Introduction
Cyclophilin (Cyp) is present in prokaryotes and
eukaryotes and is an immunophilin and a cytosolic receptor for the
immunosuppressive drug, cyclosporin A (1). In addition, Cyp possesses enzymatic
peptidyl-prolyl cis-trans isom-erase (PPIase) activity,
which is essential for protein folding in vivo (2). PPIase activity has been suggested to
facilitate protein folding, intracellular trafficking and the
maintenance of multiprotein complex stability (3). There are several isoforms of Cyp,
including CypA (4), CypB (5), CypC (6) and CypD (7). CypA is predominantly localized in the
cytoplasm and is a highly conserved protein in mammalian cells
(4,8). In our previous study (9), CypA protected cells against cellular
stresses, including hypoxia and cisplatin-induced effects, and it
was hypothesized that the protective effects of CypA were, at least
in part due, to its antioxidant activity (9). However, the antioxidant activity of
CypA remains to be fully elucidated.
Several mutations in CypA have been identified. The
CypA mutant, which carries a serine (Ser/S) instead of proline
(Pro/P) at amino acid 16, has been reported to alter the
folding/assembly pathway and its refolding intermediates have been
observed to fall into a kinetic trap in the refolding process under
the observed time course, resulting in a small fraction of
multimerized CypA (10). It has
been demonstrated that another CypA mutant, which carries alanine
(Ala/A) instead of arginine (Arg/R) at amino acid 55, retains
<1% PPIase catalytic activity compared with the CypA/wild-type
(WT) (11,12).
The present study aimed to demonstrate the
antioxidant activity of CypA by measuring the total antioxidant
capacity of the purified CypA proteins. The association between the
antioxidant activity of CypA and its PPIase catalytic activity was
also investigated.
Materials and methods
Construction of glutatione S-transferase
(GST)-CypA fusion proteins
The present study was approved by the Ethics
Committee of Kyunghee University (Seoul, South Korea). Plasmids
encoding GST fusion proteins were constructed using pGEX-KG vectors
(American Type Culture Collection, Manassas, MA, USA). All the
plasmid DNA was prepared using a modified alkaline lysis method
(10). Briefly, the mutagens were
created through site-direct mutagenesis as previously described
(13) CypA/WT, CypA/R55A, the
PPIase-defective mutate gene, and CypA/P16S, which is a mutant gene
with a more flexible structure, were digested using BamHI
and EcoRI [New England Biolab (NEB), Inc., Ipswich, MA, USA]
and ligated into the pGEX-KG expression vector. The ligation
mixture (NEB, Inc.) was used to transform an Escherichia
coli DH5α strain (Life Technologies, Grand Island, NY, USA).
All the constructs were verified by DNA sequencing.
Site-directed mutagenesis of CypA
Mutations in CypA were produced by site-directed
mutagenesis using modified rapid polymerase chain reaction (PCR)
(14). Substitution of Ser16 into
Pro and Ala55 into Arg were performed by PCR in two steps with a
MyCycler (Bio-Rad, Hercules, CA, USA). Firstly, the two fragments,
containing the sequences upstream and downstream of the Ser16 and
Ala55 residues, were amplified using primers containing the desired
mutations. A PCR mixture, total volume 50 μl [2 μg
CypA/WT template DNA; 1 μl each forward and reverse primers
(100 pmol; Macrogen, Inc., Seoul, Republic of Korea); 5 μl
10X buffer; 3 μl deoxyribonucleotide; 1 μl taq
polymerase (Takara Bio, Inc., Otsu, Japan); and 37 μl
distilled water], was used. The cycling conditions were as follows:
94°C for 5 min, followed by 25 cycles of 94°C for 1 min, 55°C for 1
min, and 72°C for 1 min, the reaction was terminated by 72°C for 10
min. The primer sequences used were as follows: R55A forward,
AAATTTGGATCCATGGTCAACCCCACCG and reverse,
GGCGGAATTCTTAGAGTTGTCCACAGTC; and P16S forward,
ACTGTAAGCTTATGGTCAACCCCACCG and reverse,
CCCGGGGATATCTTAGAGTTGTCCACAG. Secondly, the two amplified fragments
were used as templates for a second PCR reaction at the same
conditions, in which only a primer for the 5′ end of the first
fragment and a primer for the 3′ end of the second fragment were
used, resulting in a full length Pro16 and Arg55 mutated CypA.
Expression and purification of the
recombinant fusion proteins
The pGEX-KG, pGEX-KG/CypA / W T, pGEX-KG/CypA/P16S
and pGEX-KG/CypA/R55A plasmids were transformed into the DH5α E.
coli strain. The bacterial cells were grown at 37°C in 200 ml
Lysogeny broth (Life Technologies) containing ampicillin (100
μg/ml; Duchefa Biochemie, Haarlem, Netherlands) from an
overnight culture. At at absorbance600 of ~0.6
(NovaspecII; Biotek Instruments, Inc., Winooski, VT, USA), the
bacterial cells were induced using 0.1 mM isopropyl
β-d-thiogalactopy ranoside (IPTG) for 3 h at 30°C. The cells were
harvested by centrifugation at 890 × g for 15 min and resuspended
in 2 ml 1X phosphate-buffered saline (PBS; Bioworld, Bundang, South
Korea) containing 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO,
USA). The cells were maintained on ice and lyzed by sonication
(Sonicator W-375 Cell disruptor; Heat Systems Ultrasonics Inc.,
Wehnrath, Germany). Following centrifugation at 890 × g for 15 min
at 4°C, the cell debris was removed and the supernatant was
incubated overnight with washed glutathione (GSH)-agarose
(Sigma-Aldrich) at 4°C with gentle rotation (Rotamix SLRM I;
Seoulin Bioscience Co., Seoul, South Korea). The samples were
centrifuged at 100 × g for 1 min at 4°C and the GST-only or the
GST-CypA fusions bound to the GSH-agarose beads (Sigma-Aldrich)
were washed five times with ice-cold PBS containing 1% Triton
X-100. The target fusion protein was eluted from the resin using an
elution buffer, containing 20 mM reduced GSH in 100 mM Tris-HCl (pH
9.0; Duchefa Biochemie) overnight at 4°C. Following centrifugation
at 100 × g for 1 min at 4°C, the supernatant containing the fusion
proteins was mixed with 6X SDS sample buffer (Sigma-Aldrich),
containing 0.35 M Tris-HCl (pH 6.8), 10.3% SDS, 36% glycerol, 0.6%
bromophenol blue and 0.6 M dithiothreitol. The samples were
analyzed by SDS-PAGE, native-PAGE and immunoblot analysis.
Removal of the GST by selective cleavage
using thrombin
The GST tag was cleaved from the GST-CypA using a
Thrombin CleanCleave™ kit (Sigma-Aldrich) according to the
manufacturer’s instructions. The fusion protein (1 mg/ml) in
cleavage buffer containing 500 mM Tris-HCl (pH 8.0) and 100 mM
CaCl2 (Duchefa Biochemie), was incubated at 4°C with
gentle rotation overnight in the presence of 100 μl bovine
thrombin agarose beads (Sigma-Aldrich). Following the reaction, the
thrombin agarose was removed from the mixture by centrifugation at
100 × g for 1 min. The supernatant was assessed by SDS-PAGE,
native-PAGE and immunoblot analysis.
SDS-PAGE, immunoblot analysis and
native-PAGE
The total cell lysate, purified fusion proteins and
cleaved fusion proteins were separated by 12 % SDS-PAGE (Life
Technologies) and the proteins were transferred onto nitrocellulose
membranes (Pall Corp., Pensacola, FL, USA). Transfer of the
proteins was assessed by Ponceau red staining (Sigma-Aldrich) and
the membranes were subsequently blocked for 1 h at room temperature
in 3% bovine serum albumin (Sigma-Aldrich) in Tris-buffered saline
containing 10 mM Tris-HCl (pH 8.0; Life Technologies) and 150 mM
NaCl (Duchefa Biochemie), supplemented with 0.05% Tween-20 (TBST).
The nitrocellulose membrane was washed with 1X TBST three times for
15 min and incubated with the following primary antibodies: Mouse
monoclonal anti-GST (1:1,000; sc-138; Santa Cruz Biotechnology,
Inc. Dallas, TX, USA), rabbit polyclonal anti-CypA (1:1,000;
07-313; Millipore, Billerica, MA, USA), mouse monoclonal anti-HA
(1:1,000; sc-7392; Santa Cruz Biotechnology, Inc.) and mouse
polyclonal anti-GAPDH (1:1,000; csa-335; Enzo Life Sciences,
Farmingdale, NY, USA), for 1 h at room temperature. Following
washing with TBST three times for 15 min, the membrane was
incubated with horseradish peroxidase-conjugated secondary antibody
for 45 min at room temperature.
Native-PAGE analysis was performed using a 12% gel.
The stacking gel, separating gel and the running buffer were
prepared as for SDS-PAGE, however, no SDS was added and the sample
was not heated.
PPIase activity assay
The PPIase activity assay was performed, as
described previously (15–17), with the suggested substrate solvent
application (18). This assay
determines the rate of conversion of cis-to-trans in
proline-containing peptides, based on the principle that
α-chymotrypsin cleaves the peptide only when it is in the
trans conformation (18).
The N-succinyl-Ala-Ala-Pro-phenylalanine-p-nitroanilide
peptide substrate (Sigma-Aldrich) was dissolved in >99%
trifluoro-ethanol (Sigma-Aldrich) with 470 mM LiCl (Sigma-Aldrich)
to prepare a 100 mM stock solution, which was further diluted to 4
mM prior to use. α-chymotrypsin was dissolved in 1 mM HCl (Junsei
Chemical Co., Ltd, Tokyo, Japan) with 2 mM CaCl2 to
prpare a 1 M stock solution. GST-CypA was diluted into 50 mM HEPES
(Sigma-Aldrich) and 86 mM NaCl (pH 8.0), to prepare the PPIase
buffer. In a 1 ml cuvette (Sigma-Aldrich), 100 μl protein
(10 μM final concentration) was added to 890 μl
PPIase buffer. The reaction was initiated by the addition of 10
μl (40 μM) peptide substrate and α-chymotrypsin
(Sigma-Aldrich) of 0.5 μl (500 μM), followed by rapid
mixing using a pipette. The change in absorbance at 390 nm,
following the cleavage of the trans form of the peptide and
release of p-nitroaniline, was monitored using a biosciences
spectrophotometer (VICTOR; PerkinElmer, Waltham, MA, USA).
Antioxidant activity assay
The antioxidant activity was determined using a
Total Antioxidant Capacity Assay kit (BioVision Research Products,
Mountain View, CA, USA) and measured by monitoring the reduction of
Cu++ reagent by the increase of absorbance at 570 nm.
The degree of quenching of radical generation in individual
samples, indicative of the presence of antioxidant activity, was
quantified by comparison with a traditional standard trolox
(Sigma-Aldrich) and the assay results were expressed in terms of
μmol/trolox.
Cell culture and reagent
Chang human liver cells (American Type Culture
Collection) were cultured in Dulbecco’s modified Eagle’s medium (GE
Healthcare, Logan, WV, USA) supplemented with 10% (v/v) fetal
bovine serum (FBS; GE Healthcare) and antibiotics [100 U/ml
penicillin (Duchefa Biochemie) and 100 μg/ml streptomycin
sulfate (Sigma-Aldrich)] in a 5% CO2 incubator. To
induce oxidative stress, the cells were treated with cisplatin (0,
15 or 20 μmol/l) or H2O2 (0, 400 or
500 μmol/l) for 24 h.
Transfection
CypA/WT and CypA/R55A were tagged (hemagglutinin
tag, 5′-TAC CCA TAC GAC GTC CCA GAC TAC GCT-3′) at the 5′ end. The
cells were cultured in a 12-well plate at 37°C in a 5%
CO2 incubator for a
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells were transfected using Lipofectamine 2000™ reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Following transfection, cells were
also incubated in the same conditions for 18–24 h.
MTT assay
Following 24 h treatment with cisplatin or
H2O2, the cell viability was evaluated using
an MTT conversion assay in a 12-well plate. The culture medium
(DMEM supplementd with 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin sulfate) was replaced with 1 ml medium
containing 0.5 mg/ml MTT (Sigma-Aldrich; dissolved in filtration
water) and incubated for 60 min at 37°C. The medium was then
carefully aspirated from the plates and the blue-colored
tetrazolium crystals, resulting from mitochondrial enzymatic
activity, on the MTT substrate were solubilized in 150 μl
100% dimethylsulfoxide (Junsei, Tokyo, Japan). The absorbance was
measured at 595 nm in a Model 680 Microplate Reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell survival was expressed
as the percentage of absorbance relative to that of the untreated
cells.
Results
Purification of the expressed GST-CypA
fusion proteins
In order to investigate the biochemical activity of
CypA, the GST-CypA/WT, GST-CypA/P16S and GST-CypA/R55A plasmids
were expressed in E. coli. A schematic of the constructed
expression plasmids is shown in Fig.
1A. The recombinant GST-CypA fusion proteins were successfully
overexpressed by the addition of 0.1 mM IPTG in E. coli
(Fig. 1B). The GST-only and
GST-CypA fusion proteins were eluted using GSH-beads (Fig. 1B). The expression levels of
GST-CypA/WT, GST-CypA/P16S and GST-CypA/R55A were analyzed by
western blotting (Fig. 1C). The
18-kDa recombinant CypA proteins were purified following GST
cleavage with thrombin, and the purified proteins were confirmed by
western blotting using an anti-CypA antibody (Fig. 2A). A small fraction of the
CypA/P16S mutant protein has been reported to exhibit a
multimerized structure due to its low yield of refolding (10). Therefore, in order to confirm the
multimerized CypA/P16S, native-PAGE in non-denaturing conditions
was used (Fig. 2B). The results
demonstrated that a significant level of multimerized complexes of
CypA/P16S remains, consistent with the previous report (10). However, CypA/WT remained as a
monomer (Fig. 2A).
 | Figure 1(A) Schematic of the GST-CypA
constructs. (B) Western blot analysis of the purified GST-CypA
fusion protein. The total GST protein lysate and the eluted GST
protein lysate were separated by SDS-PAGE. The proteins were
detected using an anti-GST antibody. Lane 1, total GST protein
lysate; lane 2, total GST-CypA fusion protein lysate; lane 3,
eluted GST protein lysate; Lane 4, eluted GST-CypA fusion protein
lysate. (C) Western blot analysis of the purified GST-CypA/WT,
GST-CypA/P16S, and GST-CypA/R55A fusion proteins. The proteins were
detected using an anti-CypA antibody. Lane 1, eluted GST-CypA/WT;
lane 2, eluted GST-CypA/P16S; lane 3, eluted GST-CypA/R55A. GST,
glutatione S-transferase; CypA, cyclophilin A. |
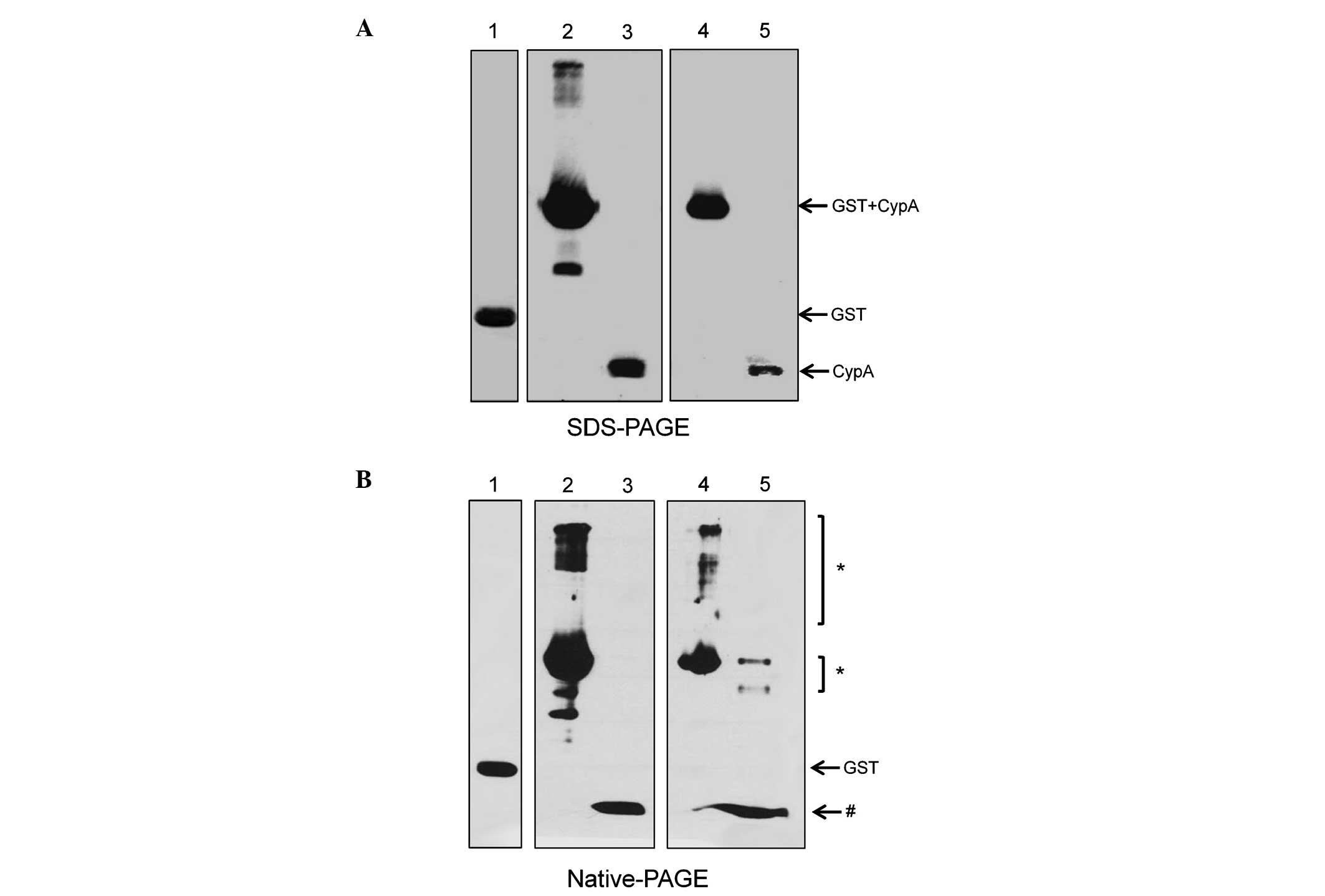 | Figure 2(A) Western blot analysis of the
recombinant CypA proteins. CypA recombinant fusion proteins were
separated by SDS-PAGE following GST cleavage. The proteins were
detected using an anti-GST antibody (lane 1) and an anti-CypA
antibody (lanes 2–5). Lane 1, eluted GST protein; lane 2, eluted
GST-CypA/WT protein; lane 3, CypA/WT protein following cleavage;
lane 4, eluted GST-CypA/P16S protein; lane 5, recombinant CypA/P16S
protein following GST cleavage. (B) Recombinant CypA fusion
proteins were separated by Native-PAGE gel. The proteins were
detected using anti-GST (lane 1) and anti-CypA (lane 2–5). Lane 1,
eluted GST protein alone; lane 2, eluted GST-CypA/WT protein; lane
3, CypA/WT protein following cleavage; lane 4, eluted GST-CypA/P16S
protein; lane 5, CypA/P16S protein following cleavage.
*Multimers of CypA protein; #monomer of CypA
protein. CypA, cyclophilin; GST, glutatione S-transferase. |
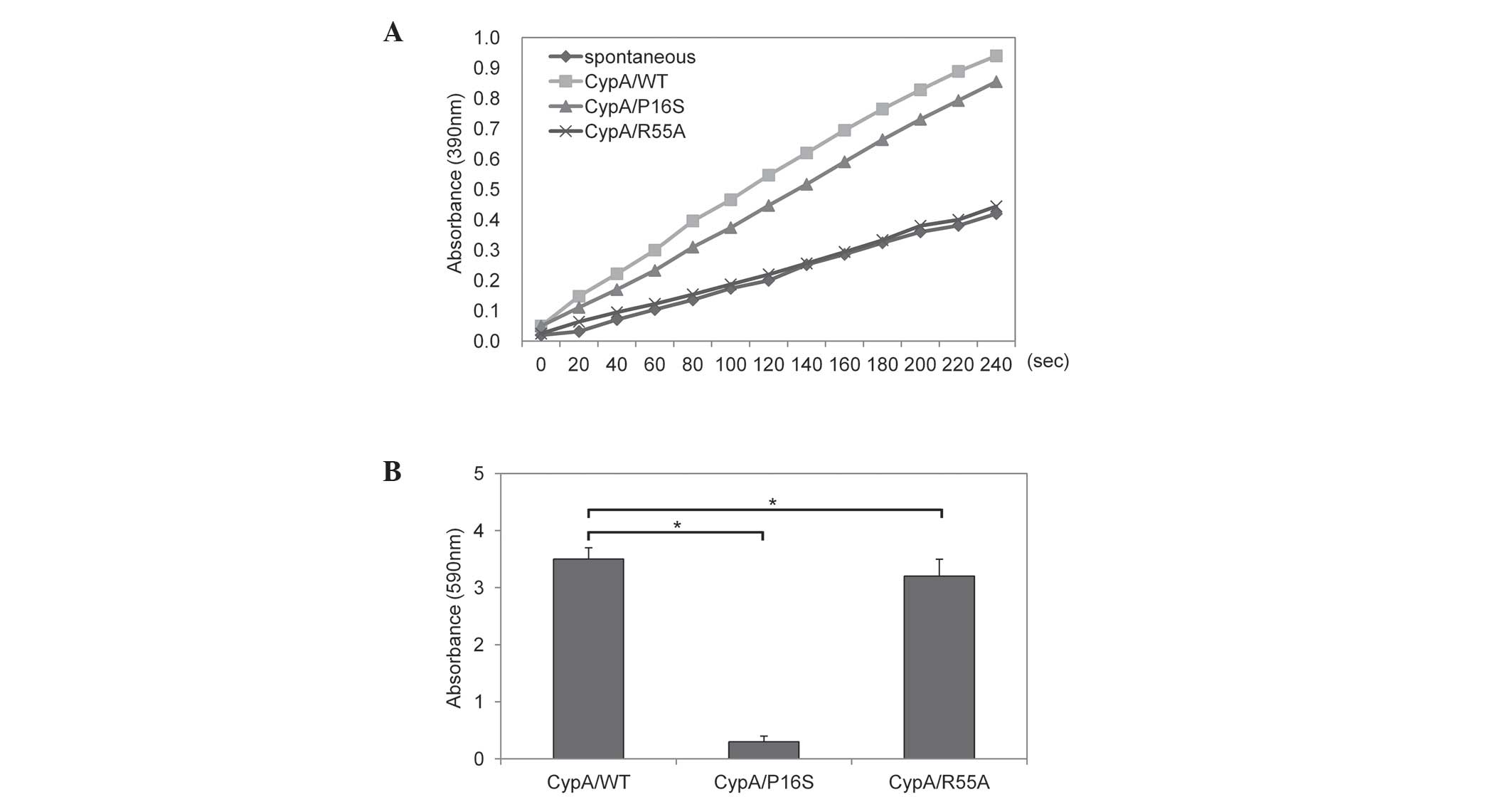
PPIase and antioxidant activity assays of
purified CypA protein
The PPIase activity of the purified CypA protein was
measured. The PPIase activity assay is based on the observation
that α-chymotrypsin cleaves the C-terminal amide bond only in the
trans X-pro conformer of the chromogenic substrate,
X-Pro-Phe-pNA. The rapid hydrolysis perturbs the cis-trans
conformational equilibrium, which enables the PPIase-catalyzed
cis-to-trans isomeriztion to be monitored. The PPIase
activity was assayed in an α-chymotrysin coupled assay. As shown in
Fig. 3A, recombinant CypA/WT and
CypA/P16S exhibited PPIase activity, although CypA/P16S
demonstrated marginally less efficient activity. Consistent with a
previous report, the CypA/R55A was defective in its PPIase
activity, as shown in Fig. 3A
(13). The total antioxidant
activity of the purified CypA protein was also measured. CypA/WT
and CypA/R55A demonstrated significantly higher antioxidant
activities, however, CypA/P16S exhibited no antioxidant activity
(Fig. 3B). GST-only was used as a
negative control.
Overexpression of CypA reduces cell death
induced by cisplatin or H2O2
To confirm the importance of the antioxidant
activity of CypA during oxidative stress, HA-tagged CypA was
expressed in Chang human liver cells (Fig. 4A). The overexpression of CypA/WT or
CypA/P16S was analyzed by western blotting (Fig. 4B). The effects of over-expressed
CypA on reactive oxygen species-mediated cell death was determined
using an MTT assay with various concentrations of
H2O2 (400 and 500 μM) for 24 h. The
CypA/WT-transfected cells demonstrated a higher survival rate
compared with the empty vector-transfected cells. By contrast, the
CypA/P16S-transfected cells exhibited a higher sensitivity to
H2O2-mediated cell death compared with the
CypA/WT-transfected cells (Fig.
4C). The chemoresistance of the CypA/WT- and
CypA/P16S-transfected cells following treatment with cisplatin were
monitored using an MTT assay. Cisplatin is known to induce
apoptosis, partly through the generation of oxidative stress
(19–21). The cells transfected with CypA/WT
had a reduced level of cell death following treatment with
cisplatin, compared with the empty vector-transfected cells. By
contrast, the CypA/P16S-transfected cells exhibited a lower
survival rate following treatment with cisplatin, compared with the
CypA/WT-transfected cells (Fig.
4D). These findings suggested that antioxidant activity is
required for the protective effects of CypA against oxidative
stress.
Discussion
CypA protects cells from several types of cellular
stress, including oxidative stress (14) and endoplasmic reticulum stress
(22). PPIase activity is reported
to be associated with several cellular functions, in addition to
its biochemical activity (23).
Several reports have demonstrated that the PPIase activity of CypA
is important (24) and our
previous study revealed that overexpression of CypA protects
several cell lines from oxidative stress in a PPIase
activity-dependent manner (9).
However, the antioxidant activity of CypA and its importance remain
to be fully elucidated. Therefore, the present study focused on the
antioxidant activity of CypA and identified for the first time, to
the best of our knowledge, its importance in the cellular response
to oxidative stress.
The antioxidant activity of recombinant CypA,
purified from bacterial extracts, was measured using a Total
Antioxidant Capacity Assay kit. The results demonstrated that a
significant level of antioxidant activity was associated with
CypA/WT and CypA/R55A, while CypA/P16S was defective in antioxidant
activity. The PPIase activity of each protein was also measured and
its association with antioxidant activity was investigated. As
shown in Fig. 2A, CypA/WT and
CypA/P16S exhibited PPIase activity, although CypA/P16S had
marginally less efficient activity. This indicated that Pro16 in
the structure of CypA does not affect the catalytic PPIase activity
since Pro16 is located at a region distant from the catalytic
center of the PPIase. In addition, these results suggested that the
antioxidant activity of CypA is independent of PPIase activity,
since CypA/R55A, which is defective in PPIase activity, exhibits
antioxidant activity. Notably, CypA/P16S formed a multimerized
complex, while CypA/WT existed as a monomer, as shown in Fig. 2B. Multimerization may inhibit the
antioxidant activity of CypA/P16S. However, further studies are
required to elucidate the association between the multimerization
of CypA and its antioxidant activity.
Our previous study demonstrated that the
overexpression of CypA/WT may be important in tumorigenesis by
reducing apoptosis under hypoxic conditions and by treatment with
cisplatin (9). Therefore, the
present study hypothesized that the overexpression of CypA/WT,
however, not CypA/P16S, may reveal a protective effect on oxidative
stress-induced cell death. As hypothesized, the CypA/WT protected
the cells from H2O2 or cisplatin-mediated
cell death, while the CypA/P16S mutant was unable to protect the
cells (Fig. 4).
In conclusion, the present study demonstrated that
antioxidant activity was associated with CypA and was independent
of PPIase activity. In addition, the antioxidant activity of CypA
was required for the protective effects of CypA against
H2O2 or cisplatin-mediated cell death. These
findings may be useful for identifying a novel chemotherapeutic
target in tumor cells, since cancer cells are usually resistant to
oxidative stress, which is induced by anti-cancer drugs, such as
Cisplatin (25).
Acknowledgments
This study was supported by grants from Kyung Hee
University (Seoul, Republic of Korea) in 2012 (no. KHU-20121733)
and the Basic Science Research Program through the National
Research Foundation of Korea, funded by the Ministry of Education
(no. NRF-2013R1A1A2060694).
References
|
1
|
Handschumacher RE, Harding MW, Rice J,
Drugge RJ and Speicher DW: Cyclophilin: a specific cytosolic
binding protein for cyclosporin A. Science. 226:544–547. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caroni P, Rothenfluh A, McGlynn E and
Schneider C: S-cyclophilin. New member of the cyclophilin family
associated with the secretory pathway. J Biol Chem.
266:10739–10742. 1991.PubMed/NCBI
|
|
3
|
Andreeva L, Heads R and Green CJ:
Cyclophilins and their possible role in the stress response. Int J
Exp Pathol. 80:305–315. 1999. View Article : Google Scholar
|
|
4
|
Wang P and Heitman J: The cyclophilins.
Genome Biol. 6:2262005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Price ER, Zydowsky LD, Jin MJ, Baker CH,
McKeon FD and Walsh CT: Human cyclophilin B: a second cyclophilin
gene encodes a peptidylprolyl isomerase with a signal sequence.
Proc Natl Acad Sci USA. 88:1903–1907. 1991. View Article : Google Scholar
|
|
6
|
Schneider H, Charara N, Schmitz R, et al:
Human cyclophilin C: primary structure, tissue distribution, and
determination of binding specificity for cyclosporins.
Biochemistry. 33:8218–8224. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carpentier M, Allain F, Haendler B, et al:
Two distinct regions of cyclophilin B are involved in the
recognition of a functional receptor and of glycosaminoglycans on T
lymphocytes. J Biol Chem. 274:10990–10998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harding MW, Handschumacher RE and Speicher
DW: Isolation and amino acid sequence of cyclophilin. J Biol Chem.
261:8547–8555. 1986.PubMed/NCBI
|
|
9
|
Choi KJ, Piao YJ, Lim MJ, et al:
Overexpressed cyclophilin A in cancer cells renders resistance to
hypoxia- and cisplatin-induced cell death. Cancer Res.
67:3654–3662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu LR, Yan X, Luo M, Guan YX and Yao SJ:
Preparation, characterization and refolding in vitro of a
recombinant human cyclophilin A mutant: effect of a single Pro/Ser
substitution on cyclophilin A structure and properties. Biotechnol
Prog. 24:302–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Helekar SA and Patrick J: Peptidyl prolyl
cistrans isomerase activity of cyclophilin A in functional
homo-oligomeric receptor expression. Proc Natl Acad Sci USA.
94:5432–5437. 1997. View Article : Google Scholar
|
|
12
|
Morita T, Kawabata T, Horikawa N, et al:
Therapeutic consideration of patients with mental diseases. Seishin
Shinkeigaku Zasshi. 94:1092–1098. 1992.In Japanese.
|
|
13
|
Zydowsky LD, Etzkorn FA, Chang HY, et al:
Active site mutants of human cyclophilin A separate peptidyl-prolyl
isomerase activity from cyclosporin A binding and calcineurin
inhibition. Protein Sci. 1:1092–1099. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gavin PD, Devenish RJ and Prescott M: FRET
reveals changes in the F1-stator stalk interaction during activity
of F1F0-ATP synthase. Biochim Biophys Acta. 1607:167–179. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fischer G, Bang H, Ludwig B, Mann K and
Hacker J: Mip protein of Legionella pneumophila exhibits
peptidyl-prolyl-cis/trans isomerase (PPlase) activity. Mol
Microbiol. 6:1375–1383. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rahfeld JU, Schierhorn A, Mann K and
Fischer G: A novel peptidyl-prolyl cis/trans isomerase from
Escherichia coli. FEBS Lett. 343:65–69. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rouvière PE and Gross CA: SurA, a
periplasmic protein with peptidyl-prolyl isomerase activity,
participates in the assembly of outer membrane porins. Genes Dev.
10:3170–3182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kofron JL, Kuzmic P, Kishore V,
Colon-Bonilla E and Rich DH: Determination of kinetic constants for
peptidyl prolyl cis-trans isomerases by an improved
spectrophotometric assay. Biochemistry. 30:6127–6134. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Guo C, Vasko MR and Kelley MR:
Implications of apurinic/apyrimidinic endonuclease in reactive
oxygen signaling response after cisplatin treatment of dorsal root
ganglion neurons. Cancer Res. 68:6425–6434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santos NA, Catao CS, Martins NM, Curti C,
Bianchi ML and Santos AC: Cisplatin-induced nephrotoxicity is
associated with oxidative stress, redox state unbalance, impairment
of energetic metabolism and apoptosis in rat kidney mitochondria.
Arch Toxicol. 81:495–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marullo R, Werner E, Degtyareva N, et al:
Cisplatin induces a mitochondrial-ROS response that contributes to
cytotoxicity depending on mitochondrial redox status and
bioenergetic functions. PLoS One. 8:e811622013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim J, Choi TG, Ding Y, et al:
Overexpressed cyclophilin B suppresses apoptosis associated with
ROS and Ca2+ homeostasis after ER stress. J Cell Sci.
121:3636–3648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunter T: Prolyl isomerases and nuclear
function. Cell. 92:141–143. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin ZG, Melaragno MG, Liao DF, et al:
Cyclophilin A is a secreted growth factor induced by oxidative
stress. Circ Res. 87:789–796. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pratibha R, Sameer R, Rataboli PV,
Bhiwgade DA and Dhume CY: Enzymatic studies of cisplatin induced
oxidative stress in hepatic tissue of rats. Eur J Pharmacol.
532:290–293. 2006. View Article : Google Scholar : PubMed/NCBI
|