Introduction
Parkinson’s disease (PD) is a prevalent
neurodegenerative disease, which is neuropathologically
characterized by the loss of substantia nigra pars compacta (SNpc)
dopaminergic neurons, resulting in disabling motor abnormalities
(1,2). Numerous studies have explored the
pathogenesis of PD; however, the etiology of PD remains to be fully
elucidated. Previous studies have indicated that mitochondrial
dysfunction upregulates the production of reactive oxygen species
(ROS), which initiates the neurodegeneration of dopaminergic cells
in vivo and in vitro (3–6).
6-Hydroxydopamine (6-OHDA), a hydroxylated analogue of dopamine,
was reported to induce ROS degeneration, therefore resulting in
models of PD in vivo and in vitro (1,7–9).
PC12 rat pheochromocytoma cells, derived from rat pheochromocytoma
tumors, have numerous properties that are similar to those of
dopaminergic neurons and are therefore widely used for studies
investigating the pathogenesis and treatment of PD (10–12).
In the present study, PC12 cells exposed to 6-OHDA served as a
typical experimental model for the investigation of PD in
vitro.
Conserved dopamine neurotrophic factor (CDNF), a
member of the mammalian mesencephalic astrocyte-derived
neurotrophic factor family, has been reported to significantly
protect and reverse the loss of dopaminergic neurons due to 6-OHDA
treatment in vivo, through the direct infusion of CDNF
proteins or CDNF-engineered vectors into the brain (2,13–16).
The present study aimed to investigate whether CDNF exhibited
comparable neuroprotective and reversal effects in 6-OHDA-treated
PC12 cells in vitro.
Materials and methods
Harvesting and identification of CDNF
protein production
Total RNA was isolated from mouse heart and skeletal
muscle using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). First-strand cDNA was synthesized
using SuperscriptII reverse transcriptase (Invitrogen Life
Technologies) and then amplified through polymerase chain reaction
(PCR). Recombinant mouse CDNF (pFastBacHTb-mCDNF) labeled with Flag
and 6X His tags (Invitrogen Life Technologies) was produced through
baculoviral expression in Sf9 insect cells (Invitrogen Life
Technologies), as described previously (12). Secreted CDNF was purified from
culture supernatants using nickel affinity chromatography followed
by anion exchange chromatography. Rats were kept at the Anhui
Province Key Laboratory of Brain Function & Brain Disease
(Anhui, China) and all experimental procedures were performed in
accordance with the Guidelines for Animal Care and Use of the
National Institutes of Health. The present study was approved by
the Ethical Commttee of Anhui Provincial Hospital (Anhui,
China).
Cell culture and drug treatment
PC12 cells were obtained from the Shanghai Institute
of Cell Biology, Chinese Academy of Sciences (Shanghai, China), and
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Cells were treated with
6-OHDA (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) at concentrations of 1, 2.5, 5, 25, 50, 100 and 150
μM and then cultured for 24 h in order to determine the
optimal concentration of 6-OHDA for inducing PD model cells. PC12
cells were pre- or post-incubated with 50, 100 and 200 nM CDNF
protein in order to investigate the protective and reversal effects
of CNDF protein on 6-OHDA-induced neurotoxicity. The groups used in
the present study were as follows: A, control; B, 6-OHDA treatment
only; C, 6-OHDA + CDNF pre-treatment; and D, 6-OHDA + CDNF
post-treatment.
MTT assay
Cell viability was measured using a colorimetric MTT
assay. This method involves the cleavage of a yellow tetrazolium
salt into a purple formazan compound through the dehydrogenase
activity of intact mitochondria. In brief, cells were washed once
with phosphate-buffered saline (PBS) prior to the addition of 0.1
ml serum-free medium containing MTT (1 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) to each well. Following incubation for 3 h, the
supernatant was removed and the formazan product obtained was
dissolved in 1 ml dimethylsulfoxide (Sigma-Aldrich) and incubated
for 15 min with agitation on a microtiter plate shaker (CFJ-II;
Shanghai Lei Yun Test Instrument Manufacturing Co., Ltd., Shanghai,
China). The absorbance was then measured at 570 nm using an enzyme
immunoassay instrument (DG5031; Shanghai Kehuai Instrument Co.,
Ltd, Shanghai, China). Cell viability was expressed as a percentage
of that of the untreated cells, which served as the control
group.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate-mediated nick end
labeling (TUNEL) staining
A TUNEL kit was purchased from Roche Diagnostics
(Basel, Switzerland). Following 24 h of culture under the
experimental conditions described above, PC12 cells were fixed with
4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 h.
Cells were then washed with PBS, treated with 0.3%
H2O2 in methanol for 10 min and then washed
with 0.1% sodium citrate containing 0.1% Triton X-100 (Santa Cruz
Biotechnology, Inc.). In order to detect nuclear DNA fragmentation,
the TUNEL reagent was applied to the fixed cells. In brief, cells
were incubated with 50 μl terminal deoxynucleotidyl
transferase (TdT) solution (45 μl equilibration buffer, 1
μl biotin-11-deoxyuridine triphosphate and 4 μl TdT
enzyme; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd,
Beijing, China) in the dark, in a humid atmosphere at 37°C for 60
min. Samples were then further incubated with 50 μl
streptavidin-horseradish peroxidase (HRP) solution (0.5 μl
streptavidin-HRP and 99.5 μl PBS; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd) in the dark for 30 min at room
temperature. In order to determine the rate of cell death, staining
for peroxidase was performed using 100 μl diaminobenzidine
(DAB) solution (5 μl of 20X DAB, 1 μl of 30%
H2O2 and 94 μl PBS; Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd) for 10 min. All samples were
washed three times with PBS and then observed under a microscope
(BX51; Olympus, Tokyo, Japan) in order to count the
positive-stained cells. A minimum of ten fields were observed and
>1,000 cells were counted in order to determine a statistically
significant percentage of apoptotic cells. Data were expressed as a
percentage of the control group, which was designated as 100%.
Apoptosis by flow cytometry analysis
In order to detect 6-OHDA-induced early apoptosis
and late apoptosis/necrosis in the presence or absence of CDNF
treatment. The collected media was centrifuged at 1,500 × g for 5
min and then supernatants placed at −80°C until further use for
in vitro assays. PC12 cells (2×105/well) were
seeded onto six-well plates and cells in the corresponding groups
were treated with CDNF. Cells were then harvested through
centrifugation at 1,500 × g for 5 min, fixed with 70% ethanol (in
PBS) at 4°C overnight. Cells were then resuspended in PBS,
containing 40 μg/ml propidium iodide (Invitrogen Life
Technologies), 0.1 mg/ml RNase (Invitrogen Life Technologies) and
0.1% Trixon X-100, and incubated in the dark for 30 min at 37°C.
Cells were analyzed using a FACSCalibur flow cytometer
(Becton-Dickinson, San Jose, CA, USA) with an argon ion laser at
488 nm. Data were collected using Cell Quest software
(Becton-Dickinson).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis between two groups was performed
using Student’s t-test with SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Harvesting and identifying CDNF
Total RNA was extracted from mouse heart and
skeletal muscle, mouse CDNF cDNA was then reverse transcribed and
amplified using PCR. Recombinant CDNF was produced using a
baculovirus expression system in Sf9 cells, where CDNF was secreted
into the culture medium and then purified (Fig. 1).
Cell viability of 6-OHDA-induced PC12
cells
In order to investigate the effect of 6-OHDA on cell
viability in PC12 cells, the cells were incubated with 6-OHDA at
concentrations of 1, 2.5, 5, 25, 50, 100 and 150 μM and then
cultured for 24 h. The viability of PC12 cells was then measured
using an MTT assay. As shown in Fig.
2, at concentrations <25 μM, 6-OHDA exhibited no
significant effect on apoptosis in PC12 cells. However, significant
increases in 6-OHDA-induced apoptosis occurred in a dose-dependent
manner at concentrations ≥25 μM in PC12 cells. In addition,
there was a marked increase in apoptosis between 50 and 100
μM (P=0.006), which resulted in the apoptotic rate exceeding
50% at 100 μM. Although no significant increase was observed
between 100 and 150 μM (P=0.076), the number of necrotic
cells in the 150 μM group was observably increased (Data not
shown), which would interfere with the investigation of the
reversal effect of CDNF protein. Therefore, in order to reduce the
toxicity of 6-OHDA, 100 μM was deemed the optimal
experimental concentration (Fig.
2).
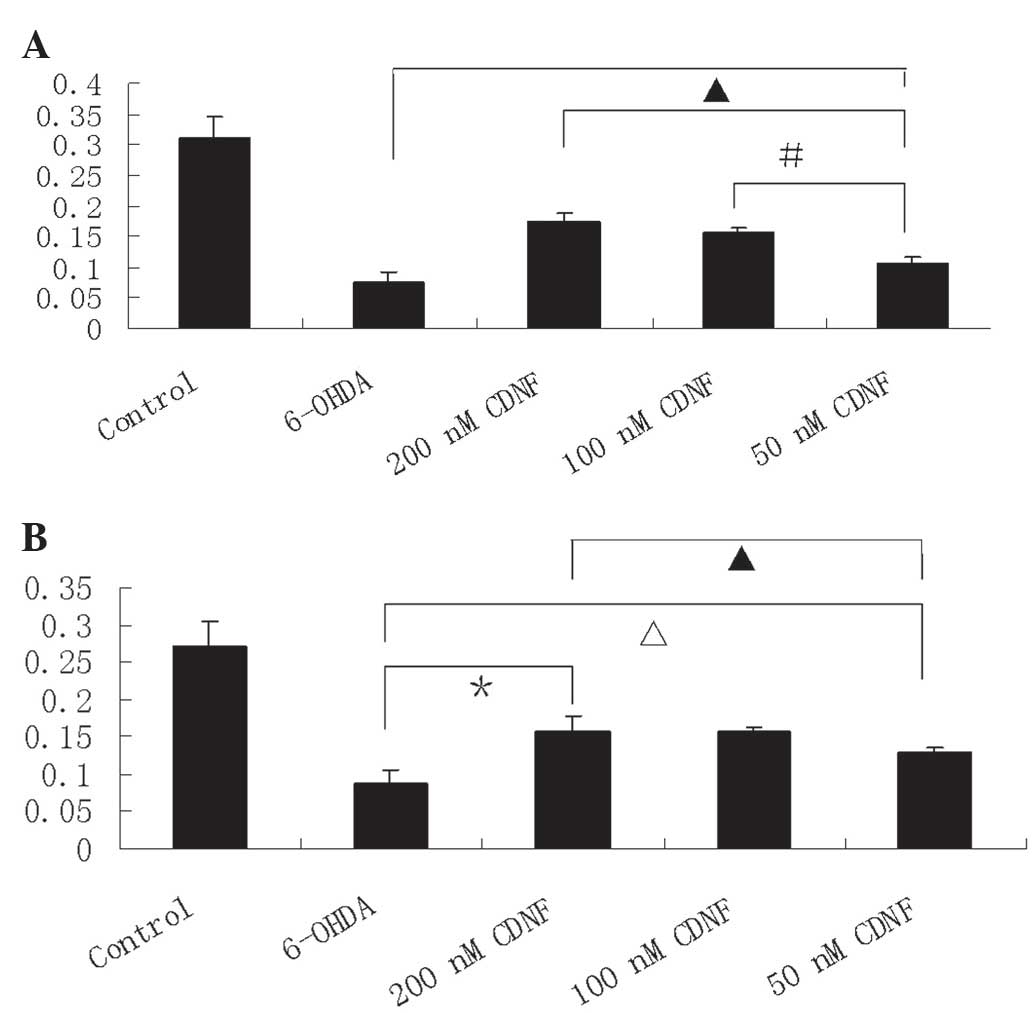
CDNF exerts protective and reversal
effects on 6-OHDA-induced PC12 cell viability
PC12 cell viability was determined using an MTT
assay. Following treatment with 6-OHDA alone (100 μM), cell
viability was significantly reduced (21.8%) compared with that of
the control group. CDNF treatment (50, 100 and 200 nM) for 30 min
prior to 6-OHDA (100 μM) exposure for 24 h resulted in the
significant increase of PC12 cell viability compared with that of
the 6-OHDA alone treatment, with survival rates of 46.6, 54.7 and
69.6% of control, respectively (Fig.
3A). In addition, PC12 cells incubated with CDNF following
exposure to 6-OHDA for 24 h demonstrated a significant increase in
cell viability compared with that of those treated with 6-OHDA
alone, with survival rates of 47.7, 57.6 and 57.5% of control,
respectively (Fig. 3B). These data
therefore indicated that CDNF prevented and reversed the effects of
6-OHDA on cell viability.
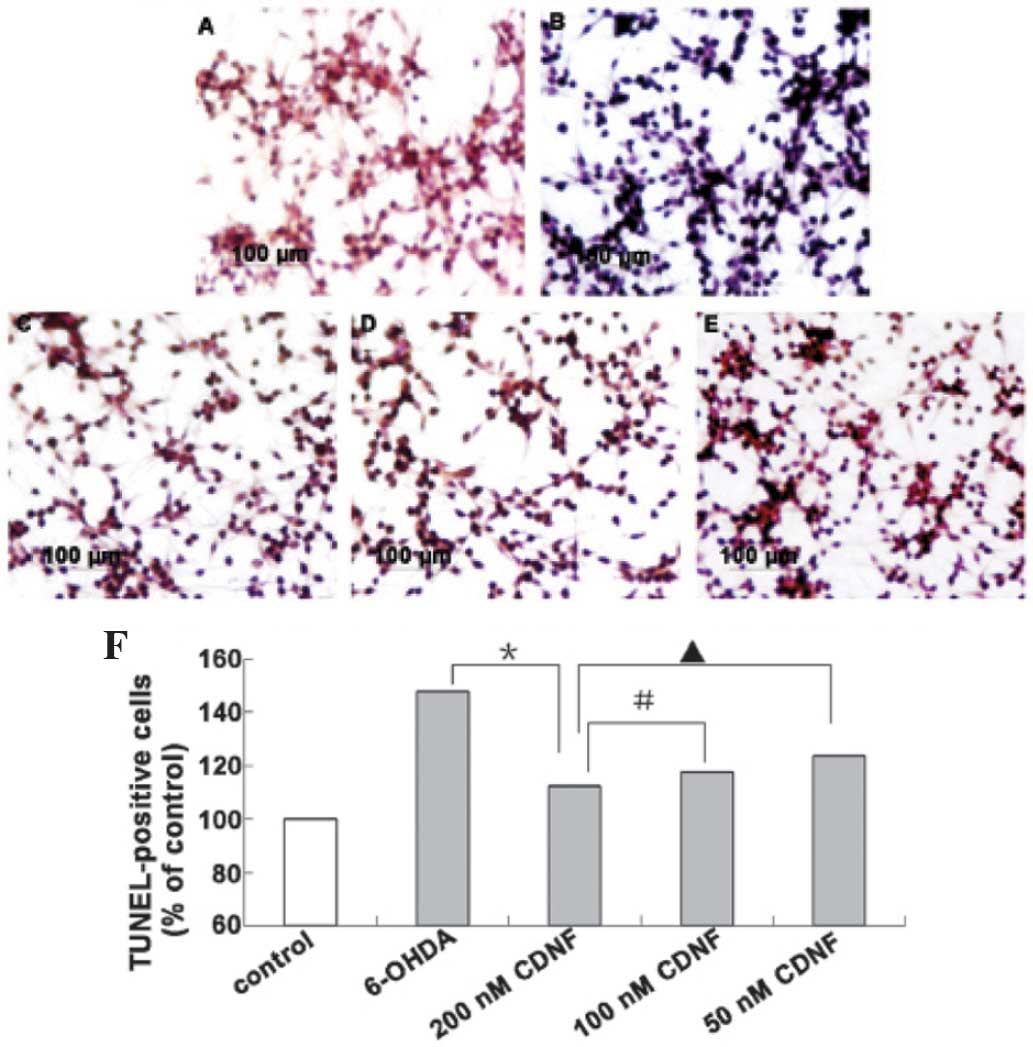
Attenuation of 6-OHDA-induced apoptosis
by CDNF
A distinctive biochemical characteristic of
apoptotic cell death is the occurrence of internucleosomal DNA
fragmentation. In the present study, apoptotic cells were assessed
using TUNEL staining, a widely used method for detecting DNA
fragmentation in situ. As shown in Fig. 4 and 5, following exposure to 100 μM
6-ODHA, the number of TUNEL-positive PC12 cells was significantly
increased compared with that of the control group (P<0.05). In
addition, the anti-apoptotic effects of CDNF (200, 100 and 50 nM)
were examined in PC12 cells prior to and following 6-OHDA-induced
apoptosis. As shown in Fig. 4,
treatment of PC12 cells with CDNF (200, 100 and 50 nM) for 30 min
prior to 6-OHDA exposure for 24 h resulted in a significant
decrease in the number of TUNEL-positive cells compared with that
of the 6-OHDA only group, with apoptotic rates of 112.47%, 117.87%
and 123.65% of control, respectively. Furthermore, when incubated
with CDNF following exposure to 6-OHDA for 24 h, the number of
TUNEL-positive cells was also markedly decreased compared with that
of the 6-OHDA only group, with apoptotic rates of 116.98%, 121.65
and 128.12% of control, respectively (Fig. 5).
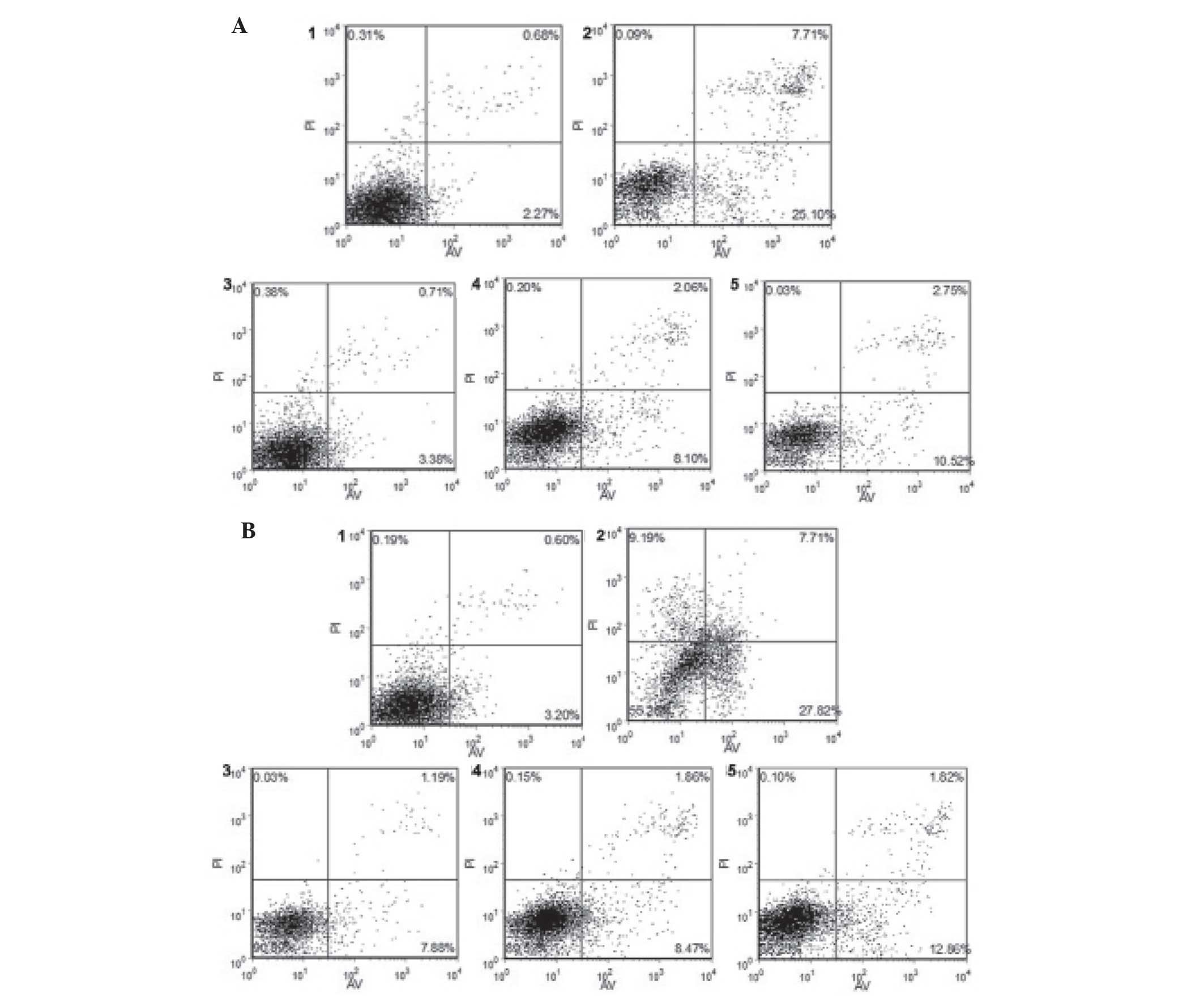
Flow cytometric analysis of the effects
of CDNF on 6-OHDA-induced apoptosis in PC12 cells
In order to examine the effects of 6-OHDA and CDNF
on apoptosis in PC12 cells, flow cytometric analysis was performed.
The results showed that following treatment with 6-OHDA alone,
~39.79% of cells were apoptotic at 24 h; however, pre-treatment of
6-OHDA-induced PC12 cells with CDNF (200, 100 and 50 nM)
significantly reduced the apoptotic rates to 5.62, 10.36 and
13.31%, respectively (Fig. 6A). In
addition, following incubation with CDNF protein (200, 100 and 50
nM) post-exposure to 6-OHDA for 24 h resulted in significantly
decreased apoptotic rates of 9.1%, 10.48% and 14.78%, respectively
(Fig. 6B).
Discussion
The present study demonstrated that 6-OHDA induced
the decrease of PC12 cell viability to 21.8%, which was in
accordance with a previous study by Gorman et al (17). In addition, the present study
demonstrated that CDNF prevented and reversed the effect of 6-OHDA
on PC12 cell apoptosis in a dose-dependent manner, suggesting a
neuroprotective function in neuronal cells in vitro.
Previous studies have elucidated that neurotrophic
factors, including BDNF and GDNF, may prevent neurodegeneration
(18,19). In addition, GDNF was previously
reported to improve symptoms in patients with PD; however, this
potential treatment was found to have serious adverse effects and
low clinical benefit (20–22). These findings encouraged novel
studies into potential neurotrophic factors that may slow down or
reverse the progression of neuronal degeneration. Lindholm et
al (13) reported that CDNF
exerted neuroprotective and reversed the neurodegenerative effects
of dopaminergic neurons in vivo, which significantly
decreased the loss of dopaminergic neurons in rat models induced by
6-OHDA and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, at the
pathological and behavioral levels (2,13,14).
The aim of the present study was to evaluate the neuroprotective
effects of CDNF in vitro. PC12 cells pre-cultured with CDNF
prior to 6-OHDA treatment exhibited increased cell viability, in a
dose-dependent manner, as detected using an MTT assay. The maximum
protective effect on cell viability was observed at a pre-culture
concentration of 200 nM CDNF, which increased cell viability to
69.6%. In addition, pre-treatment with CDNF decreased the number of
TUNEL-positive cells and the apoptotic rate of PC12 cells in a
dose-dependent manner. Furthermore, in the present study, PC12
cells were cultured with 6-OHDA for 24 h and then post-treated with
various concentrations of CDNF. These results showed that cell
viability was also increased compared with that of the control
group. In addition, the reversal effects of CDNF were detected
using TUNEL-staining and flow cytometry; however, the protective
effects were marginally inferior to those of the CDNF
pre-treatment. By contrast, the neuroprotective effect on cell
viability of CDNF in vitro (200 nM, 69.6%) was markedly less
potent than the reported effect of CDNF in vivo (96%)
(13). The explanation for this
may be due to the fact that the PC12 cell model induced using
6-OHDA is an acute model of PD, which is not completely consistent
with the chronic progress of neurodegeneration in vivo
(23–25). In addition, single cell models do
not replicate the survival microenvironment of dopaminergic neurons
in vivo. Another explanation may be that CDNF proteins
stimulate other cytokines or signal pathways, which may be involved
in neuroprotection in vivo. Notably, in the present study,
the reversal effect of post-treatment with CDNF on cell viability
(200 nM, 57.5%) was comparable to that of the reported effect in
vivo (58%) (13). This may be
attributed to the fact that 6-OHDA treatment led to later stages of
apoptosis and necrosis in the PC12 cells pre-cultured with 6-OHDA,
as shown in Fig. 5, whereas the
earlier stages of apoptosis were more prominent following
pre-treatment with CDNF. This therefore indicated that CDNF was
unable to reverse the late stages of apoptosis and necrosis in PC12
cells.
Furthermore, the results of the present study also
demonstrated that the preventive and reversal effects of CDNF
occurred in a dose-dependent manner, regardless of pre- and
post-incubation, which was consistent with those of other
neurotrophic factors (26,27). In addition, the cell morphology
observed by TUNEL-staining and cell apoptosis as determined using
flow cytometry also demonstrated the dose-dependent neuroprotective
and reversal effects of CDNF; in particular, the effects of CDNF on
the protection against early apoptosis.
In conclusion, the results of the present study
showed that CDNF exerted neuroprotective and reversal effects on
6-OHDA-induced PC12 cells in a dose-dependent manner. Therefore
CDNF may have therapeutic potential for PD.
Acknowledgments
The present study was supported by grants from the
Foundation of the Department of Education of Anhui Province (grant
no. KJ2010B381), the Foundation of Natural Science of Anhui
Province (grant no. 11040606Q11) and the National Natural Science
Fund (grant no. 81100960).
References
|
1
|
Han B, Hu J, Shen J, Gao Y, Lu Y and Wang
T: Neuroprotective effect of hydroxysafflor yellow A on
6-hydroxydopamine-induced Parkinson’s disease in rats. Eur J
Pharmacol. 714:83–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Airavaara M, Harvey BK, Voutilainen MH, et
al: CDNF protects the nigrostriatal dopamine system and promotes
recovery after MPTP treatment in mice. Cell Transplant.
21:1213–1223. 2012. View Article : Google Scholar
|
|
3
|
Chaturvedi RK and Flint Beal M:
Mitochondrial diseases of the brain. Free Radic Biol Med. 63:1–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopert P, Day BJ and Patel M: Thioredoxin
reductase deficiency potentiates oxidative stress, mitochondrial
dysfunction and cell death in dopaminergic cells. PLoS One.
7:e506832012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cantu D, Fulton RE, Drechsel DA and Patel
M: Mitochondrial aconitase knockdown attenuates paraquat-induced
dopaminergic cell death via decreased cellular metabolism and
release of iron and H2O2. J Neurochem.
118:79–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calabrese V, Lodi R, Tonon C, et al:
Oxidative stress, mitochondrial dysfunction and cellular stress
response in Friedreich’s ataxia. J Neurol Sci. 233:145–162. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prinz A, Selesnew LM, Liss B, Roeper J and
Carlsson T: Increased excitability in serotonin neurons in the
dorsal raphe nucleus in the 6-OHDA mouse model of Parkinson’s
disease. Exp Neurol. 248:236–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadan O, Bahat-Stromza M, Barhum Y, et al:
Protective effects of neurotrophic factor-secreting cells in a
6-OHDA rat model of Parkinson disease. Stem Cells Dev.
18:1179–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olds ME, Jacques DB and Kpoyov O:
Behavioral/neurophysiological investigation of effects of combining
a quinolinic acid entopeduncular lesion with a fetal mesencephalic
tissue transplant in striatum of the 6-OHDA hemilesioned rat.
Synapse. 49:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Offen D, Sherki Y, Melamed E, Fridkin M,
Brenneman DE and Gozes I: Vasoactive intestinal peptide (VIP)
prevents neurotoxicity in neuronal cultures: relevance to
neuroprotection in Parkinson’s disease. Brain Res. 854:257–262.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang LZ, Sun WC and Zhu XZ: Ethyl pyruvate
protects PC12 cells from dopamine-induced apoptosis. Eur J
Pharmacol. 508:57–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chagkutip J, Govitrapong P, Klongpanichpak
S and Ebadi M: Mechanism of 1-methyl-4-phenylpyridinium-induced
dopamine release from PC12 cells. Neurochem Res. 30:633–639. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindholm P, Voutilainen MH, Laurén J, et
al: Novel neurotrophic factor CDNF protects and rescues midbrain
dopamine neurons in vivo. Nature. 448:73–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Voutilainen MH, Bäck S, Peränen J, et al:
Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat
model of Parkinson’s disease. Exp Neurol. 228:99–108. 2011.
View Article : Google Scholar
|
|
15
|
Bäck S, Peränen J, Galli E, et al: Gene
therapy with AAV2-CDNF provides functional benefits in a rat model
of Parkinson’s disease. Brain Behav. 3:75–88. 2013. View Article : Google Scholar
|
|
16
|
Ren X, Zhang T, Gong X, Hu G, Ding W and
Wang X: AAV2-mediated striatum delivery of human CDNF prevents the
deterioration of midbrain dopamine neurons in a 6-hydroxy-dopamine
induced parkinsonian rat model. Exp Neurol. 248:148–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gorman AM, Szegezdi E, Quigney DJ and
Samali A: Hsp27 inhibits 6-hydroxydopamine-induced cytochrome c
release and apoptosis in PC12 cells. Biochem Biophys Res Commun.
327:801–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stahl K, Mylonakou MN, Skare Ø,
Amiry-Moghaddam M and Torp R: Cytoprotective effects of growth
factors: BDNF more potent than GDNF in an organotypic culture model
of Parkinson’s disease. Brain Res. 1378:105–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gyárfás T, Knuuttila J, Lindholm P,
Rantamäki T and Castrén E: Regulation of brain-derived neurotrophic
factor (BDNF) and cerebral dopamine neurotrophic factor (CDNF) by
anti-parkin-sonian drug therapy in vivo. Cell Mol Neurobiol.
30:361–368. 2010. View Article : Google Scholar
|
|
20
|
Gill SS, Patel NK, Hotton GR, O’Sullivan
K, et al: Direct brain infusion of glial cell line-derived
neurotrophic factor in parkinson disease. Nat Med. 9:589–595. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel NK, Bunnage M, Plaha P, Svendsen CN,
Heywood P and Gill SS: Intraputamenal infusion of glia cell
line-derived neurotrophic factor in PD: a two-year outcome study.
Ann Neurol. 57:298–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lang AE, Gill S, Patel NK, et al:
Randomized controlled trial of intraputamenal glial cell
line-derived neurotrophic factor infusion in Parkinson disease. Ann
Neurol. 59:459–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Black YD, Xiao D, Pellegrino D, Kachroo A,
Brownell AL and Schwarzschild MA: Protective effect of metabotropic
glutamate mGluR5 receptor elimination in a 6-hydroxydopamine model
of Parkinson’s disease. Neurosci Lett. 486:161–165. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winner B, Desplats P, Hagl C, et al:
Dopamine receptor activation promotes adult neurogenesis in an
acute Parkinson model. Exp Neurol. 219:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon IH, Choi HS, Shin KS, et al: Effects
of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12
cells and a rat model of Parkinson’s disease. Neurosci Lett.
486:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim Y, Li E and Park S: Insulin-like
growth factor-1 inhibits 6-hydroxydopamine-mediated endoplasmic
reticulum stress-induced apoptosis via regulation of heme
oxygenase-1 and Nrf2 expression in PC12 cells. Int J Neurosci.
122:641–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Peng C, Li L, Ming M, Yang D and Le
W: Glial cell-derived neurotrophic factor protects against
proteasome inhibition-induced dopamine neuron degeneration by
suppression of endoplasmic reticulum stress and caspase-3
activation. J Gerontol A Biol Sci Med Sci. 62:943–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|