Introduction
Asthma is a chronic inflammatory airway disease. It
affects over 300 million individuals worldwide with an expected
increase of 100 million by 2025 (1,2). The
pathophysiological characteristics of allergic asthma, including
chronic pulmonary eosinophilia, airway hyperresponsiveness (AHR) to
a variety of nonspecific spasmogenic stimuli, excessive airway
mucus production and elevated serum immunoglobulin E (IgE) levels
are all associated with aberrant T-helper 2 (Th2) cell responses.
Th2 cells are known to secrete interleukin (IL)-4, -5, -9 and -13.
These cytokines, particularly IL-4, -5 and -13, have been
documented to have a relatively important role in asthma
progression. Th2 cell differentiation is driven by the
transcription factor GATA-binding protein 3 (GATA-3), a member of
the GATA family of zinc finger proteins (3). This transcription factor is known as
the master regulator of Th2-cell differentiation. GATA-3 is
suppressed by T-bet expressed in T cells, a Th1-specific
transcription factor, which is hypothesized to induce interferon
(IFN)-γ production while inhibiting IL-4 production (4).
Afzelin (Fig. 1) is
a flavonol glycoside found in Ficus palmata and Nymphaea
odorata. Previously, it has been found to inhibit lipid
peroxidation and cyclooxygenase (COX)-1 and COX-2 in vivo.
It is the rhamnoside of kaempferol, which has been documented to
suppress inflammatory-cell infiltration in a mouse model of asthma
(5). A previous study indicated
that afzelin inhibits the growth of breast cancer cells through
stimulating apoptosis, while being relatively non-toxic to normal
cells (6). However, the effects of
afzelin on asthma phenotypes have remained to be elucidated. The
present study was performed to investigate the anti-asthmatic
effect of afzelin and its mechanism of action in a mouse model of
asthma.
Materials and methods
Experimental animals
A total of 30 female BALB/c mice (five weeks old,
25–30 g) were attained from the animal house of the Capital Medical
University (Beijing, China), and maintained under controlled
conditions, temperature (24±2°C), relative humidity (60±10%) and
photoperiod (12-h light/dark cycle). The room was well ventilated
(>10 air changes/h) with fresh air, as per the Committee for the
Purpose of Control and Supervision on Experiments on Animals
guidelines. Animals were fed on a standard pellet diet and
sterilized water was provided ad libitum. Animals acclimated
for seven days were used for the pre-clinical studies. Approval of
the animal experimental protocols was obtained from the ethics
committee of the Capital Medical University (Beijing, China).
Reagents
Chicken egg albumin (OVA, grade V), aluminium
hydroxide gel (alum) and dexamethsone (Dexa),
acetyl-β-methylcholine chloride (methacholine) and protease
inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Antibodies used for western blotting were purchased from
Cell Signaling Technology (Beverly, MA, USA). Afzelin (purity, 99%)
was acquired from Chirochem (Daejeon, Korea). All other chemicals
and reagents were commercially obtained from Sigma-Aldrich and were
of the highest quality.
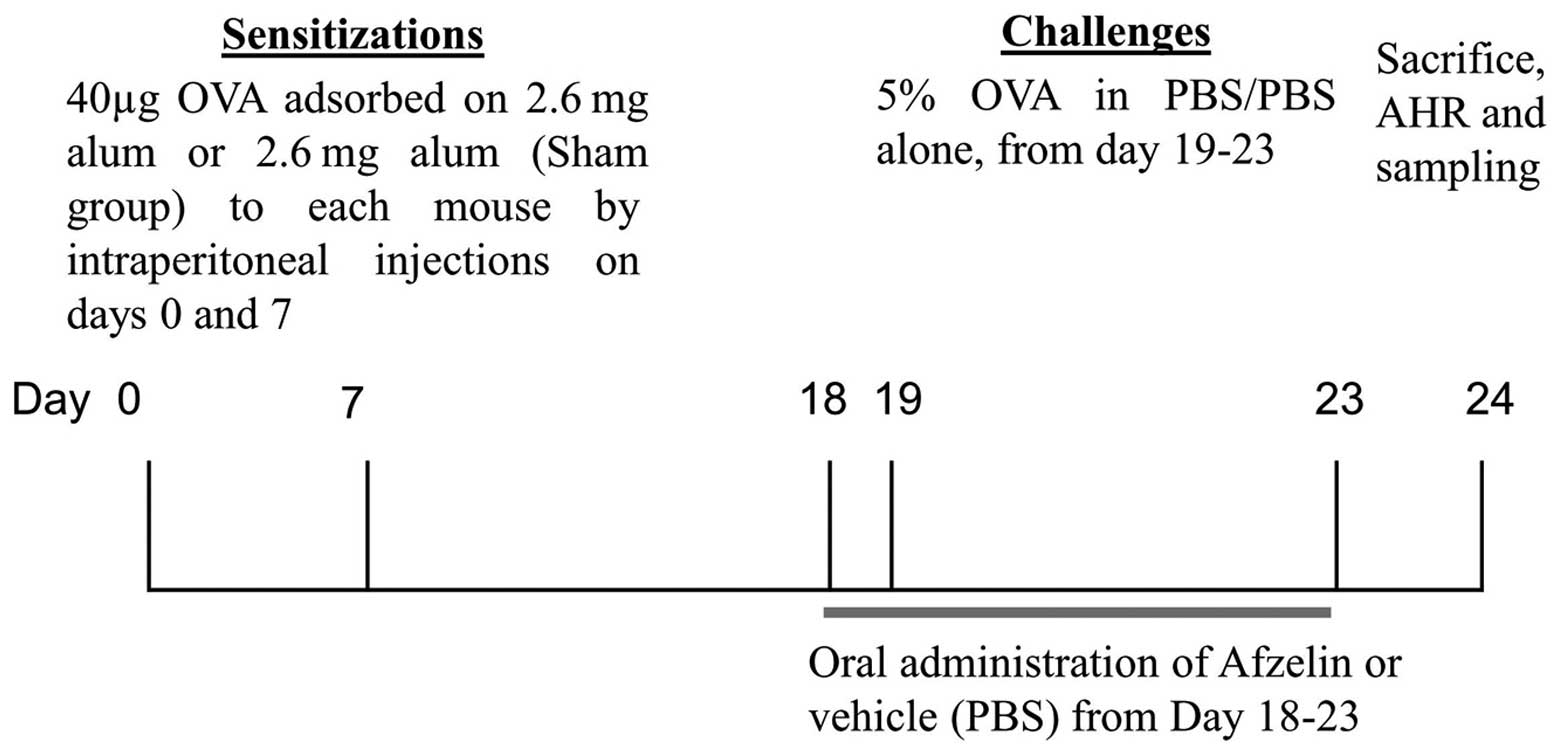
Segregation of animals and dosing
schedule
Mice were segregated into six groups (six mice in
each group) following acclimation; each group was termed according
to sensitization/challenge/treatment: Group 1,
SHAM/phosphate-buffered saline (PBS)/Vehicle (Veh; normal
controls); group 2, OVA/OVA/Veh (OVA controls, OVA-sensitized and
OVA-challenged); group 3, OVA/OVA/Dexa [OVA-sensitized,
OVA-challenged and Dexa-treated (0.75 mg/kg)]; and groups 4–6,
OVA/OVA/afzelin [OVA-sensitized, OVA-challenged and afzelin-treated
(0.1, 1 and 10 mg/kg)]. The test compounds and the Dexa were
administered orally, once daily from day 19 to day 23 (Fig. 2) (7). PBS was used as a vehicle.
Sensitization, airway OVA challenging and
treatment
The animals were sensitized intraperitoneally with
40 μg OVA plus 2.6 mg aluminum hydroxide in 200 μl
PBS on days 0 and 7. Mice were then challenged from days 19 to 23
(5 min per day) with 5% OVA in PBS (OVA groups) or PBS
(Sham/PBS/Veh) as described previously with certain modifications
(8). Mice were administered the
test drug and Dexa once a day from days 19 to 23. Mice were
sacrificed on day 24 by heart puncture under ether anesthesia
(Sigma-Aldrich), and bronchoalveolar lavage was performed to
evaluate lung eosinophilia.
Evaluation of AHR
AHR, in the form of airway resistance was estimated
in anesthetized mice using the FlexiVent system (Synol High-Tech,
Beijing, China), which uses a computer-controlled mouse ventilator
and integrates with respiratory mechanics, as described previously
(9). Final results were expressed
as airway resistance with increasing concentrations of methacholine
(Mch; 0, 2, 4, 8, 12 and 16 mg/ml).
Bronchoalveolar lavage fluid (BALF)
collection
After mice were bled and sacrificed following
anesthesia with ether, BALF was collected for differential cell
counting and measurement of cytokines. This was performed by
cannulating the upper part of the trachea and lavaging three times
with 0.5 ml PBS containing 0.05 mM EDTA (7). The BALF was centrifuged at 4,000 × g
at 4°C for 3 min and the cells were separated from the fluid. The
supernatant was stored at −70°C until use. The cells were
re-suspended in PBS containing 0.05 mM EDTA and the total cell
number was determined by using a hemocytometer. The differential
BAL cells were counted using microscopy (MCL-3000; MCALON, Beijing,
China) following cytospin preparations and Giemsa staining (Giemsa
stain modified, Sigma-Aldrich).
Cytokine measurement
Cytokine measurement was performed from serum
samples of animals. Levels of cytokines IL-5, -13 and -4 as well as
IFN-γ were determined using ELISA (R&D Systems, Minneapolis,
MN, USA). The ELISAs were performed as per the manufacturer’s
instructions.
Measurement of OVA-specific IgE
Each well of a microtiter plate (Abcore, Ramona, CA,
USA) was coated with 5 μg OVA in 100 μl PBS overnight
at 4°C. Following three washes, nonspecific sites were blocked with
0.5% Tween 20 (Abcore) in PBS. Mouse sera in duplicate were added
to the Ag-coated wells, the plates were incubated and bound IgE was
detected with biotinylated anti-mouse IgE (BD Pharmingen, San
Diego, CA, USA). Streptavidin-peroxidase conjugates (Takara
Biotechnology Co., Ltd., Dalian, China) were added and the bound
enzymes were detected with the addition of a tetramethylbenzidine
substrate system (BD Pharmingen) and absorbance was read at 450 nm
using an ultraviolet spectrophotometer (UV-3600; Shimadzu
Corporation, Kyoto, Japan). Absorbance was converted to arbitrary
units.
Western blot analysis
The lungs were homogenized in a homogenizing buffer
[1% NP-40, 150 mM NaCl, 50 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich),
phenylmethylsulfonyl fluoride and complete protease inhibitor
cocktail (Bio-Rad Laboratories, Inc., Hercules, CA, USA)]. Protein
estimation of the samples was performed according to the Bradford
method (10). For western
blotting, 30 μg protein was denatured at 100°C for 5 min in
Tris-glycine SDS (Abcore) sample loading buffer. Protein samples
were loaded onto 10% SDS gels and resolved at 70 V (300 mA) for 3 h
and then electro-transferred onto a polyvinylidene difluoride
membrane (Bio-Rad Laboratories, Inc.) in transfer buffer using a
Mini Transblot electrophoretic transfer cell (Bio-Rad Laboratories,
Inc.) for 90–120 min at 150 V. Membranes were blocked in 5%
fat-free dry milk (Abcore) dissolved in Tris-buffered saline and
Tween 20. for 2.5 h at room temperature. Anti-GATA3 and anti-T-bet
mouse polyclonal antibodies (1:1,000 dilution; Bio-Rad
Laboratories, Inc.) were used to determine expression of their
corresponding proteins, and a monoclonal β-actin antibody was used
as the loading control (Sigma-Aldrich) (11). After incubation with the primary
antibodies overnight at 4°C the membranes were incubated with goat
anti-mouse immunoglobulin G secondary antibody (1:5,000 dilution;
Bio-Rad Laboratories, Inc.) for 1 h at 25°C. The blots were
visualized with a chemiluminescent detection system (ECL; GE
Healthcare Australia, Rydalmere, Australia) according to the
manufacturer’s instructions.
Histological examination
After BALF was obtained, the left lung was removed,
fixed in 10% neutral buffered formalin for 24 h and then the
specimens were dehydrated and embedded in paraffin in a standard
manner. In order to perform histological examination, 5-μm
sections of fixed embedded tissues were cut and stained with
hematoxylin & eosin (Abcore) according to routine laboratory
procedures (12). Histological
analyses were performed by pathologists blinded to the treatment
groups. For each mouse, five airway sections, distributed
throughout the left lung, were analyzed with the use of a light
microscope (MCL-3000) attached to an image-analysis system
(Image-Pro Plus 4.0; Media Cybernetics, Minneapolis, MN, USA). The
images were then cropped and corrected for brightness and contrast,
but otherwise were not manipulated (7).
Statistical analysis
Groups were analyzed using a one-way analysis of
variance followed by Dunnett’s multiple comparison tests to examine
differences between OVA-challenged as well as PBS- and afzelin-and
Dexa-treated groups. P<0.05 was considered to indicate a
statistically significant difference. Values are presented as the
mean ± standard error of the mean for each group.
Results
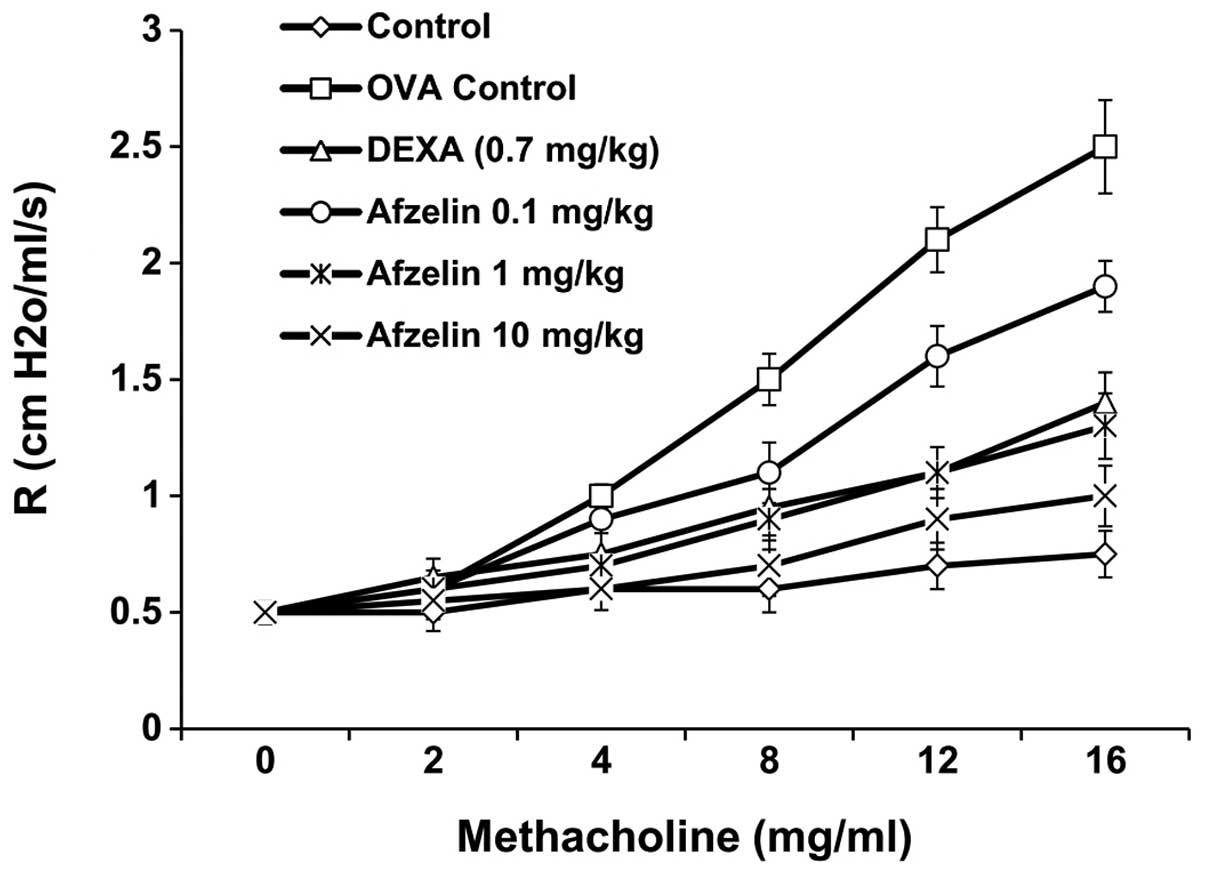
Afzelin decreases AHR in experimental
asthma
To examine the effect of afzelin on AHR, airway
resistance was measured in anaesthetized mice by invasive
whole-body plethysmography. No significant difference was found in
baseline airway resistance among the six groups. The airway
resistance generated by administration of Mch at doses of 0–16
mg/ml significantly increased in the OVA and afzelin (0.1
mg/kg)-treated groups. However, the control, Dexa- and afzelin (1
and 10 mg/kg)-treated groups exhibited a sharp decrease in airway
resistance (Fig. 3).
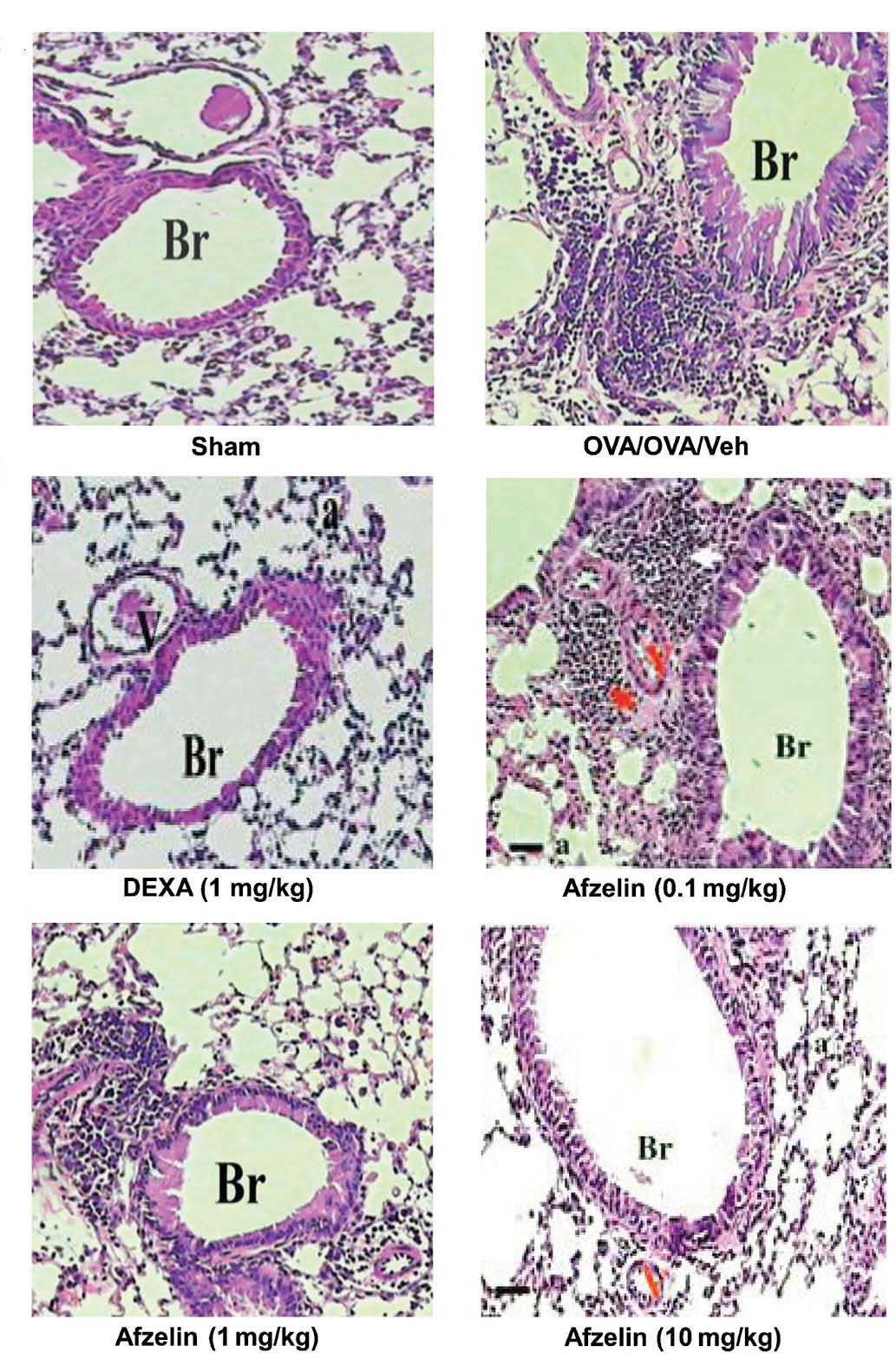
Afzelin attenuates airway
inflammation
Apart from macrophages, only few inflammatory cells
were detected in the control group. However, a significant increase
in total cell number was observed in OVA-sensitized and challenged
animals, when compared with those in the control mice. The effect
of afzelin on allergen-induced inflammatory cell infiltration was
assessed in animals treated with three different doses of afzelin.
As shown in Table I, afzelin at 1
and 10 mg/kg suppressed allergen-induced inflammatory cell
infiltration. However, in the case of the 0.1 mg/kg-treated group,
infiltration of inflammatory cells was not reduced. The
anti-inflammatory effect of afzelin was further demonstrated by
histological examination of hematoxylin & eosin-stained
sections (Fig. 4). A marked
affluence of inflammatory cells into the airway was observed in
OVA-sensitized/challenged mice, but not in the PBS-treated control
mice. Mice treated with afzelin exhibited a marked diminution in
inflammatory cell infiltration into the airways.
 | Table IEffect of afzelin on total cell count
and differential cell count. |
Table I
Effect of afzelin on total cell count
and differential cell count.
| Treatment | Total count
(×104/ml) | Differential count
(%)
|
|---|
| Macro | Mono | Eosino | Neutro |
|---|
| SHAM | 3.1±1.1 | 51.3±5.5 | 49.6±4.5 | – | – |
| OVA/OVA/Veh | 49.2±10.0 | 7.4±1.1 | 13.6±4.0 | 61.7±4.9 | 12.9±3.3 |
| Dexa (0.7 mg/kg) | 23.5±8.3 | 26.8±7.6 | 31.9±13.0 | 22.3±11.0 | 8.0±3.8 |
| Afzelin (0.1
mg/kg) | 35.6±11.2 | 17.9±6.9 | 38.2±2.3 | 52.2±4.4 | 9.4±2.3 |
| Afzelin (1
mg/kg) | 17.7±6.3 | 38.9±3.3 | 32.2±2.7 | 15.9±1.5 | 5.3±1.2 |
| Afzelin (10
mg/kg) | 15.5±10.2 | 47.0±13.0 | 42.5±9.3 | 11.5±3.2 | 3.3+1.1 |
Afzelin affects Th1 and Th2 cytokine
release
Measurement of the Th2 cytokines IL-4, -5 and -13
was performed in the serum collected from the mice. Mice treated
with the test compound afzelin demonstrated no significant change
in cytokine release when compared with those in the control at
doses of 1 and 10 mg/kg (Fig. 5).
However, cytokine levels measured in the 0.1 mg/kg-treated group of
animals exhibited a significant variation from the control group.
Afzelin increased the release of IFN-γ, a Th1 cytokine, indicating
that it affects T-cell differentiation, which was further supported
by its effect on GATA3 and T-bet.
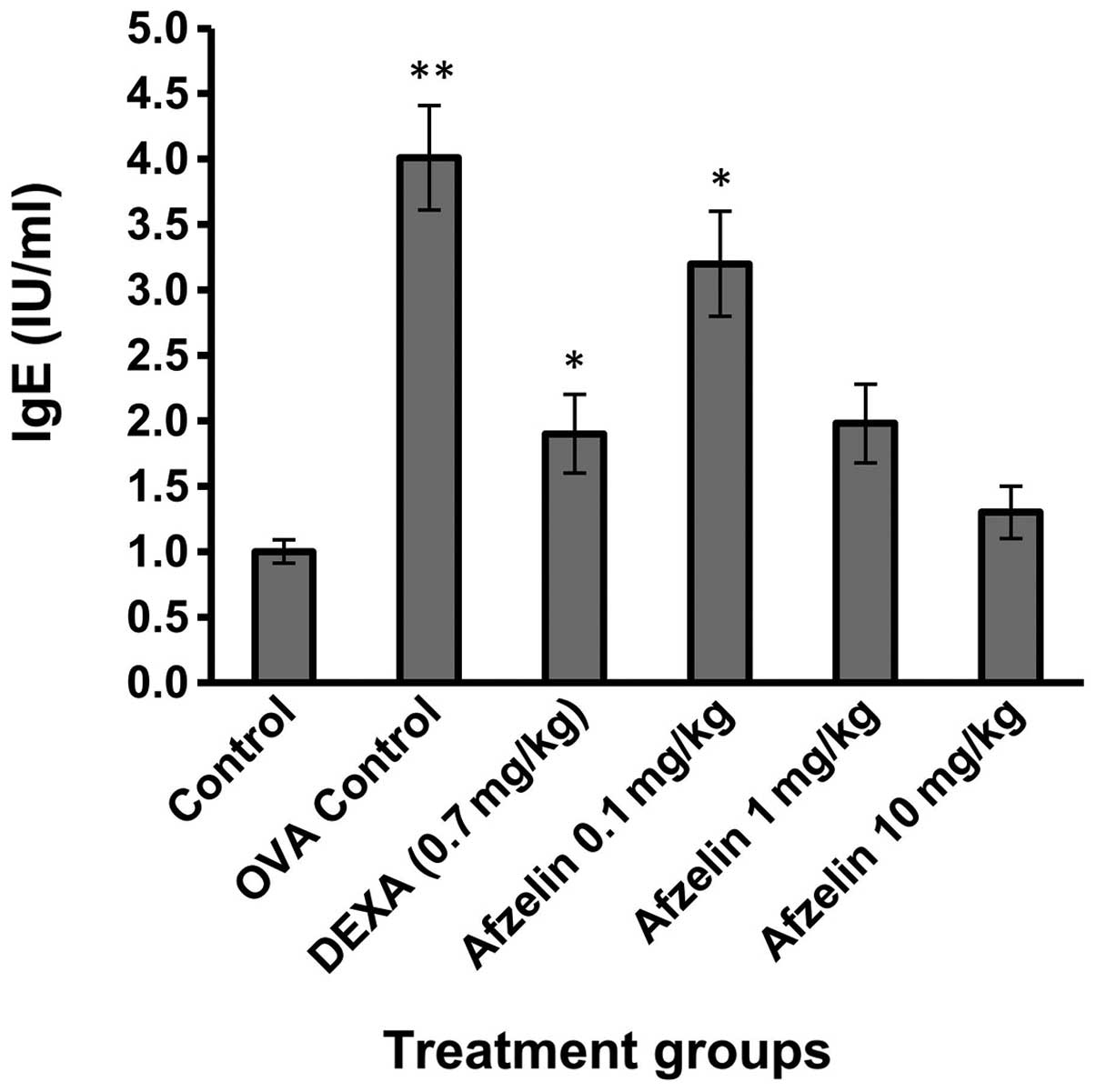
Afzelin reduces OVA-specific IgE
levels
OVA-specific IgE levels were elevated in the OVA
group when compared with those in the control group (Fig. 6). Treatment with afzelin (1 and 10
mg/kg) demonstrated no significant change in OVA-specific IgE
levels as compared with those in the control group.
Afzelin alters the expression of T-bet
and GATA-3 in the lungs
Expression levels of T-bet and GATA-3 in the lungs
were altered in OVA control mice, Dexa-treated mice and
afzelin-treated mice. However, no change was observed in the
expression levels of any of these proteins in control animals.
Treatment with afzelin increased expression of T-bet, while at the
same time decreasing GATA-3 expression in a dose-dependent manner
(Fig. 7).
Discussion
In the present study, the effects of afzelin on
allergen-induced airway inflammation and immune response in acute
experimental asthma were assessed. It was found that administration
of afzelin markedly reduced Th2 cytokine levels and OVA-specific
IgE, and suppressed airway inflammatory cell infiltration induced
by allergens, resulting in a decreased number of eosinophils and
total inflammatory cells in BALF. Lung histology validated the
effect of afzelin on airway inflammation. These findings suggested
that afzelin is an anti-asthmatic agent and may be beneficial for
the treatment of allergic asthma.
It is widely accepted that T cells have an important
role in the injurious lung immune responses associated with asthma
(13,14). CD4+ Th cells can be
divided into Th1 and Th2 groups, functionally based on the various
types of cytokine they produce. The different patterns of T-cell
differentiation generate the different inflammatory effectors and
those inflammatory effectors are correlated with the extent and
type of damage observed in the airways (15–17).
Under normal physiological conditions, the ratio of Th1 to Th2
cells is maintained at an appropriate level. Once the balance
between Th1 and Th2 is disrupted, disease may occur (18). The two major Th-specific
transcription factors T-bet and GATA-3, which regulate the
expression of the cytokine genes, are characteristics of Th1 or Th2
and have crucial roles in Th-cell differentiation. It has been
reported that a change in the T-bet/GATA-3 ratio reflects a change
in the Th1/Th2 balance (18–20).
Therefore, the T-bet/GATA-3 ratio may be used to evaluate the
immune balance of Th1/Th2 responses in asthma (4). In addition, increased IL-4 production
is correlated with excessive Th2-cell responses and increased IFN-γ
levels are associated with excessive Th1 cell responses (21). In the present study, the ratio of
T-bet to GATA-3 decreased in the asthma group compared with that in
the control group and was partly reverted in the afzelin treatment
groups. At an equal pace, IL-4 production was depressed and IFN-γ
levels increased in the treatment groups. This change was more
prominent in the 1 and 10 mg/kg afzelin-treated groups. Treatment
with Dexa had similar effects to those of 1 mg/kg afzelin. The
pathophysiology of AHR is complex as numerous factors contribute to
its development. Allergen-induced airway inflammation is important
among these factors, and Th2 cytokines, particularly IL-4, are
critical in allergic inflammation and development of AHR (22).
In the present study, no significant difference was
found in baseline airway resistance among the six groups. The
airway resistance generated by administration of Mch at 30–270
μg/kg was significantly increased in the OVA group and the
afzelin (0.1 mg/kg)-treated group. However, the control group,
Dexa- and afzelin (1 and 10 mg/kg)-treated groups revealed a sharp
decrease in airway resistance. In conclusion, the present study
indicated that afzelin is promising as a beneficial medication for
the treatment of asthma through ameliorating allergic
responses.
References
|
1
|
Global Initiative for Asthma (GINA),
Global Strategy for Asthma Management and Prevention, GINA: 2014,
http://www.ginasthma.org.
Accessed December 15, 2014.
|
|
2
|
Masoli M, Fabian D, Holt S and Beasley R:
The global burden of asthma: executive summary of the GINA
Dissemination Committee report. Allergy. 59:469–478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu J, Yamane H, Cote-Sierra J, Guo L and
Paul WE: GATA-3 promotes Th2 responses through three different
mechanisms: induction of Th2 cytokine production, selective growth
of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res.
16:3–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nath P, Leung SY, Williams AS, et al:
Complete inhibition of allergic airway inflammation and remodelling
in quadruple IL-4/5/9/13-/-mice. Clin Exp Allergy. 37:1427–1435.
2007.PubMed/NCBI
|
|
5
|
Gong JH, Shin D, Han SY, Kim JL and Kang
YH: Kaempferol suppresses eosionphil infiltration and airway
inflammation in airway epithelial cells and in mice with allergic
asthma. J Nutr. 142:47–56. 2012. View Article : Google Scholar
|
|
6
|
Diantini A, Subarnas A, Lestari K, et al:
Kaempferol-3-O-rhamnoside isolated from the leaves of Schima
wallichii Korth. inhibits MCF-7 breast cancer cell proliferation
through activation of the caspase cascade pathway. Oncol Lett.
3:1069–1072. 2012.PubMed/NCBI
|
|
7
|
Mabalirajan U, Dinda AK, Kumar S, et al:
Mitochondrial structural changes and dysfunction are associated
with experimental allergic asthma. J Immunol. 181:3540–3548. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Philippe P, Pierre C, Danuta R, Bart NL
and Martin O: Protein tyrosine phosphatases regulate asthma
development in a murine asthma model. J Immunol. 182:1334–1340.
2009. View Article : Google Scholar
|
|
9
|
Du Q, Zhou LF, Chen Z, Gu XY, Huang M and
Yin KS: Imiquimod, a toll-like receptor 7 ligand, inhibits airway
remodelling in a murine model of chronic asthma. Clin Exp Pharmacol
Physiol. 36:43–48. 2009. View Article : Google Scholar
|
|
10
|
Jones CG, Daniel Hare J and Compton SJ:
Measuring plant protein with the Bradford assay: 1. Evaluation and
standard method. J Chem Ecol. 15:979–992. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seung-Hyung K, Bok-Kyu K and Young-Cheol
L: Antiasthmatic effects of Hesperidin, a potential Th2 cytokine
antagonist, in a mouse model of allergic asthma. Mediators Inflamm.
2011:4854022011.
|
|
12
|
Borges O, Borchard G, Sousa AD, Junginger
HE and Cordeiro-da-Silva A: Induction of lymphocytes activated
marker CD69 following exposure to chitosan and alginate
biopolymers. Int J Pharm. 337:254–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson GP and Coyle AJ: TH2 and
‘TH2-like’ cells in allergy and asthma: pharmacological
perspectives. Trends Pharmacol Sci. 15:324–332. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coyle AJ, Le Gros G, Bertrand C, et al:
Interleukin-4 is required for the induction of lung Th2 mucosal
immunity. Am J Respir Cell Mol Biol. 13:54–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morrisey EE, Ip HS, Lu MM and Parmacek MS:
GATA-6: a zinc finger transcription factor that is expressed in
multiple cell lineages derived from lateral mesoderm. Dev Biol.
177:309–322. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arceci RJ, King AA, Simon MC, Orkin SH and
Wilson DB: Mouse GATA-4: a retinoic acid-inducible GATA-binding
transcription factor expressed in endodermally derived tissues and
heart. Mol Cell Biol. 13:2235–2246. 1993.PubMed/NCBI
|
|
17
|
Cho SH, Stanciu LA, Holgate ST and
Johnston SL: Increased interleukin-4, interleukin-5, and
interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma.
Am J Respir Crit Care Med. 171:224–230. 2005. View Article : Google Scholar
|
|
18
|
Szabo SJ, Sullivan BM, Peng SL and
Glimcher LH: Molecular mechanisms regulating Th1 immune responses.
Ann Rev Immunol. 21:713–758. 2003. View Article : Google Scholar
|
|
19
|
Szabo SJ, Sullivan BM, Stemmann C,
Satoskar AR, Sleckman BP and Glimcher LH: Distinct effects of T-bet
in Th1 lineage commitment and IFN-gamma production in CD4 and CD8 T
cells. Science. 295:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Har rington LE, Hatton RD, Mangan PR, et
al: Interleukin 17-producing CD4+ effector T cells develop via a
lineage distinct from the T helper type 1 and 2 lineages. Nat
Immunol. 6:1123–1132. 2005. View
Article : Google Scholar
|
|
21
|
Zhang DH, Cohn L, Ray P, Bottomly K and
Ray A: Transcription factor GATA-3 is differentially expressed in
murine Th1 and Th2 cells and controls Th2-specific expression of
the intrleukin-5 gene. J Biol Chem. 272:21597–21603. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cohn L, Tepper JS and Bottomly K:
IL-4-independent induction of airway hyperresponsiveness by Th2,
but not Th1, cells. J Immunol. 161:3813–3816. 1998.PubMed/NCBI
|