Introduction
The unfolded protein response (UPR) is an adaptive
cellular response, which is involved in the attenuation of protein
synthesis to prevent distorted protein aggregation and the
activation of endoplasmic reticulum (ER)-associated distorted
protein degradation to accelerate the elimination of protein
aggregates (1–5). Conversely, extended UPR activation
causes ER stress, which leads to apoptosis and activation of
activating transcription factor (ATF) (6). ATF6 activates ATF4, a central
transcriptional activator, which activates the apoptotic factor,
C/EBP homologous protein (CHOP) (6). The UPR also activates caspase-12
(7–9) and disrupts the Ca2+
balance in the ER (10–12). The UPR apoptotic pathway generates
reactive oxygen species (ROS), which are derived from the
UPR-associated oxidative protein folding machinery in the ER
through protein disulfide isomerase (PDI), coenzyme endoplasmic
oxidoreductin-1-like (Ero1-L) and decreased levels of glutathione
(13–15).
Previous studies have revealed that the UPR is
associated with aging and potentially UPR-induced age-related
disease, including cataracts (16–18).
Age-related cataracts (ARCs), a major type of cataract, are
considered to occur as a result of normal aging processes. Aging,
combined with environmental and genetic stresses, is considered to
be the predominant contributor to the pathogenesis of lens
oxidation, crystallin modification and aggregation (19). However, several of the pathogenic
mechanisms involved in ARC remain to be elucidated. Oxidative
stress is considered to be one of the cellular conditions
associated with the development of ARC (20,21).
This is induced by a range of factors, including diabetes,
malnutrition, systemic and ocular disease processes, pollutants,
drugs, heavy metals, ionizing radiation, glucose and changes in the
oxygenation of cells (20,21). These stress factors result in the
generation of ROS (16,22,23).
Based on emerging evidence of UPR activation and its
association with aging, the present study aimed to investigated
whether UPR activation occurs in aging human lens cells, which may
be involved in the formation of ARCs.
Materials and methods
Age-related human lenses and cell
culture
Human lenses aged between 50 and 90 years were
obtained from The Eye Bank of Shandong Eye Institute (Qingdao,
China) for the detection of UPR proteins in the lens. The human
lens epithelial cell (HLEC) line was used for in vitro
experiments. HLECs were previously stored in liquid nitrogen and
then maintained in 25 mM glucose Dulbecco’s modified Eagle’s medium
(DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) with 10%
fetal calf serum at 37°C in 5% atmospheric oxygen. The HLECs were
pre-cultured overnight in 5 mM glucose DMEM in a CO2
incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
use in the subsequent experiments.
ROS and apoptosis measurements
The HLECs were cultured for 12, 24, 48 and 72 h
prior to harvesting to determine the levels of ROS and apoptosis.
The cells (~5×106) were collected by centrifugation and
subsequently resuspended in 250 μl phosphate-buffered saline
(PBS; pH 7.4), containing 20 μM dihydrorhodamine. After 30
min incubation at room temperature in the dark, the cells were
washed using PBS and resuspended in 500 μl PBS for
fluorescence activated cell sorting (FACS) analysis using a BD FACS
Aria™ II (BD Biosciences, Franklin Lakes, NJ, USA). The measure the
levels of apoptosis, the cells were counterstained with propidium
iodide (2 μg/ml) at 4°C. The cells were filtered through a
50 μm nylon mesh (BD Biosciences) to remove cell clumps and
were seeded into FACS tubes for FACS analysis to assess levels of
apoptosis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the HLECs,
following treatment with or without varying concentrations of
homocysteine using a Quick-RNA™ MicroPrep solution (Zymo Research
Corporation, Orange, CA, USA) according to the manufacturer’s
instructions. Subsequently, the purified total RNA was reverse
transcribed using iScript™ Reverse Transcription supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer’s instructions. The reverse transcribed RNA was
analyzed by RT-qPCR using a SsoFast™ EvaGreen supermix (Bio-Rad
Laboratories, Inc.). The primer sequences were designed for optimal
design using OligoPerfect™ Designer software (Invitrogen Life
Technologies), according to the manufacturer’s instructions and
were synthesized commercially (Invitrogen Life Technologies). The
primer sequences used were as follows: Binding immunoglobulin
protein (BIP), forward (5′-GGAAAGAAGGTTACCC ATGC-3′) and reverse
(5′-TTAGGCCAGCAATAGTTCCA-3′); inositol-requiring enzyme-1 (IRE1),
forward (5′-AGACTTTGTCATCGGCCTTT-3′) and reverse (5′-TGTATTCTGTTCGC
CCAAGA-3′); ATF6 forward (5′-GATTAAAGGCTGCCCTCTCA-3′) and reverse
(5′-GCCTCTGGTTCTCTGACACA-3′); PKR-like eukaryotic initiation factor
2a kinase (PERK), forward (5′-CACCAGAGAAGTGGCAAGAA-3′) and reverse
(5′-CATCCATTGGGCTAGGAGAG-3′) and β-actin forward
(5′-CCAACCGCGAGAAGATGA-3′) and reverse (5′-CCAGA
GGCGTACAGGGATAG-3′). The PCR conditions included: 2 min at 95°C, 20
sec at 95°C, 20 sec at the annealing temperature, 30 sec at 72°C
for 35 cycles and 10 min at 72°C. Each reaction was performed in
triplicate in three independent experiments. A standard curve (18S
rRNA) was constructed from a dilution series of a reference cDNA
sample and was included in each RT-qPCR run to correct for possible
variations in product amplification. The relative copy numbers were
obtained using the standard curve and were normalized to the values
obtained for β-actin, the internal control. The fold change in
expression levels were obtained using the 2−∆∆CT method
(24).
Western blot analysis
The HLECs were lysed in radioim-munoprecipitation
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). The
soluble proteins (10–20 μg/l) were separated using SDS-PAGE
and were subsequently blotted onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
using 5% non-fat dry milk for 1 h at room temperature prior to
incubation overnight with primary antibodies at 4°C. The primary
antibodies included: Mouse monoclonal antibodies against Bip
(1:1,000) and IRE1 (1:2,000); rabbit polyclonal ATF6 (1:2,000) and
PERK (1:3,000; BD Biosciences); rabbit polyclonal antibodies
against ATF4 (1:500) and catalase (1:2,000); mouse monoclonal
antibodies against CHOP (1:1,000), Erol-Lα (1:1,000), Erol-Lβ
(1:500), PDI (1:2,000) and glutathione reductase (1:2,000),
superoxide dismutase (SOD; 1:3,000; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and mouse monoclonal antibodies against GAPDH
(1:4,000) obtained from Novus Biological (Littleton, CO, USA). The
membranes were washed and subsequently incubated with secondary
antibodies, including horseradish peroxidase (HRP) conjugated goat
anti-rabbit IgG (1:5,000) and goat anti-mouse IgG-HRP (1:5,000;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature.
Luminol reagent (0.1 ml per cm2 of membrane; Thermo
Fisher Scientific) was applied to the membranes and the bands were
detected using a Bio-rad XRS system (Bio-Rad Laboratories, Inc.).
Antibodies against BIP, IRE1, ATF6, PERK (BD Biosciences), ATF4,
CHOP, Ero1-Lα, Ero1-Lβ, PDI, glutathione reductase (GR), catalase,
and superoxide dismutase (SOD), from Santa Cruz Biotechnology,
Inc., (Santa Cruz, CA, USA) and GAPDH from Novus Biological,
(Littleton, CO, USA) were used. The intensity of each band was
normalized to GAPDH.
Statistical analysis
The data are expressed as the mean ± standard
deviation and the statistical significance was evaluated using
Student’s t-test with SPSS (version 15.0) software (SPSS, Inc.,
Chigago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
ROS production and apoptosis
Following exposure of the HLECs to calcimycin for
12, 24, 48 and 72 h, a gradual increase was observed in the
production of ROS, with a significant increase at 48 h. Similarly,
apoptosis was observed after 24 h and a significant increase was
observed after 72 h. ROS production and apoptosis were measured
using FACS analysis. ROS production was determined by
dihydrorhodamine staining and propidium iodide staining was used to
measure apoptotic cell population. The percentage of staining with
the respective dyes is shown in Fig.
1A.
mRNA expression levels of UPR-associated
genes
The mRNA expression levels of UPR-associated genes
were measured using RT-qPCR. The mRNA expression level of BIP
gradually increased after 24 h of exposure, with a significant
10-fold increase after 72 h (Fig.
1B). BIP is considered a marker for UPR activation, which
triggers the activation of other UPR-associated proteins. The mRNA
expression levels of UPR-associated genes, including IRE1, ATF6 and
PERK, began increasing at 48 h, with a maximum 8-fold increase at
72 h (Fig. 1B), indicating the
effect of UPR activation in the HLECs following exposure to
calcimycin, the ER stressor.
Activation of UPR-associated proteins in
the HLECs
The western blot analysis of UPR-associated proteins
revealed that the level of BIP gradually increased in the HCLCs
after 12 h of exposure to calcimycin. A significant increase in the
protein level of BIP was identified after 24 h of exposure. The
levels of other UPR proteins were elevated after 24 h of exposure,
indicating activation of the UPR in the HLECs (Fig. 2A). As the duration of exposure
increased, the levels of the UPR proteins also increased, which was
correlated with the activation of the UPR observed in aging human
lenses. As a result of UPR activation, the levels of the proteins
involved in the protein folding machinery, including PDI and
Ero1-Lα, decreased. Conversely, the protein levels of Ero1-Lβ, a
counter regulator for Ero1-Lα, increased (Fig. 2B). This confirmed that HLECs
exposed to calcimycin led to activation of the UPR.
 | Figure 2Activation of the UPR in the HLECs.
(A) Western blot analysis of the levels of UPR signaling proteins
BIP, IRE1, ATF6 and PERK. (B) Western blot analysis of the levels
of UPR-associated proteins, which were expressed following UPR
activation. UPR, unfolded protein response; HLECs, human lens
epithelial cells; ROS, reactive oxygen species; BIP, binding
immunoglobulin protein; IRE1, inositol-requiring enzyme-1; ATF,
activating transcription factor; PERK, PKR-like eukaryotic
initiation factor 2a kinase, Ero1-L, endoplasmic
oxidoreductin-1-like; PDI, protein disulfide isomerase. |
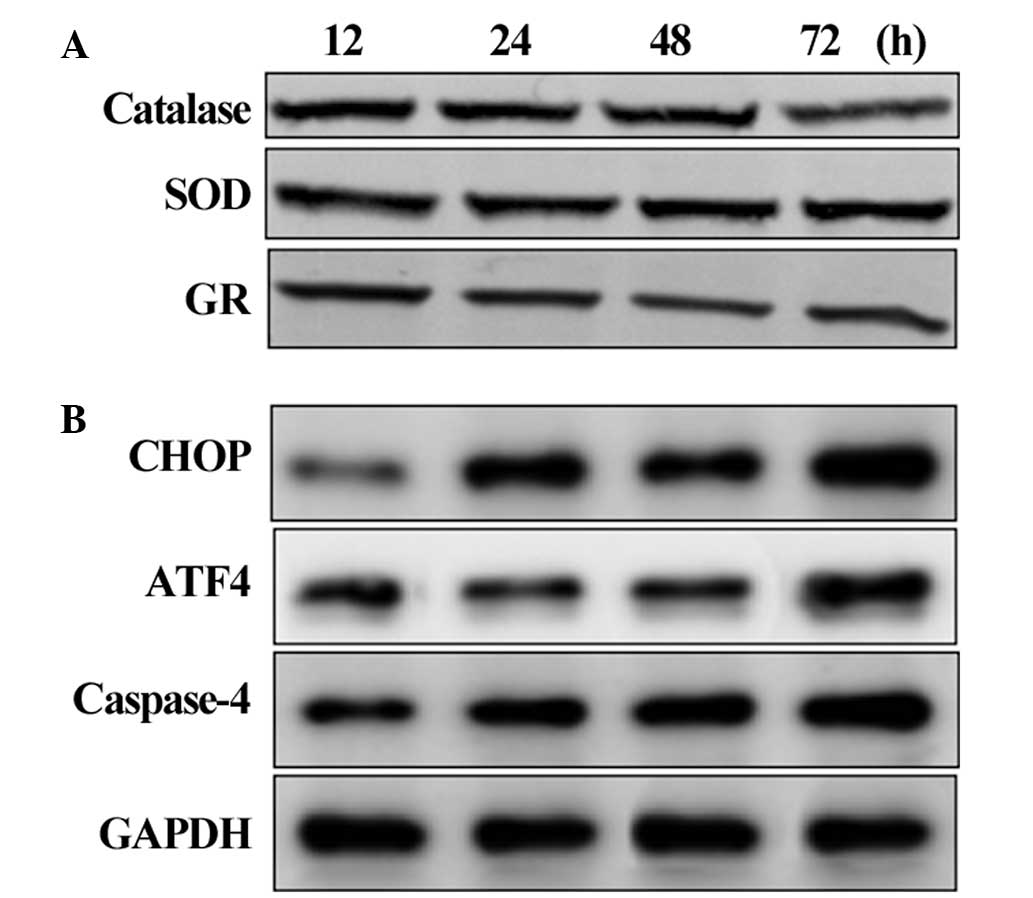
Suppression of the antioxidant system and
activation of apoptosis
The protein levels of antioxidant and apoptotic
signaling proteins were assessed in the HLECs exposed to
calcimycin. The protein levels of the antioxidants catalase, SOD
and GR decreased after 48 and 72 h of exposure, indicating that UPR
activation suppressed the antioxidant level and produced ROS
(Fig. 3A). The levels of the CHOP,
ATF4 and caspase-4 apoptotic signaling proteins gradually increased
after 24 h and increased markedly at 72 h (Fig. 3B). This suggested that the
activation of UPR triggers the ER stress-mediated apoptotic
signaling pathway in HLECs.
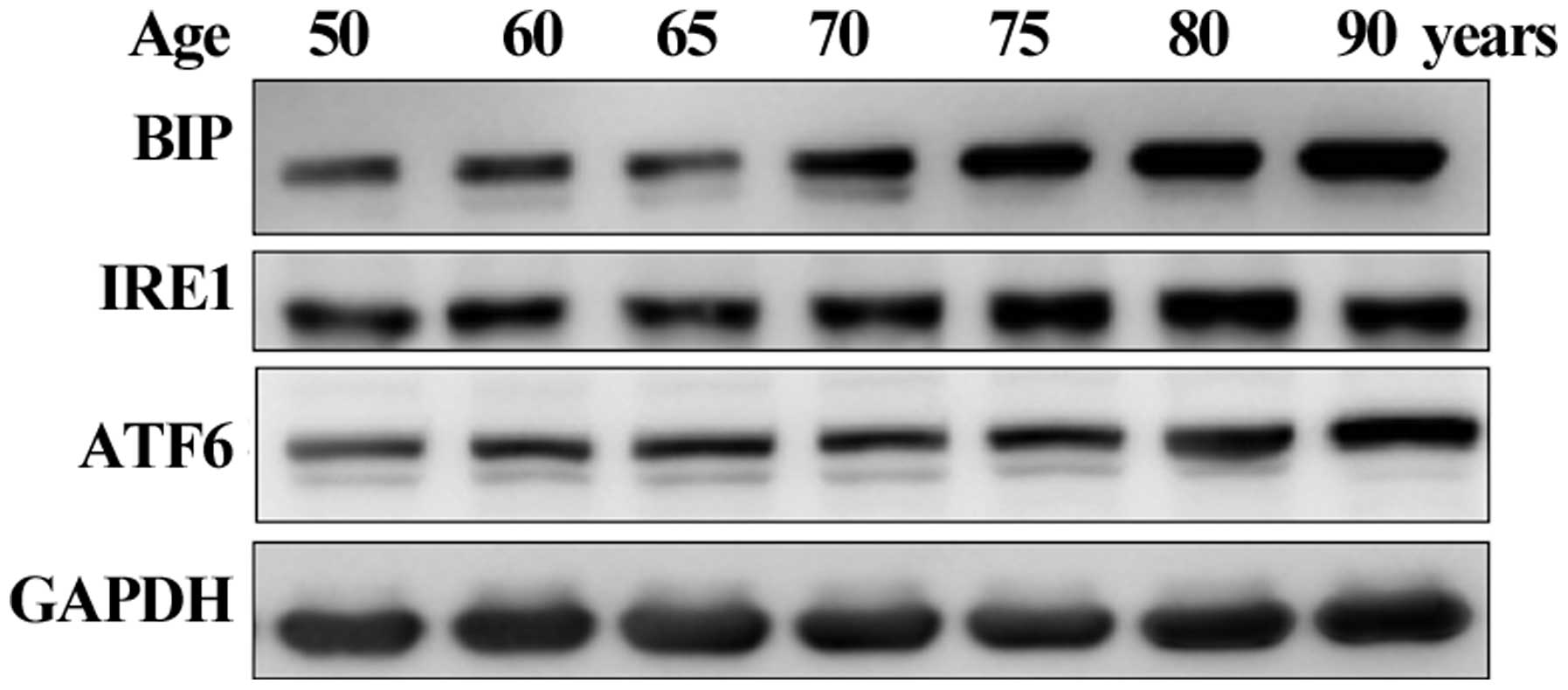
Age-related activation of the
UPR-associated proteins in human lenses
Based on the in vitro results, the present
study aimed to confirm whether UPR activation is observed in human
lenses aged between 50 and 90 years old. The protein expression of
BIP was observed in all the lenses and significantly elevated in
lenses aged ≥70 years. The IRE1 and ATF6 UPR proteins gradually
increased with age (Fig. 4). These
results confirmed that the UPR was activated in the human lenses.
Therefore, activation of the UPR was involved in the production of
ROS, decreased antioxidant levels and activation of the apoptotic
pathway, ultimately leading to lens oxidation or the formation of
cataracts.
Discussion
Cataract formation is a multifactorial disease
occurring predominantly in older individuals based on their
exposure to internal or external stressors. Cataractogenic factors
are responsible for the generation of ROS and the failure of the
antioxidant system in the lens epithelial cells, leading to lens
oxidation or cataract formation (25). Generally, ROS are generated in the
mitochondria of the cell, however, lens epithelial cells lacks
mitochondria and the generation of ROS in the lens cells remains to
be elucidated (26,27). The present study revealed that ER
stress induced the activation of the UPR and that chronic
activation of the UPR leads to the generation of ROS and causes
apoptosis. At higher levels of calcimycin, misfolded proteins
accumulated in the ER, which can then induce the UPR (28,29).
In the present study, HLECs exposed to calcimycin,
an ER stressor, for 24 h led to the formation of ROS and, after 48
h, apoptosis occurred. It was hypothesized that this may be due to
activation of the UPR. To investigate this hypothesis, gene
expression levels were assessed after 12, 24, 48 and 72 h of
calcimycin exposure. The initial responses of the UPR are the
phosphorylation of three ER-associated transmembrane proteins;
PERK, IRE1 and ATF6, which are cleaved and separated from the ER
membrane (16). These responses
lead to translational and transcriptional arrest and a reduction in
protein production in order to recover from the accumulation of
unfolded proteins in the ER. However, in the presence of continued
ER stress, the apoptotic pathway is activated by the central
transcriptional activators, ATF4 and ATF6, which subsequently
activate the apoptotic factor CHOP (30). Consistent with these results, the
present study revealed a significant increase in the mRNA and
protein levels of IRE1, ATF6, PERK 72 h after exposure. The levels
of BIP, which is considered to be an ER stress marker, were
increased after 24 h. A similar pattern of protein expression
levels were identified in the 50–90 year old lenses, in which an
age-dependent increase in UPR-associated protein levels was
observed. These results demonstrated that UPR activation is common
in aging lenses and may trigger the formation of ARC.
To elucidate whether activation of the UPR is
involved in altering the antioxidant system to cause apoptosis, the
levels of antioxidants and other UPR-associated proteins involved
in apoptosis were assessed. The UPR also activates caspase-4
(caspase-12 in rodents) resulting in apoptosis (29,31)
and generates excess levels of ROS, driven by PDI, Ero1-Lα and
Ero1-Lβ (13–15). The production of ROS decreases the
levels of cytosolic glutathione and contributes to an additional
source of ROS from the mitochondria (32) resulting in apoptosis. The results
from the present study were consistent with these findings. The
initial step of UPR activation triggered the survival pathway by
increasing the levels of PDI and Ero1-Lβ. Notably, the levels of
Ero1-Lα decreased during the exposure period. The protein levels of
catalase, SOD and GR decreased in the cells exposed for >24 h.
Similarly, activation of the UPR induced the apoptotic pathway,
which was confirmed by elevated protein levels of ATF4, CHOP and
caspase-4.
In conclusion, the present study demonstrated that
the UPR was activated in HLECs, which was confirmed in human aged
lenses. Furthermore, UPR activation generated excess levels of ROS
and altered the antioxidant system. Prolonged exposure to UPR
activation triggered apoptotic pathway signaling proteins, leading
to lens oxidation or the formation of ARC. Thus, UPR activation is
important in the formation of ARC.
References
|
1
|
Kozutsumi Y, Segal M, Normington K,
Gething MJ and Sambrook J: The presence of malfolded proteins in
the endoplasmic reticulum signals the induction of
glucose-regulated proteins. Nature. 332:462–464. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brewer JW and Diehl JA: PERK mediates
cell-cycle exit during the mammalian unfolded protein response.
Proc Natl Acad Sci USA. 97:12625–12630. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prostko CR, Brostrom MA, Malara EM and
Brostrom CO: Phosphorylation of eukaryotic initiation factor (eIF)
2 alpha and inhibition of eIF-2B in GH3 pituitary cells by
perturbants of early protein processing that induce GRP78. J Biol
Chem. 267:16751–16754. 1992.PubMed/NCBI
|
|
4
|
Shi Y, Vattem KM, Sood R, An J, Liang J,
Stramm L, et al: Identification and characterization of pancreatic
eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved
in translational control. Mol Cell Biol. 18:7499–7509.
1998.PubMed/NCBI
|
|
5
|
Tirasophon W, Welihinda AA and Kaufman RJ:
A stress response pathway from the endoplasmic reticulum to the
nucleus requires a novel bifunctional protein
kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev.
12:1812–1824. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tinhofer I, Anether G, Senfter M, Pfaller
K, Bernhard D, Hara M, et al: Stressful death of T-ALL tumor cells
after treatment with the anti-tumor agent Tetrocarcin-A. FASEB J.
16:1295–1297. 2002.PubMed/NCBI
|
|
9
|
Xie Q, Khaoustov VI, Chung CC, Sohn J,
Krishnan B, Lewis DE, et al: Effect of tauroursodeoxycholic acid on
endoplasmic reticulum stress-induced caspase-12 activation.
Hepatology. 36:592–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaufman RJ, Scheuner D, Schroder M, Shen
X, Lee K, Liu CY, et al: The unfolded protein response in nutrient
sensing and differentiation. Nat Rev Mol Cell Biol. 3:411–421.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER stress-mediated apoptosis: the story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar
|
|
12
|
Ji C, Deng Q and Kaplowitz N: Role of
TNF-alpha in ethanol-induced hyperhomocysteinemia and murine
alcoholic liver injury. Hepatology. 40:442–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haynes CM, Titus EA and Cooper AA:
Degradation of misfolded proteins prevents ER-derived oxidative
stress and cell death. Mol Cell. 15:767–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu BP, Ho-Schleyer SC, Travers KJ and
Weissman JS: Biochemical basis of oxidative protein folding in the
endoplasmic reticulum. Science. 290:1571–1574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikesugi K, Yamamoto R, Mulhern ML and
Shinohara T: Role of the unfolded protein response (UPR) in
cataract formation. Exp Eye Res. 83:508–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elanchezhian R, Palsamy P, Madson CJ, et
al: Low glucose under hypoxic conditions induces unfolded protein
response and produces reactive oxygen species in lens epithelial
cells. Cell Death Dis. 3:e3012012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elanchezhian R, Palsamy P, Madson CJ,
Lynch DW and Shinohara T: Age-related cataracts: homocysteine
coupled endoplasmic reticulum stress and suppression of
Nrf2-dependent antioxidant protection. Chem Biol Interact.
200:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michael R and Bron AJ: The ageing lens and
cataract: a model of normal and pathological ageing. Philos Trans R
Soc Lond B Biol Sci. 366:1278–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harding JJ: Free and protein-bound
glutathione in normal and cataractous human lense. Biochem J.
117:957–960. 1970.PubMed/NCBI
|
|
21
|
Lou MF: Redox regulation in the lens. Prog
Retin Eye Res. 22:657–682. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikesugi K, Mulhern ML, Madson CJ, et al:
Induction of endoplasmic reticulum stress in retinal pericytes by
glucose deprivation. Curr Eye Res. 31:947–953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mulhern ML, Madson CJ, Danford A, Ikesugi
K, Kador PF and Shinohara T: The unfolded protein response in lens
epithelial cells from galactosemic rat lenses. Invest Ophthalmol
Vis Sci. 47:3951–3959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shichi H: Cataract formation and
prevention. Expert Opin Investig Drugs. 13:691–701. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bloemendal H, de Jong W, Jaenicke R,
Lubsen NH, Slingsby C and Tardieu A: Ageing and vision: structure,
stability and function of lens crystallins. Prog Biophys Mol Biol.
86:407–485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang L1, Tang D, Yappert MC and Borchman
D: Oxidation-induced changes in human lens epithelial cells 2.
Mitochondria and the generation of reactive oxygen species. Free
Radic Biol Med. 41:926–936. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schröder M: The unfolded protein response.
Mol Biotechnol. 34:279–290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puustjärvi T, Blomster H, Kontkanenv M,
Punnonen K and Terasvirta M: Plasma and aqueous humour levels of
homocysteine in exfoliation syndrome. Graefes Arch Clin Exp
Ophthalmol. 242:749–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arap MA, Lahdenranta J, Mintz PJ, Hajitou
A, Sarkis AS, Arap W and Pasqualini R: Cell surface expression of
the stress response chaperone GRP78 enables tumor targeting by
circulating ligands. Cancer Cell. 6:275–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang HC, Nguyen T and Pickett CB:
Phosphorylation of Nrf2 t Ser-40 by protein kinase C regulates
antioxidant response element-mediated transcription. J Biol Chem.
277:42769–42774. 2002. View Article : Google Scholar : PubMed/NCBI
|