Introduction
Ankylosing spondylitis (AS) is a chronic
inflammatory disease that primarily affects the axial skeleton,
peripheral joints and the attachment of ligaments and entheses. The
predominant clinical feature of this disease is inflammatory lower
back pain, and over time certain patients develop spinal immobility
and ankylosis (1,2). AS occurs predominately in males
(3). The prevalence of AS is
0.2–0.54% among the Han Chinese population, which accounts for 92%
of the whole Chinese population (4–6),with
similar rate of prevalence in Europe and America. The clinical
manifestations, severity, and risk of developing the disease vary
by ethnicity, geography and gender, with a prevalence that is
higher in young males and lower in certain populations, such as
Japan (7,8).
Despite a long standing knowledge of the familial
associations of AS, the fundamental pathogenetic mechanism remains
undefined. A number of cytokines have been shown to have critical
roles in the pathogenesis of AS, including inflammatory cytokines
and bone metabolism-related factors. Interleukin (IL)-17 and IL-23
are cytokines associated with inflammation, autoimmunity and
defense against bacteria. The elevated levels of IL-17 and IL-23 in
patients with AS indicates that they have critical roles in the
pathogenesis of AS (9,10). Vascular endothelial growth factor
(VEGF) has a crucial role in angiogenesis, which is important for
the pathogenesis of chronic inflammatory diseases in joints.
Angiogenesis is a potential novel target for therapeutic
intervention in inflammatory joint disease. The disease status of
AS has been shown to be associated with elevated VEGF plasma levels
(11). Prostaglandin E2 (PGE2) is
an important factor for osteoblast differentiation in AS. PGE2 has
anabolic effects on bones and promotes proliferation and
differentiation of osteoblasts, thereby inducing the expression of
bone sialoprotein and bone alkaline phosphatase (BAP) (12). The above inflammatory factors are
closely associated with the pathogenesis and prognosis of AS.
In addition, numerous bone metabolism-associated
factors are involved in AS. DKK-1 is an inhibitory molecule that
regulates the Wnt pathway, which controls osteoblastogenesis, and
the potential role of DKK-1 in AS has been explored (13). Besides, a number of other bone
metabolism-associated factors are involved in the formation of AS,
including the receptor activator of nuclear factor κB ligand
(RANKL), osteoprotegerin (OPG), bone alkaline phosphatase (BAP) and
bone morphogenetic protein-2 (BMP-2). Other factors reported to be
closely associated with the pathogenesis of AS include matrix
metalloproteinase (MMP)-3 and cross-linked telopeptide of type II
collagen (CTX-II), which have been recommended as predictors for
AS. Serum metalloproteinase MMP-3 has been shown to be effective
for predicting AS progression (14) and urinary CTX-II is an important
index of cartilage degradation (15). The above biomarkers may provide
information that promotes understanding of the prognosis, disease
activity, and pathogenesis of AS (16). Thus, these biomarkers are important
indexes when evaluating the efficacy of drugs in treating AS.
Tripterygium glycosides tablet (TGTs), is a product
of a Traditional Chinese Medicinal plant comprising of triptolides,
which have been used in China for the long-term treatment of
inflammatory conditions such as rheumatoid arthritis, various skin
disorders, chronic nephritis and AS. In recent years, TGT has been
used to treat patients with active AS with improved efficiency.
However, the mechanism is far from understood.
The aim of the current study was to explore the
effects of TGT in the treatment of active AS and identify the
possible mechanisms. A total of 36 patients with active AS (AS
group) were enrolled and treated with TGT (20 mg, 3 times/day) for
12 weeks, while 21 healthy, age- and gender-matched volunteers were
used as a control group. The levels of several serum biomarkers
(DKK-1, IL-17, RANKL, OPG, BAP, CTX-II, MMP-3, PGE2, BMP-2, and
VEGF) were assessed in patients with AS prior and subsequent to TGT
treatment and in healthy controls.
Materials and methods
Study population
Ethical approval was obtained from the Human
Research Ethics Committee at the Affiliated Hospital of Nanjing
University of Traditional Chinese Medicine (Nanjing, China), and
participants provided written informed consent. TGT (cat. no.
0802102) was purchased from Deeng Pharmaceutical Company (Zhejiang,
China). In the study, 36 patients with AS and 21 unrelated healthy
controls who were age- and gender-matched were recruited at the
Affiliated Hospital of Nanjing University of Traditional Chinese
Medicine between January 2010 and January 2013. All patients and
controls were of Han Chinese origin. All the patients with AS were
treated with 20 mg TGT three times per day for 12 weeks; no other
treatments were given to the patients. The diagnosis of AS was been
made by experienced rheumatologists; all diagnoses satisfy the
modified New York criteria (17).
Subjects with rheumatoid arthritis, inflammatory bowel disease,
psoriasis or other autoimmune diseases were excluded from the AS
and the control groups. Subjects with additional use of
nonsteroidal anti-inflammatory drugs (NSAIDs) or other drugs were
excluded from the AS and control groups. The patients were
followed-up with for 12 weeks. Peripheral blood samples (2 ml) were
obtained from the patients prior and subsequent to TGT treatment
for 12 weeks. The samples were collected in heparin-containing
tubes (Becton Dickinson, Heidelberg, Germany) and all samples were
immediately stored at −70°C until further processing.
Basic data acquisition
The Bath AS disease activity index (BASDAI) was
administered to all the patients with AS using questionnaires, as
it is the most widely used tool for the assessment of AS functional
status and disease activity (18).
Serum assays for ESR (erythrocyte sedimentation rate) and CRP
(C-reactive protein) were performed on patients with AS prior and
subsequent to TGT treatment. ESR was determined using the
Westergren method (19) and CRP
was determined using an ELISA kit (Kehua Biotech, Shanghai, China).
The normal range of ESR is 0–15 mm/h and the normal range of CRP is
0–10 mg/l.
Serum dickkopf homolog 1 (DKK1) and IL-17
levels
Reverse transcription-polymerase chain
reaction (RT-PCR)
Fifteen patients with AS prior and subsequent to TGT
treatment were randomly selected for RT-PCR analysis. The
expression levels of DKK1 and IL-17 were measured by RT-qPCR. Total
RNA was extracted from serum using TRIzol® (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
Primer sequences (Sangon Biotech, Shanghai, China) and RT-PCR
product length of the investigated factors were listed as follows:
Forward, 5′-CCAACGCTATCAAGAACCTGC-3′, and reverse,
5′-TGACCGGAGACAAACAGAACC-3′ for DKK1 (461 bp); forward,
5′-ACAACCGATCCACCTCACC-3′, and reverse, 5′-CAGCCCACGGACACCAGTA-3′
for IL-17 (232 bp); forward, 5′-AAGGTCGGAGTCAACGGATTT-3′, and
reverse, 5′-AGATGATGACCCTTTTGGCTC-3′ for human (h)GAPDH (352 bp).
cDNA was amplified by PCR in a thermal cycler (Eppendorf, Hamburg,
Germany). Thirty-two cycles were performed for hGAPDH and the other
primer pairs. The PCR steps were as follows: 1 cycle at 95°C for 30
sec for initial denaturation; followed by 40 cycles at 95°C for 3
sec and 60°C for 30 sec.
Amplification products were analyzed by
electrophoresis and the intensity of the PCR products was
quantified using Quantity One software (Bio-Rad, Hercules, CA,
USA).
ELISA assay
The activity levels of serum DKK1 and IL-17 were
measured using a commercial human DKK1 ELISA kit (Hufeng
Biotechnology, Shanghai, China) and a human IL-17 ELISA kit
(eBioscience, San Diego, CA, USA) according to the manufacturer’s
instructions.
Levels of biomarkers in serum (RANKL,
OPG, BAP, CTX-II, MMP-3, PGE2, BMP-2 and VEGF)
Serum levels of RANKL, OPG, BAP, CTX-II, MMP-3,
PGE2, BMP-2 and VEGF were measured using commercial human ELISA
kits according to the manufacturer’s instructions (RANKL, OPG, BAP
and CTX-II from Hufeng Biotechnology; MMP-3, PGE2, BMP-2 and VEGF
from KeyGen Biotechnology, Nanjing, China). The serum levels of
these factors in the AS group prior or subsequent to TGT treatment
were compared with the control group.
Statistical analysis
Statistical analyses were performed using the
statistical software Statistical Packages for Social Sciences
(SPSS) version 14.0 (SPSS, Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical features
The BASDAI, ESR and CRP of the patients with AS
prior and subsequent to TGT treatment are recorded in Table I. Prior to TGT treatment, the
BASDAI score in 20 cases was >50 mm, and average score of BASDAI
of all the patients with AS (51.94±11.57) was higher than normal
range. After 12 weeks of TGT treatment, the BASDAI score was
significantly reduced (24.47±13.01). Prior to treatment, the level
of ESR and CRP were higher than those of the normal controls. After
12 weeks of TGT treatment, the levels of ESR and CRP were
significantly decreased to normal range.
 | Table IBASDAI, ESR and CRP for patients with
AS before and after TGT treatment. |
Table I
BASDAI, ESR and CRP for patients with
AS before and after TGT treatment.
| Basic index | Before TGT
treatment | After TGT
treatment |
|---|
| BASDAI (mm) | 51.94±11.57 | 24.47±13.01a |
| ESR (mm/h) | 31.18±15.63 | 15.87±14.65a |
| CRP (mg/l) | 16.30±11.28 | 6.10±6.88a |
DKK1 and IL-17 RNA level in serum
The RNA levels of DKK1 and IL-17 in
serum of the groups are presented in Fig. 1, which shows the increased DKK1 RNA
expression and decreased IL-17 RNA expression following TGT
treatment for patients with AS.
DKK1 and IL-17 protein level in
serum
The protein levels of DKK1 and IL-17 in serum of the
groups are shown in Fig. 2. Prior
to TGT treatment, there was a significant reduction in DKK1
expression (P<0.05) and significant increase in IL-17 expression
(P<0.05) in the AS group compared with the control group.
Following TGT treatment for six weeks, the serum DKK1 level in
patients with AS were significantly upregulated (P<0.05), and
the serum IL-17 protein level in patients with AS was significantly
downregulated (P<0.05).
Levels of biomarkers in serum (RANKL,
OPG, BAP, CTX-II, MMP-3, PGE2, BMP-2 and VEGF)
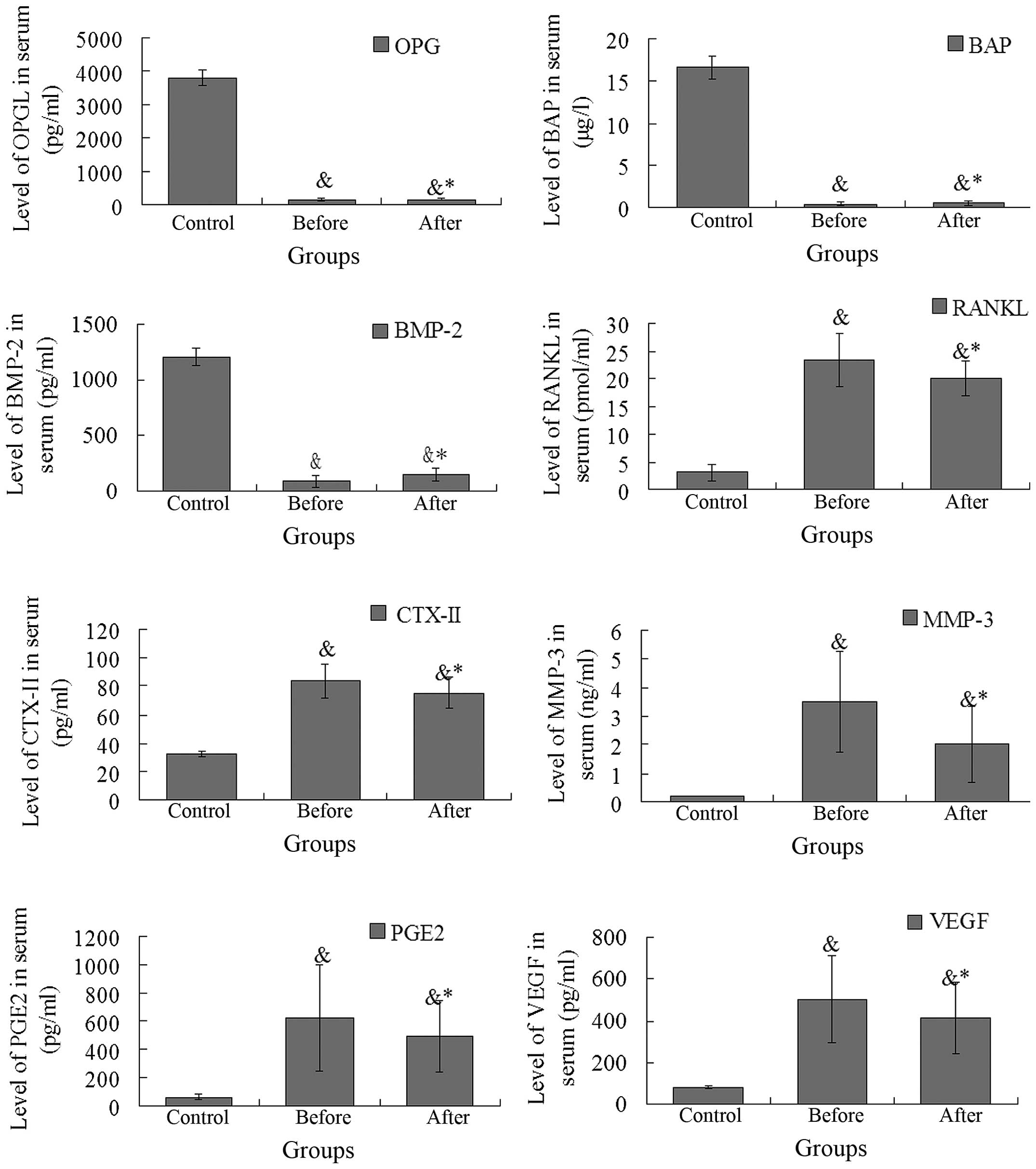
The expression levels of RANKL, OPG, BAP, CTX-II,
MMP-3, PGE2, BMP-2, and VEGF in patients with AS prior and
subsequent to TGT treatment are shown in Fig. 3. A reduction in serum OPG, BAP and
BMP-2 levels were observed in patients with AS compared with those
of the control subjects, and increased serum RANKL, CTX-II, MMP-3,
PGE2, and VEGF levels were observed in patients with AS compared
with those of the control subjects. Following TGT treatment in
patients with AS, there was a significant increase in the
expression of OPG, BAP and BMP-2 (all P<0.01), and a significant
decrease in the expression of RANKL, CTX-II. MMP-3, PGE2 and VEGF
(P<0.01, P<0.01, P<0.01, P<0.05 and P<0.01,
respectively).
Discussion
AS is a chronic inflammation of the sacroiliac
joints, spine and peripheral joints. NSAIDs are the first stage of
medication in treating the pain and stiffness associated with AS.
However, NSAIDs have significant side effects, in particular,
damage to the gastrointestinal tract. When NSAID treatment is
insufficient, second line medications, sometimes referred to as
disease modifying anti-rheumatic drugs, are used, including
Sulfasalazine and Methotrexate. Tumor necrosis factor (TNF)
blockers are one of the most promising medications for treating AS.
However, TNF blockers may cause serious side effects, including
reactivating latent tuberculosis and neurological problems.
TGT is a product of a Traditional Chinese Medicinal
plant that has been used in China for the long-term treatment of
inflammatory conditions including rheumatoid arthritis, various
skin disorders, chronic nephritis and AS. More recently, TGT has
been used to treat patients with active AS and find an improved
efficiency. However, the mechanism of action is unclear. In the
present study, the expression levels of biomarkers were examined in
patients with AS prior and subsequent to TGT treatment, and the
possible mechanisms of TGT in the treatment of AS were revealed.
Firstly, we investigated the anti-inflammatory effect of TGT on
patients with AS. The inflammation caused by AS is associated with
the pathogenetic condition and prognosis of AS, that is, patients
with AS with higher inflammation level may have worse functional
status. Cansu et al (20)
found that patients with AS with higher ESR or CRP levels had
higher BASFI scores, and that the levels of CRP were significantly
associated with a high BASFI. In the present study, we found an
elevated ESR level, CRP level and BASDAI score in patients with AS
compared with those of the healthy controls, which is consistent
with published results. Following TGT treatment, it was shown that
the BASDAI score and the ESR and CRP levels of the patients with AS
were significantly reduced to a normal level (all P<0.05),
demonstrating the possible anti-inflammatory effect of TGT on AS.
Furthermore, significantly higher serum levels of IL-17, VEGF and
PGE2 were observed in patients with AS compared with those of the
control subjects (all P<0.05), and the levels were revealed to
be significantly reduced following TGT treatment for 12 weeks
(P<0.01, P<0.01 and P<0.05, respectively). The results
demonstrated that TGT may regulate inflammatory factors, such as
IL-17, and inhibit the production of PGE2, which leads to an
anti-inflammatory effect on AS. The changes in the levels of
inflammatory cytokines in the serum further demonstrated the
possible anti-inflammatory effect of TGT on AS.
Additionally, it was determined that TGT may be a
potent regulator of new bone formation in patients with AS. The
inflammation caused by rheumatoid arthritis (RA) induces bone
destruction, while inflammation caused by AS may induce bone
destruction and new bone formation. Bone destruction is associated
with large amounts of new bone formation in patients with AS. This
is because bone repair occurs after bone destruction, however,
while acute lesions resolve completely, more advanced lesions are
associated with new bone formation, characterized by fusion of the
spine and joints such as sacroiliac joint, clinical stiffness and
formation of osteophyma (21).
Therefore, inhibiting new bone formation is another potential
strategy for the treatment of AS.
Prostaglandins (PGE), bone morphogenetic protein
(BMP) and Wnt proteins are the primary factors with essential roles
in this bone formation process. PGE2, which has anabolic effects on
bones and promotes proliferation, is an important factor for
osteoblast differentiation in AS; inducing the expression of bone
sialoprotein and bone alkaline phosphatase (BAP) (12). PGE2 synergizes with BMP-2, a member
of the transforming growth factor/BMP protein family in inducing
bone formation (22). In addition,
Wnt proteins have been identified as potent inducers of bone
regeneration (23). On the basis
of inhibiting new bone formation, the mechanisms of current drugs
for treating AS are as follows: i) TNF blockers. TNF induces the
expression of Wnt, so TNF blockers improve the signs and symptoms
of AS, but can not change radiographic progression. ii) Dkk-1
inducers. Dkk-1 is an inhibitory molecule that regulates the Wnt
pathway, which controls osteoblastogenesis, and the potential role
of Dkk-1 in AS has been explored (13). The Wnt signaling pathway is
inhibited by inducing DKK1, thus the new bone formation is
inhibited. iii) PGE2 inhibitors. PGE2 downregulates Dkk-1 and
sclerostin to increase the activity of the Wnt pathway, thus lead
to the new bone formation. By inhibiting the expression of PGE2,
certain drugs have shown great efficacy for treating AS, for
example NSAIDs. In the present study, significantly lower serum
DKK1 levels (RNA/protein) were observed in patients with AS
compared with those of the healthy controls which is consistent
with the results of Diarra et al (13), in addition to significantly higher
serum PGE2 levels. Following TGT treatment for 12 weeks, the Dkk-1
levels (RNA/protein) were significantly upregulated and the PGE2
levels were significant downregulated in patients with AS,
demonstrating that TGT may be a potent inhibitor of new bone
formation in patients with AS. However, the BMP-2 level in patients
with AS was observed to be significantly increased following TGT
treatment. We speculate that this is because BMP-2 is regulated by
additional signaling pathways, such as intracellular Smad signaling
(24). The efficiency of TGT at
regulating BMP-2 requires further study.
Thirdly, it was determined that the efficacy of TGT
in the treatment of AS may be associated with its bone protective
effect. A number of bone metabolism-related factors are also
involved in the formation of AS. RANKL, a novel member of the
tumor-necrosis factor family, has a key role in osteoclast
formation and activation. RANKL is expressed by osteoblast lineage
cells and binds with two different receptors, the first of which is
OPG, and the second is RANK, which is expressed on the surface of
osteoclast lineage cells. The binding of RANKL and RANK on
osteoclasts or osteoclast precursor cell activates the
transcription factor NF-kB and protein kinase JNK, which promote
osteoclast proliferation, differentiation, maturation and bone
resorption activity (25). OPG,
belonging to the TNF receptor superfamily, is the natural
antagonist of RANKL receptor, blocking the linkage of RANKL and
RANK, thereby inhibiting the biological effects of RANKL, including
such as bone resorption, osteoclast differentiation, activation and
induction of apoptosis. OPG is reported to be associated with poor
physical mobility and to reflect systemic inflammation in AS
(26). Therefore, RANKL promotes
osteoclast genesis, while OPG prevents bone erosion (27). In the present study, a
significantly lower OPG level and a higher RANKL level were
observed in patients with AS compared with those of control
subjects. Following treatment with TGT, a significant increase in
the OPG level and a reduction in the RANKL level were observed in
patients with AS, which shows the potential bone protective effect
of TGT by regulating RANKL/OPG signal pathway in patients with
AS.
To improve understanding of the progression of AS
and the effects of TGT on treating AS, several other serum
biomarkers for evaluating the AS progression were measured in the
study, including BAP, MMP-3 and CTX-II. BAP, the bone-specific
isoform of alkaline phosphatase, has been shown to be a sensitive
and reliable indicator of bone metabolism (28). In the current study, a reduction in
the serum BAP level was observed in patients with AS compared with
that of control subjects, and a significantly increased serum BAP
level was found in patients with AS following treatment with TGT.
Furthermore, the results revealed significantly higher serum levels
of MMP-3, CTX-II and VEGF in patients with AS compared with control
subjects, and the levels were significantly reduced following
treatment with TGT. Serum MMP-3 has been shown to be an effective
predictor of AS progression (14)
and urinary CTX-II is an important index of cartilage degradation
(15). Patients with AS have been
reported to have significantly higher mean urinary and serum CTX-II
levels compared with those of control subjects (29,30),
which is consistent with the present results. In conclusion, TGT is
effective at improving the signs and symptoms of patients with AS,
and the changes in serum biomarkers demonstrate the mechanisms that
may be associated with the anti-inflammatory effect, inhibition of
new bone formation and a potential bone protective effect.
Acknowledgments
This study was supported by grants from the Fund for
the Talents in Traditional Chinese Medicine of Jiangsu Province
(no. LJ200907) and Jiangsu Province Administration of Traditional
Chinese Medicine (no. LZ11014). The authors would like to thank
Professor David Yu (Medical Center at the University of California,
Los Angeles, Doctor of Medicine and Lifetime Professor) for his
technical support and suggestions.
References
|
1
|
Rolle AS, Zimmermann B and Poon SH:
Etanercept-induced Henoch-Schönlein purpura in a patient with
ankylosing spon-dylitis. J Clin Rheumatol. 19:90–93. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calin A, Brophy S and Blake D: Impact of
sex on inheritance of ankylosing spondylitis: a cohort study.
Lancet. 354:1687–1690. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng SC, Liao Z, Yu DT, et al: Epidemiology
of spondyloarthritis in the people’s republic of china: review of
the literature and commentary. Semin Arthritis Rheum. 37:39–47.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rubin LA, Amos CI, Wade JA, et al:
Investigating the genetic basis for ankylosing spondylitis: linkage
studies with the major histocompatibility complex region. Arthritis
Rheum. 37:1212–1220. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng QY, Chen R and Darmawan J: Rheumatic
diseases In China. Arthritis Res Ther. 10:172008. View Article : Google Scholar
|
|
7
|
Zochling J and Smith EU: Sero negative
spondyloarthritis. Best Pract Res Clin Rheumatol. 24:747–756. 2010.
View Article : Google Scholar
|
|
8
|
Reveille JD: Epidemiology of
spondyloarthritis in North America. Am J Med Sci. 341:284–286.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wendling D, Cedoz JP, Racadot E, et al:
Serum IL-17, BMP-7 and bone turnover markers in patients with
ankylosing spondylitis. Joint Bone Spine. 74:304–305. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mei Y, Pan F, Gao J, et al: Increased
serum IL-17 and IL-23 in the patient with ankylosing spondylitis.
Clin Rheumatol. 30:269–273. 2011. View Article : Google Scholar
|
|
11
|
Goldberger C, Dulak J, Duftner C, et al:
Vascular endothelial growth factor (VEGF) in ankylosing
spondylitis-a pilot study. Wien Med Wochenschr. 152:223–225. 2002.
View Article : Google Scholar
|
|
12
|
Samoto H, Shimizu E, Matsuda-Honjyo Y, et
al: Prostaglandin E2 stimulates bone sialoprotein (BSP) expression
through cAMP and fibroblast growth factor 2 response elements in
the proximal promoter of the rat BSP gene. J Biol Chem.
278:28659–28667. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daoussis D, Liossis SN, Solomou EE, et al:
Evidence that DKK-1 is dysfunctional in ankylosing spondylitis.
Arthritis Rheum. 62:150–158. 2010. View Article : Google Scholar
|
|
14
|
Maksvmowych WP, Landewé R, Conner-Spady B,
et al: Serum matrix metalloproteinase 3 is an independent predictor
of structural damage progression in patients with ankylosing
spon-dylitis. Arthritis Rheum. 56:1846–1853. 2007. View Article : Google Scholar
|
|
15
|
Christgau S, Garnero P, Fledelius C, et
al: Collagen type II C-telopeptide fragments as an index of
cartilage degradation. Bone. 29:209–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maksvmowych WP: What do biomarkers tell us
about the pathogenesis of ankylosing spondylitis? Arthritis Res
Ther. 11:1012009. View
Article : Google Scholar
|
|
17
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garrett S, Jenkinson T, Whitelock HC, et
al: A new approach to defining disease status in AS: the bath
ankylosing spondylitis disease activity index. J Rheumatol.
21:2286–2291. 1994.PubMed/NCBI
|
|
19
|
Westergren A: Studies of the suspension
stability of the blood in pulmonary tuberculosis. Acta Med Scand.
54:247–282. 1921. View Article : Google Scholar
|
|
20
|
Cansu DU, Calışır C, Savaş Yavaş U, et al:
Predictors of radio-graphic severity and functional disability in
Turkish patients with ankylosing spondylitis. Clin Rheumatol.
30:557–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maksymowych WP, Morency N, Conner-Spady B,
et al: Suppression of inflammation and effects on new bone
formation in ankylosing spondylitis: evidence for a window of
opportunity in disease modification. Ann Rheum Dis. 72:23–28. 2013.
View Article : Google Scholar
|
|
22
|
Zhang X, Schwarz EM, Young DA, et al:
Cyclooxygenase-2 regulates mesenchymal cell differentiation into
the osteoblast lineage and is critically involved in bone repair. J
Clin Invest. 109:1405–1415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minear S, Leucht P, Jiang J, et al: Wnt
proteins promote bone regeneration. Sci Transl Med. 2:29–30. 2010.
View Article : Google Scholar
|
|
24
|
Lories RJ, Daans M, Derese I, et al:
Noggin haploinsufficiency differentially affects tissue responses
in destructive and remodeling arthritis. Arthritis Rheum.
54:1736–1746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O’Gradaigh D, Ireland D, Bord S, et al:
Joint erosion in rheumatoid arthritis: interactions between tumour
necrosis factor α, interleukin 1 and receptor activator of nuclear
factor κb ligand (RANKl) regulate osteoclasts. Ann Rheum Dis.
63:354–359. 2004. View Article : Google Scholar
|
|
26
|
Chen CH, Chen HA, Liao HT, et al: Soluble
receptor activator of nuclear factor-kappab ligand (RANKl) and
osteoprotegerin in ankylosing spondylitis: OPG is associated with
poor physical mobility and reflects systemic inflammation. Clin
Rheumatol. 29:1155–1161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao L, Zhu T, Zhao D, et al:
Adeno-associated virus-mediated osteoprotegerin gene transfer
protects against joint destruction in a collagen-induced arthritis
rat model. Joint Bone Spine. 79:482–487. 2012. View Article : Google Scholar
|
|
28
|
Kress BC: Bone alkaline phosphatase:
methods of quantitation and clinical utility. J Clin Ligand Assay.
21:139–148. 1998.
|
|
29
|
Park MC, Chung SJ, Park YB, et al: Bone
and cartilage turnover markers, bone mineral density and
radiographic damage in men with ankylosing spondylitis. Yonsei Med
J. 49:288–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poddubnyy D, Denis Congad, et al:
Predictive and protective value of biomarkers in patients with
ankylosing spondylitis who are at high risk of radiographic spinal
progression. Arthritis Rheum. 63:13382011.
|