cells under identical conditions. After five
days, all the cells were harvested in the 24-well flat-bottom
plates. For intracellular cytokine staining, cells were exposed for
4 h to phorbol 12-myristate 13-acetate (PMA; 50 ng/ml;
Sigma-Aldrich, St. Louis, MO, USA), ionomycin (500 ng/ml;
Sigma-Aldrich), Golgi-Stop (1 μl/1.5 ml; BD Biosciences) and
Golgi-Plug (1 μl/ml; BD Biosciences) as described in a
previous study by our group (19).
For immunosuppression analysis, the fluorochrome-conjugated
antibodies mouse monoclonal anti-human CD4 (cat. no. 47-0047; 0.6
μg/ml; eBioscience), mouse monoclonal anti-human IFN-γ (cat.
no. 502506; 5.0 μg/ml; BioLegend, San Diego, CA, USA) and
rat monoclonal anti-human IL-2 (cat. no. 500342; 2.5 μg/ml;
BioLegend, San Diego, CA, USA) were used for staining at 4°C for 40
min. Flow cytometric analyses were performed as described
above.
Induction assay of VIP
The healthy CD14+ mononuclear cells and
CD4+ T cells were isolated from healthy donor PBMCs
using Ficoll density gradient centrifugation and magnetic-activated
cell sorting as described (9,20).
The induction capacity of VIP was analyzed in a co-culture assay
with purified healthy CD14+ mononuclear cells
(1.5×105) in the presence of different concentration of
pretreated VIP (Null, 10−7 M and 10−6 M; EMD
Chemicals, Inc., Gibbstown, NJ, USA) for three days, as VIP has
been observed to induce the emergence of T-regulatory cells (Treg)
with suppressive activity on effector T cells and 10−7 M
VIP was suggested in the induction assay in their studies (18). Subsequently, pretreated
CD14+ mononuclear cells (1×105) were
harvested for co-culture with allogeneic CD4+ T cells
(3×105) in the presence of anti-CD3/anti-CD28
stimulation. After five days, all cells were harvested and
incubated in the presence of PMA, ionomycin, Golgi-Plug and
Golgi-Stop at 37°C and 5% CO2 for 4 h. For
immunosuppressive analysis, fluorochrome-conjugated antibodies,
including anti-CD4, anti-IFN-γ and anti-IL-2 were used for staining
and flow cytometric analysis were performed as described above.
Determination of IL-10 expression using
ELISA
For determination of IL-10 responses of
CD4+ T cells affected by VIP-induced CD14+
PBMCs, co-culture supernatants from the VIP induction assay were
removed and assessed using ELISA (Human IL-10 ELISA kit, Invitrogen
Life Technologies, Carlsbad, CA, USA). To further confirm the
hypothesis that IL-10 secretion is involved in CD4+
T-cell immune tolerance, rat anti-human IL-10 (cat. no. 16-7108;
15.0 μg/ml; eBioscience) was added into the VIP-pretreated
CD14+ PBMCs and CD4+ T-cell co-culture system
in an induction assay in vitro. After five days of
co-culture, anti-CD4, anti-IFN-γ and anti-IL-2 were stained and
flow cytometric analysis was performed for detecting whether
anti-IL-10 can reverse the immunosuppressive capacity of
VIP-induced CD14+HLA-DR−/low MDSCs.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Comparisons were made using Student’s t-test for ELISA and the
Wilcoxon test for the other data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Frequency of
CD14+HLA-DR−/low cells is increased in
gastric cancer tissue
In general, phenotypic features are considered the
hallmark in identifying MDSCs; however, there is no uniform marker
for human MDSCs. Previously, CD14 and HLA-DR markers have been
partially used to characterize MDSCs in human cancer and
identification of more specific surface markers would facilitate
the understanding of the origin and functional activities of MDSCs
(9,21). CD33+ and
CD11b+ expression was suggested as possible surface
markers of monocytic MDSCs (22).
However, it is noteworthy that PBMCs were widely used for
phenotypic analysis of human cancer (9,21–22).
Additional experiments towards MDSCs in the tumor microenvironment
in humans are required. To investigate the features of
immune-associated cells in the tumor microenvironment, the
frequency of CD14+HLA-DR−/low cells in
mononuclear cells isolated from tumor-infiltrating tissue were
analyzed in gastric cancer patients. As controls, adjacent normal
tissue was assigned as the non-tumor infiltrating group. In the
present study, paired tissue was acquired from the same gastric
cancer patient to avoid errors caused by individual differences.
Representative dot plots of tumor-infiltrating tissue and adjacent
normal tissue are shown in Fig.
1A. As shown in Fig. 1B, there
was a significant increase in the frequency of CD14+
HLA-DR−/low cells in tumor-infiltrating tissue as compared with
that in adjacent normal tissue (13.2±8.0% versus 8.3±2.8%, n=19).
As is generally consistent with the results in human cancer PBMCs
(9), it was additionally suggested
that although the CD14+HLA-DR−/low cells
observed in tumor and non-tumor infiltrating tissue exhibited
common surface markers, the phenotypic heterogeneity and functional
difference in immune suppression should be considered (23).
CD14+ HLA-DR−/low
MDSCs are potent suppressors of CD4+ T cells in regard
to IFN-γ and IL-2 production
As the suppressive capacity of T-cell immune
responses is considered critical in MDCS identification,
CD14+HLA-DR−/low cells in the present study
were analyzed using a suppression assay. In hepatocellular
carcinoma patients, CD14+HLA-DR−/low cells
apparently suppressed T-cell responses in IFN-γ production, whereas
CD14+HLA-DR+ cells failed to suppress IFN-γ
secretion. Therefore, the immunosuppressive function of
CD14+ mononuclear cells isolated from tumor PBMCs is
predominantly supplied by CD14+HLA-DR−/low
cell immunosuppression. Subsequently, it was assessed whether
CD14+ mononuclear cells from tumor infiltrating tissue
of gastric cancer patients are more immunosuppressive to
CD4+ T-cell responses, compared with those from
non-tumor infiltrating tissue. As a result, CD14+
mononuclear cells from tumor infiltrating tissue suppressed IFN-γ
expression of healthy donor CD4+ T cells, compared with
CD14+ mononuclear cells isolated from non-tumor
infiltrating tissue (16.2±1.3% versus 8.1±2.3%, n=6; Fig. 2A and B). In addition, as a
pleiotropic cytokine with important effects on innate and adaptive
immunity, a reduction in IL-2 expression indicated deficient T-cell
function and tumor progression. As shown in the tumor
microenvironment, CD14+ mononuclear cells suppress IL-2
production of CD4+ T cells, indicating that
CD14+ HLA-DR−/low MDSCs obtained
immunosuppressive capacity, whereas CD14+ mononuclear
cells from adjacent normal tissue failed to suppress IL-2
production (13.7±2.1% versus 8.2±2.5%, n=6; Fig. 2C and D). The aforementioned
findings confirmed that CD14+HLA-DR−/low
MDSCs caused immune-promoting cytokine downregulation in T-cell
immune deficiency in gastric cancer tissue.
VIP is involved in the induction of human
CD14+HLA-DR−/low MDSCs
Previously, VIP was considered to have potent
anti-inflammatory effects and was demonstrated to induce the
release of IL-10 and downregulate the number of IFN-γ+
natural killer (NK) and NKT cells, which subsequently inhibited the
cytolytic activity of NK cells (24). One hypothesis is that VIP exerts
crucial effects in the pathogenesis of various human tumors,
including the initiation, expansion and activation of diverse
immune tolerance-associated cells, then trigger the
anti-inflammation and tolerance mechanism. To investigate the
capacity of VIP in inducing CD14+HLA-DR−/low
MDSCs and whether the induced MDSCs acquired a suppressive function
against CD4+ T cells, freshly sorted healthy
CD14+ PBMCs were incubated with VIP at different
concentrations. Subsequently, the pretreated CD14+ PBMCs
and healthy CD4+ T cells were co-cultured and the
suppressive function was measured by IFN-γ and IL-2 downregulation
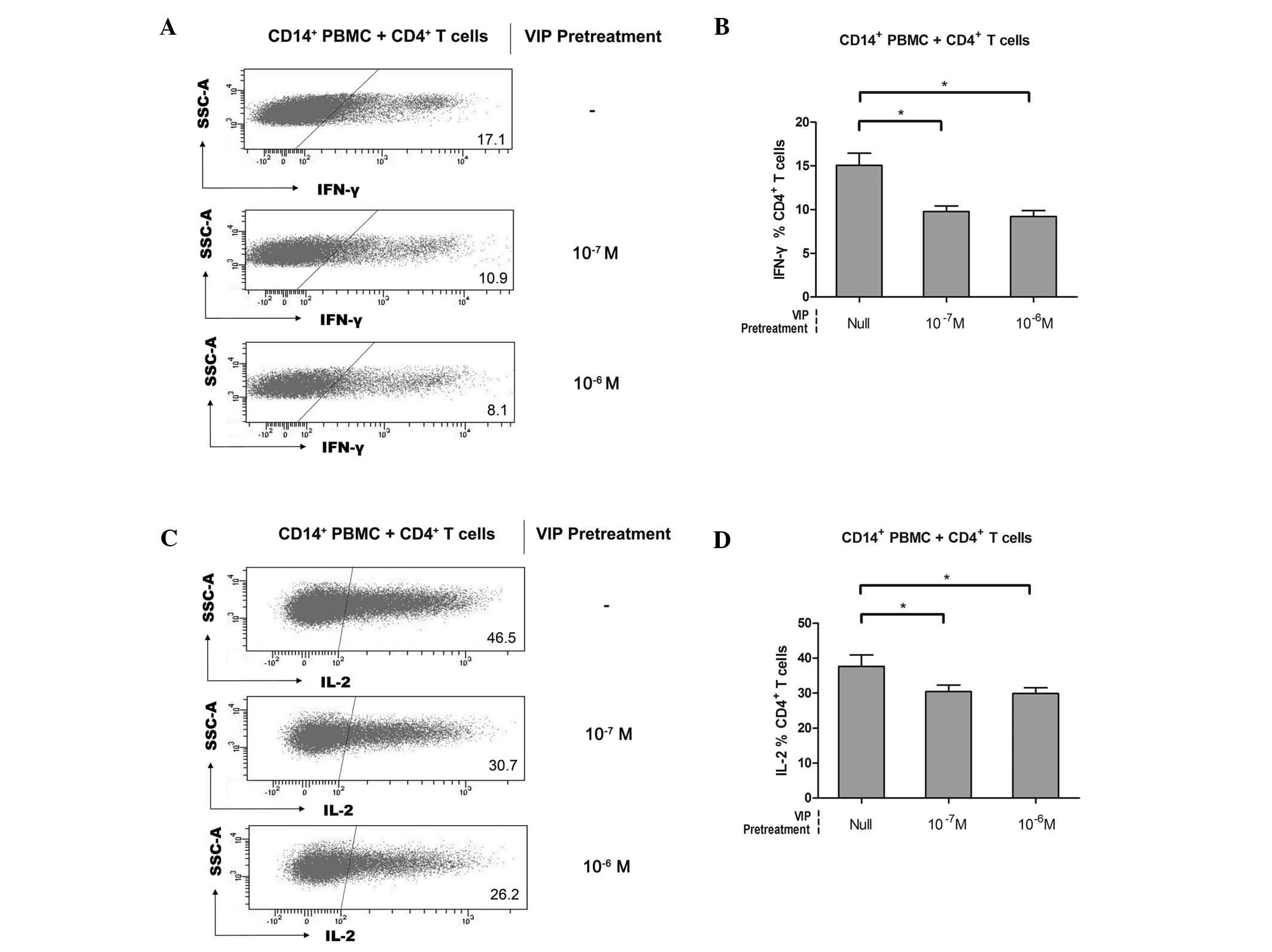
in CD4+ T cells. As a control, null-VIP-pretreated
CD14+ PBMCs were used. Compared with the null-VIP
pretreatment group, IFN-γ production of CD4+ T cells was
inhibited following co-culturing with 10−7 or
10−6 M VIP-pretreated CD14+ PBMCs (15.1±3.1%
versus 9.8±1.4% versus 9.2±1.5%, n=5; Fig. 3A and B). In addition, a similar
suppressive effect was detected on the IL-2 production of
CD4+ T cells (37.7±7.2% versus 30.4±4.3% versus
29.9±3.7%, n=5; Fig. 3C and D).
Accordingly, it was suggested that VIP-pretreated CD14+
PBMCs acquired similar suppressive features to those of
CD14+ mononuclear cells isolated from the
tumor-infiltrating tissue of gastric cancer tissue. Thus, it was
considered that they differenciated into
CD14+HLA-DR−/low MDSCs, which constituted the
suppressive effectors. However, the induction effect was observed
to be VIP dose-independent (Fig. 3B
and D). The receptor saturation effect may exist in the
VIP-mediated induction of CD14+HLA-DR−/low
MDSCs as VIP receptors, including VIP receptor (VPAC)1, VPAC2 and
procaspase-activating compound 1 receptors (18,24).
In agreement with this finding, CD4+ T cells incubated
in the presence of VIP expressed significantly lower levels of the
effector cytokines IL-2, IL-4, IFN-γ and TNF-α (18). Results from these studies suggested
the involvement of VIP in immune tolerance through the
CD14+HLA-DR−/low MDSC induction effect.
Involvement of IL-10 secretion in immune
suppression by VIP-induced CD14+HLA-DR−/low
MDSCs
Several studies have recently proposed the
possibility of IL-10 dependent-Treg induction and macrophage
inactivation, which may indirectly inhibit tumor cell cytotoxicity
mediated by NK cells (25,26). In addition, a more robust type 1
response with increased levels of IFN-γ and decreased levels of
IL-10 was observed in a zoledronic acid-treated pancreatic
adenocarcinoma murine model due to impaired intratumoral MDSC
accumulation and increased recruitment of T cells to the tumor
(27). Thus, it was hypothesized
that IL-10 secretion was altered in CD4+ T cell response
deficiency caused by VIP-induced
CD14+HLA-DR−/low MDSCs. As was expected,
elevated levels of IL-10 were identified in the supernatants of the
co-culture system of VIP-pretreated CD14+ PBMCs and
CD4+ T cells (215.7±15.2 pg/ml versus 263.3±29.2 pg/ml
or 333.8±81.4 pg/ml; Fig. 4A),
compared with those in CD14+ PBMCs without VIP
pretreatment. Of note, VIP dose-independence was observed again as
no difference was revealed between the 10−7 M and
10−6 M VIP pretreatment groups (Fig. 4A). It was further hypothesized that
the CD4+ T-cell response deficiency may rely on IL-10
secretion when co-cultured with VIP-pretreated CD14+
PBMCs. Therefore, anti-IL-10 was added into the co-culture system
and the suppressive progress was reversed and convalescent levels
of effector cytokines, IFN-γ and IL-2 were observed. In samples
subjected to VIP pretreatment, IFN-γ and IL-2 expression were
downregulated (Fig. 4B and D).
Statistical data are shown correspondingly in Fig. 4C and E. In anti-IL-10 cases,
10−7 M VIP was selected in pretreatment owing to the
dose-independent effect in CD4+ T-cell response
deficiency between the 10−7 M and 10−6 M VIP
pretreatment groups. These findings indicated that the
immunosuppressive effect of VIP-induced CD14+
HLA-DR−/low MDSCs on CD4+ T cells is IL-10
secretion-dependent.
 | Figure 4IL-10 secretion is involved in the
immunosuppression of VIP-induced CD14+ PBMC to
CD4+ T-cell responses. In the VIP induction assay, the
co-culture supernatant of CD14+ PBMC and CD4+
T cells was harvested, then its IL-10 concentration was analyzed
using ELISA. (A) Cumulative results from eight independent
experiments. Furthermore, freshly sorted CD14+ PBMC from
healthy donors were pretreated with VIP at different concentrations
(Null, 10−7 M or 10−6 M); these pretreated
CD14+ PBMC were then co-cultured with healthy donor CD4+
T cells with/without anti-IL-10 presence. A total of four groups
were categorized depending on their VIP pretreatment and anti-IL-10
presence (No VIP + No anti-IL-10, 10−7 M VIP + No
anti-IL-10, 10−6 M VIP + No anti-IL-10, 10−7
M VIP + 15 μg/ml anti-IL-10). (B and D) After five days
co-culture and stimulation in the presence of anti-CD3/anti-CD28,
IFN-γ or IL-2 expression of CD4+ T cells determined and
representative dot plot or (C and E) cumulative results from five
independent experiments are shown, respectively. Values are
expressed as the mean ± standard deviation (n=5).
*P<0.05. PBMCs, peripheral blood mononuclear cells;
CD, cluster of differentiation; IL, interleukin; IFN, interferon;
VIP, vasoactive intestinal peptide. |
Discussion
MDSCs have been identified as a potent suppressor of
tumor immunity and therefore have potential for cancer
immunotherapy. They arise from myeloid progenitor cells that do not
terminally differentiate into mature status under the induction by
tumor-secreted and host-secreted factors. Different subsets of
MDSCs perform different features in morphology, phenotype, gene
expression and mechanism of immune tolerance.
In mice, the phenotypic features of these cells were
initially defined as Gr1+CD11b+ and recent
studies have unraveled the actual complexity of this population and
the existence of granulocytic and monocytic MDSC subsets by
distinguishing them into a
CD11b+Ly6G+Ly6Clow and
CD11b+Ly6G−Ly6Chigh phenotype
(28,29). However, this complexity is more
marked in a human setting, where heterogeneous populations of
myeloid cells with variable phenotype and immunosuppressive
features have been described in different tumors. As a result,
numerous MDSC-associated surface markers involved in human tumors
were reported in a recent study (10). In humans, monocytic MDSCs are
frequently defined as cells expressing the common monocytic marker
CD14, but lacking markers of the expression of mature myeloid and
lymphoid cells as the major histocompatibility complex class II
molecule HLA-DR (9,30). Although a similarity between the
CD14 phenotype and morphology exists between monocytic MDSCs and
inflammatory monocytes, these cell populations are functionally
distinct; monocytic MDSCs are highly immunosuppressive, expressing
high levels of inducible nitric oxide synthase (iNOS) and arginase
(ARG)1, although these two proteins are not coordinately
upregulated in inflammatory monocytes (31). In the analysis of the peripheral
blood leukocytes, a previous study observed that there was no
difference in the percentage of CD14+ monocytes in
non-small cell lung cancer (NSCLC) patients and healthy controls,
whereas the percentage and absolute number of the circulating
CD14+HLA-DR−/low subset was significantly
increased in NSCLC patients compared with that in healthy controls
(4). This further confirmed
negative or low expression of HLA-DR as a phenotypic definition of
immunosuppressive MDSCs. In a similar way to HLA-DR, the IL-13
receptor α1 chain has also been applied for distinguishing
suppressive from non-suppressive myeloid cells, in the same way as
Treg was distinguished from activated T effector cells by FoxP3 and
CD39 (11,32). In the present study, a novel subset
of CD14+HLA-DR−/low MDSCs was described in
the tumor microenvironment of gastric cancer patients. CD14 and
HLA-DR were previously used as surface molecules of monocytic
MDSCs, not only in gastrointestinal tumors, but also in others
(33). In another study on the
ability of human tumor cell lines to induce MDSCs from healthy
donor PBMCs, induced MDSCs were characterized into two distinct
subsets: CD33+HLA-DR−/low and
CD11b+HLA-DR−/low (22). In addition, MDSCs have also been
identified within a CD15+ population in bone marrow and
peripheral circulation of pancreatic adenocarcinoma patients
(27). On the basis that monocytic
MDSCs are discrepantly immunosuppressive compared with granulocytic
MDSCs, CD33 is possibly not a discriminatory surface marker for
MDSCs, as no difference was observed when MDSCs were further gated
for CD33 and CD11b positivity, while
CD11b+CD33+ and
CD11b+CD33− populations obtained an equal
suppressive capacity (8).
Recently, several other surface molecules have been used to
identify additional subsets of suppressive MDSCs, including CD83
and DC-Sign (markers associated with mature or differentiated
cells), with marked expression on
CD14+HLA-DR−/low cells in cancer patients
(30). Lechner et al
(22) demonstrated that
CD33+ MDSCs are generated when particular cytokines are
present in various cancer cell lines. In addition, a particular
surface marker was presented in a different proportion in the gated
CD14+HLA-DR−/low population; therefore, it
was hypothesized that diversity is subsistent between surface
markers even if they represent the same population. Therefore,
monocytic MDSCs are a population with universal features of
phenotypic heterogeneity in gastric cancer.
On the matter of origin, MDSCs are not as simple as
a group of migrating myeloid precursor cells, but a cluster of
cells that arise from myeloid progenitor cells and do not
terminally differentiate into mature status under the induction of
tumor-secreted and host-secreted factors under pathological
conditions and then obtain an immune suppressive function.
Demonstration of the universal nature of solid tumors induced by
MDSCs indicated that healthy donor PBMCs can be induced to form
immunosuppressive MDSCs via cytokines created whilst bearing a
tumor (22). Additionally,
CD14+ PBMCs from healthy donors acquired MDSC-like
immunosuppressive features following culturing with human
glioblastoma cell lines, which tend to promote the apoptosis of
autologous T cells (34). From
these findings, it was hypothesized that normal human
CD14+ PBMCs would adopt an MDSC-like suppressive
behavior in response to exposure to tumor-secreted cytokines or
host-secreted tumor-induced factors.
The types of factors in pathological conditions
involved in the generation of MDSCs, including GM-CSF, IL-6, IL-1β,
VEGF, PGE2 and TNFα, were characterized by their capability to
transfer the differentiation of myeloid progenitor cells to immune
tolerance status (11,12). More recently, a series of studies
revealed that VIP can modulate innate and adaptive immunity,
demonstrating a predominant anti-inflammatory action against
macrophages, promoting a positive T helper(Th)2/Th1 cytokine
balance and enhancing the production of Treg (35). In addition, numerous lines of
evidence suggested that VIP and its receptors, which are highly
expressed in breast tumor cells and lung cancer, have an important
role in the pathogenesis of tumors (14,36).
Furthermore, natural anti-VIP antibodies, which cause suppression
of VIP, may have a protective role against breast and prostate
cancer (37). Therefore, it is
important to determine the undercover mechanism underlying VIP
immunosuppression. In previous studies, IL-2 has been observed to
have a critical role in T-cell proliferation, IFN-γ production and
cytotoxicity and has been applied in cancer immunotherapy as it can
enhance various immune responses, including the generation of
antigen-specific T cells, survival rates of memory CD8+
T cells and induction of the cytotoxic T lymphocytes and
lymphokine-activated killer cells against tumor cells (38,39).
In addition, IFN-γ downregulation is considered critical for T-cell
dysfunction. Alongside the results of a previous study by our group
(20), the present study supports
a crucial role for VIP as a promoter of immune tolerance. It
suppresses secretion of IFN-γ and IL-2 as well as immune responses
through inducing normal CD14+ PBMCs to monocytic MDSCs.
Although the mechanisms involved in the suppressive effect of
monocytic MDSCs on the immune system remain controversial, direct
or indirect mechanisms possibly involved in the suppressive impact
of MDSC on immunity, including the positive correlation between
MDSC and Treg levels and inhibiting effect of
CD14+HLA-DR−/low MDSCs on autologous NK-cell
cytotoxicity and cytokine secretion (18,24).
In previous studies, the ability of MDSCs in promoting
CD4+CD25+FoxP3+ Treg cells in
vivo was described, which contributes to the indirect
immunosuppression of T-cell responses by MDSCs (26,33).
Furthermore, the involvement of VIP in immune tolerance through the
induction of Treg was confirmed (18,40).
Of note, this Treg-inducing progression involved TGFβ-dependent and
-independent pathways (34,41).
Dendritic cells (DCs) are a type of effective immune cell and it
has been confirmed that anti-inflammatory IL-10 interferes with DC
maturation (42). Upregulation of
IL-10 and poor stimulation of allogeneic T cells was observed in
the differentiation from DC to tolerogenic DC (43). In addition, it was suggested that
CD14+HLA-DR−/low monocytes were capable of
inhibiting T-cell proliferation and DC maturation (44). Thus, MDSCs may inhibit T-cell
responses and DC activity by upregulating the production of
IL-10.
To expose the details of IL-10 involvement in MDSC
activation, Sinha et al (24) demonstrated that MDSCs impaired
immunity by promoting a type 2 response, in which CD4+
T-cell and CD8+ T-cell responses were skewed through
interacting with macrophages to increase IL-10 expression (24). In addition, macrophage-produced
IL-12 was shown to promote NK activity and MDSCs may indirectly
mediate a reduction in the production of IL-12 by macrophages,
relying on their IL-10 production; thus, increased IL-10 production
may also indirectly inhibit tumor cell cytotoxicity mediated by NK
cells, which is a type of important effector cell in tumor immunity
(45). As a result, MDSCs directly
suppress adaptive and innate anti-tumor immunity and facilitate
tumor growth through their cross-talk with macrophages. The present
study demonstrated that VIP immunosuppression on CD4+ T
cells is mediated by CD14+HLA-DR−/low MDSC
induction, which is consistent with increased secretion of IL-10.
According to initial studies, IL-10 is a pleiotropic cytokine with
important immunoregulatory functions and it possesses potent
anti-inflammatory properties (46); it represses the expression of
inflammatory cytokines, including TNF-α, IL-6 and IL-1β in
macrophages. IL-10 and its receptor may be involved in tyrosine
phosphorylation of signal transducer and activator of transcription
(STAT)3 by the receptor-associated Janus kinase (JAK)1 and tyrosine
kinase 2, then subsequently trigger JAK-STAT pathways (46–48).
Several different signaling pathways in MDSCs were apparently
relevant to transcription factors of the STAT family, mainly
comprising STAT1, STAT3 and STAT6. Recent findings indicated that
the characteristics of pathological processes may recruit different
subpopulations of MDSCs with different mechanisms and targets of
suppression. As described in the review by Gabrilovich and Nagaraj
(7), the granulocytic subset of
MDSCs was found to express high levels of ROS and low levels of NO,
whereas the monocytic subset expressed low levels of ROS and high
levels of NO, and the two subsets expressed ARG1. In a study by
Kusmartsev et al (49),
MDSCs from STAT−/− mice failed to upregulate the
expression of ARG1 and iNOS and therefore did not inhibit T-cell
responses, which suggested that STAT1 was the main transcription
factor involved in the upregulation of ARG1 and iNOS expression by
monocytic MDSCs in the tumor microenvironment. In addition, it has
been demonstrated that MDSCs may inhibit T-cell function through
Th2 cytokine IL-13 as another pathway independent of ARG1, iNOS or
ROS (50). All of the above
indicates a complex interaction network between MDSCs and T-cell
responses. Drawing conclusions from the present study requires the
consideration whether VIP is involved in the pathological processes
above. Thus far, the present study provided the presumption that
IL-10 is involved in the generation of MDSCs from
CD14+HLA-DR−/low cells treated with VIP,
which may be accompanied by STAT family activation; however,
further investigations are required to examine the signaling
pathway associated with MDSC generation in human gastric
cancer.
In conclusion, an increased frequency of
immunosuppressive MDSCs in the tumor microenvironment of gastric
cancer was reported in the present study. MDSCs characterized by a
CD14+HLA-DR−/low phenotype in these patients
may suppress tumor-specific CD4+ T-cell responses,
confirming a crucial immunosuppressive pathway of MDSCs developed
in gastric cancer patients. In the present study, it was further
proposed that VIP, a novel cytokine, which can induce the
differentiation to MDSCs which have a immunosuppressive function
via the upregulation of IL-10 production, leading to suppression of
the T-cell response. In addition, the results of the present study
suggested the differentiation of gastric cancer cells into
monocytic MDSCs. Although the present study does not rule out every
conceivable pathway, it provided a clear association between VIP,
monocytic MDSC and T-cell dysfunction. Further analysis of the
mechanism of VIP-induced MDSC immune suppression may assist in the
search for novel targets for cancer immunotherapy.
Acknowledgments
The present study was supported by the National
Science Fund (grant nos. 81101825, 81272424 and 81172294), the
Research Fund for the Doctoral Program of Higher Education of China
(grant nos. 20100142120033 and 20120142110072) and Fundamental
research funds for the central universities (grant nos. 2011JC080,
2014QN039 and 2014QN055).
Abbreviations:
|
MDSC
|
myeloid-derived suppressor cell
|
|
VIP
|
vasoactive intestinal peptide
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
IL
|
interleukin
|
|
PGE
|
prostaglandin E
|
|
TNF
|
tumor necrosis factor
|
|
VEGF
|
vascular endothelial growth
factor
|
|
TGF
|
transforming growth factor
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
IFN
|
interferon
|
|
PAC
|
pituitary adenylate cyclase
|
|
iNOS
|
inducible nitric oxide synthase
|
|
ARG
|
arginase
|
|
ROS
|
reactive oxygen species
|
|
JAK
|
janus kinase
|
|
STAT
|
signal transducers and activators of
transcription
|
References
|
1
|
Almand B, Clark JI, Nikitina E, et al:
Increased production of immature myeloid cells in cancer patients:
a mechanism of immunosuppression in cancer. J Immunol. 166:678–689.
2001. View Article : Google Scholar
|
|
2
|
Bronte V, Serafini P, Apolloni E and
Zanovello P: Tumor-induced immune dysfunctions caused by myeloid
suppressor cells. J Immunother. 24:431–446. 2001. View Article : Google Scholar
|
|
3
|
Young MR, Petruzzelli GJ, Kolesiak K,
Achille N, Lathers DM and Gabrilovich DI: Human squamous cell
carcinomas of the head and neck chemoattract immune suppressive
CD34(+) progenitor cells. Hum Immunol. 62:332–341. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX
and Guo YJ: Increased CD14(+)HLA-DR (−/low) myeloid-derived
suppressor cells correlate with extrathoracic metastasis and poor
response to chemotherapy in non-small cell lung cancer patients.
Cancer Immunol Immunother. 62:1439–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zea AH, Rodriguez PC, Atkins MB, et al:
Arginase-producing myeloid suppressor cells in renal cell carcinoma
patients: a mechanism of tumor evasion. Cancer Res. 65:3044–3048.
2005.PubMed/NCBI
|
|
6
|
Hanson EM, Clements VK, Sinha P, Ilkovitch
D and Ostrand-Rosenberg S: Myeloid-derived suppressor cells
downregulate L-selectin expression on CD4+ and
CD8+ T cells. J Immunol. 183:937–944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duffy A, Zhao F, Haile L, et al:
Comparative analysis of monocytic and granulocytic myeloid-derived
suppressor cell subsets in patients with gastrointestinal
malignancies. Cancer Immunol Immunother. 62:299–307. 2013.
View Article : Google Scholar
|
|
9
|
Hoechst B, Ormandy LA, Ballmaier M, et al:
A new population of myeloid-derived suppressor cells in
hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T
cells. Gastroenterology. 135:234–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khaled YS, Ammori BJ and Elkord E:
Myeloid-derived suppressor cells in cancer: recent progress and
prospects. Immunol Cell Biol. 91:493–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lechner MG, Liebertz DJ and Epstein AL:
Characterization of cytokine-induced myeloid-derived suppressor
cells from normal human peripheral blood mononuclear cells. J
Immunol. 185:2273–2284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kusmartsev S and Gabrilovich DI: Effect of
tumor-derived cytokines and growth factors on differentiation and
immune suppressive features of myeloid cells in cancer. Cancer
Metastasis Rev. 25:323–331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandez-Martinez AB, Collado B, Bajo AM,
Sanchez-Chapado M, Prieto JC and Carmena MJ: Vasoactive intestinal
peptide induces cyclooxygenase-2 expression through nuclear
factor-kappaB in human prostate cell lines Differential
time-dependent responses in cancer progression. Mol Cell
Endocrinol. 270:8–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valdehita A, Carmena MJ, Bajo AM and
Prieto JC: RNA interference-directed silencing of VPAC1 receptor
inhibits VIP effects on both EGFR and HER2 transactivation and VEGF
secretion in human breast cancer cells. Mol Cell Endocrinol.
348:241–246. 2012. View Article : Google Scholar
|
|
15
|
Sotomayor S, Carmena MJ, Schally AV, et
al: Transactivation of HER2 by vasoactive intestinal peptide in
experimental prostate cancer: Antagonistic action of an analog of
growth-hormone-releasing hormone. Int J Oncol. 31:1223–1230.
2007.PubMed/NCBI
|
|
16
|
Couvineau A and Laburthe M: VPAC
receptors: structure, molecular pharmacology and interaction with
accessory proteins. Br J Pharmacol. 166:42–50. 2012. View Article : Google Scholar :
|
|
17
|
Laburthe M, Couvineau A and Tan V: Class
II G protein-coupled receptors for VIP and PACAP: structure, models
of activation and pharmacology. Peptides. 28:1631–1639. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pozo D, Anderson P and Gonzalez-Rey E:
Induction of alloantigen-specific human T regulatory cells by
vasoactive intestinal peptide. J Immunol. 183:4346–4359. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu K, Kryczek I, Chen L, Zou W and Welling
TH: Kupffer cell suppression of CD8+ T cells in human
hepatocellular carcinoma is mediated by B7-H1/programmed death-1
interactions. Cancer Res. 69:8067–8075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kryczek I, Wu K, Zhao E, et al: IL-17+
regulatory T cells in the microenvironments of chronic inflammation
and cancer. J Immunol. 186:4388–4395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vuk-Pavlovic S, Bulur PA, Lin Y, et al:
Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer.
Prostate. 70:443–455. 2010.
|
|
22
|
Lechner MG, Megiel C, Russell SM, et al:
Functional characterization of human Cd33+ and Cd11b+
myeloid-derived suppressor cell subsets induced from peripheral
blood mononuclear cells co-cultured with a diverse set of human
tumor cell lines. J Translat Med. 9:902011. View Article : Google Scholar
|
|
23
|
Youn JI and Gabrilovich DI: The biology of
myeloid-derived suppressor cells: the blessing and the curse of
morphological and functional heterogeneity. Eur J Immunol.
40:2969–2975. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JM, Southerland L, Hossain MS, et al:
Absence of vasoactive intestinal peptide expression in
hematopoietic cells enhances Th1 polarization and antiviral
immunity in mice. J Immunol. 187:1057–1065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sinha P, Clements VK, Bunt SK, Albelda SM
and Ostrand-Rosenberg S: Cross-talk between myeloid-derived
suppressor cells and macrophages subverts tumor immunity toward a
type 2 response. J Immunol. 179:977–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang B, Pan PY, Li Q, et al: Gr-1+CD115+
immature myeloid suppressor cells mediate the development of
tumor-induced T regulatory cells and T-cell anergy in tumor-bearing
host. Cancer Res. 66:1123–1131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porembka MR, Mitchem JB, Belt BA, et al:
Pancreatic adenocarcinoma induces bone marrow mobilization of
myeloid-derived suppressor cells which promote primary tumor
growth. Cancer Immunol Immunother. 61:1373–1385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Movahedi K, Guilliams M, Van den Bossche
J, et al: Identification of discrete tumor-induced myeloid-derived
suppressor cell subpopulations with distinct T cell-suppressive
activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poschke I, Mougiakakos D, Hansson J,
Masucci GV and Kiessling R: Immature immunosuppressive
CD14+HLA-DR−/low cells in melanoma patients
are Stat3hi and overexpress CD80, CD83 and DC-sign. Cancer Res.
70:4335–4345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Colombo MP and Piconese S:
Regulatory-T-cell inhibition versus depletion: the right choice in
cancer immunotherapy. Nat Rev Cancer. 7:880–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brimnes MK, Vangsted AJ, Knudsen LM, et
al: Increased level of both CD4+FOXP3+ regulatory T
cells and CD14+HLA-DR (−)/low myeloid-derived suppressor cells and
decreased level of dendritic cells in patients with multiple
myeloma. Scand J Immunol. 72:540–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodrigues JC, Gonzalez GC, Zhang L, et al:
Normal human monocytes exposed to glioma cells acquire
myeloid-derived suppressor cell-like properties. Neuro Oncol.
12:351–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonzalez-Rey E and Delgado M: Vasoactive
intestinal peptide and regulatory T-cell induction: a new mechanism
and therapeutic potential for immune homeostasis. Trends Mol Med.
13:241–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szilasi M, Buglyo A, Treszl A, Kiss L,
Schally AV and Halmos G: Gene expression of vasoactive intestinal
peptide receptors in human lung cancer. Int J Oncol. 39:1019–1024.
2011.PubMed/NCBI
|
|
37
|
Veljkovic M, Dopsaj V, Dopsaj M, et al:
Physical activity and natural anti-VIP antibodies: potential role
in breast and prostate cancer therapy. PloS one. 6:e283042011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lippitz BE: Cytokine patterns in patients
with cancer: a systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boytim M1, Lilly P, Drouvalakis K, et al:
A human class II MHC-derived peptide antagonizes
phosphatidylinositol 3-kinase to block IL-2 signaling. J Clin
Invest. 105:1447–1453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szema AM, Hamidi SA, Golightly MG, Rueb TP
and Chen JJ: VIP Regulates the Development & Proliferation of
Treg in vivo in spleen. Allergy Asthma Clin Immunol. 7:192011.
View Article : Google Scholar
|
|
41
|
Dumitriu IE, Dunbar DR, Howie SE, Sethi T
and Gregory CD: Human dendritic cells produce TGF-beta 1 under the
influence of lung carcinoma cells and prime the differentiation of
CD4+CD25+Foxp3+ regulatory T cells. J Immunol.
182:2795–2807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vicari AP, Chiodoni C, Vaure C, et al:
Reversal of tumor-induced dendritic cell paralysis by CpG
immunostimulatory oligonucleotide and anti-interleukin 10 receptor
antibody. J Exp Med. 196:541–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Toscano MG, Delgado M, Kong W, Martin F,
Skarica M and Ganea D: Dendritic cells transduced with lentiviral
vectors expressing VIP differentiate into VIP-secreting
tolerogenic-like DCs. Mol Ther. 18:1035–1045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Laborde RR, Lin Y, Gustafson MP, Bulur PA
and Dietz AB: Cancer vaccines in the world of immune suppressive
monocytes (CD14HLA-DR Cells): The gateway to improved responses.
Front Immunol. 5:1472014. View Article : Google Scholar
|
|
45
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Williams LM, Ricchetti G, Sarma U, Smallie
T and Foxwell BM: Interleukin-10 suppression of myeloid cell
activation-a continuing puzzle. Immunology. 113:281–292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu X, Chen J, Wang L and Ivashkiv LB:
Crosstalk among Jak-STAT, Toll-like receptor and ITAM-dependent
pathways in macrophage activation. J Leukoc Biol. 82:237–243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Finbloom DS and Winestock KD: IL-10
induces the tyrosine phosphorylation of tyk2 and Jak1 and the
differential assembly of STAT1 alpha and STAT3 complexes in human T
cells and monocytes. J Immunol. 155:1079–1090. 1995.PubMed/NCBI
|
|
49
|
Kusmartsev S, Nagaraj S and Gabrilovich
DI: Tumor-associated CD8+T cell tolerance induced by bone
marrow-derived immature myeloid cells. J Immunol. 175:4583–4592.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gabitass RF, Annels NE, Stocken DD, Pandha
HA and Middleton GW: Elevated myeloid-derived suppressor cells in
pancreatic, esophageal and gastric cancer are an independent
prognostic factor and are associated with significant elevation of
the Th2 cytokine interleukin-13. Cancer Immunol Immunother.
60:1419–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|