Introduction
Inflammatory bowel disease (IBD) is a chronic
intestinal disorder comprising Crohn’s disease (CD) and ulcerative
colitis (UC). The major clinical features of IBD include diarrhea,
rectal bleeding, abdominal pain and malnutrition (1). The prevalence of IBD has markedly
increased in recent years in China and other countries (2,3),
which has significantly affected the quality of life of the
affected patients. Currently, the diagnosis and assessment of IBD
relies predominantly on a combination of taking a patient history
and physical examination in association with laboratory,
endoscopic, histological and radiographic investigations. In order
to reliably diagnose IBD, specific biomarkers that may reflect the
activity and progression of this disease are required (2,4). In
addition, the identification of potential biomarkers has the
potential to improve current understanding of the pathogenesis of
the disease and provide novel therapeutic targets.
Pentraxin 3 (PTX3) has been established as a
multimeric inflammatory mediator, which shares structural homology
with hepatic short pentraxins, including C-reactive protein (CRP)
and the serum amyloid P component (5,6).
PTX3 is produced at sites of inflammation and is considered to
identify inflammatory disease more effectively than CRP (7,8).
However, the putative role of PTX3 in the development of CD remains
to be fully elucidated. The current study aimed to investigate the
potential of PTX3 as a novel biomarker of CD.
Materials and methods
Patients and samples
The present, single-center, study was performed
between 2007 and 2009 at the Department of Gastrointestinal
surgery, Qianfoshan Hospital of Shandong Province (Jinan, China).
The diagnoses of CD and UC were based on conventional clinical,
radiological, endoscopic and histopathological observations
(9). Serum was collected from all
the patients prior to electronic colonoscopy (n=240) and biopsy
samples were obtained from the patients with CD in order to confirm
diagnosis. The controls included age and gender-matched patients
with UC (n=240) and healthy volunteers (n=80). The patient
characteristics are shown in Tables
I and II.
 | Table IPatient characteristics of each
group. |
Table I
Patient characteristics of each
group.
| Group | Active CD
(n=139) | CD in remission
(n=101) | UC (n=240) | CRP-negative subgroup
(n=143) |
|---|
| Male/female
ratio | 76/63 | 50/51 | 130/110 | 75/68 |
| Age (years) | 36.29±10.32 | 35.18±13.46 | 37.47±15.36 | 37.45±13.98 |
| Weight (kg) | 59.14±12.63 | 62.80±13.61 | 61.44±12.29 | 58.62±11.83 |
| Height (cm) | 164.09±10.42 | 166.71±11.15 | 165.23±9.78 | 165.60±11.81 |
 | Table IIDisease distribution. |
Table II
Disease distribution.
| Distribution | Active CD
(n=139) | CD in remission
(n=101) |
|---|
| Isolated ileal | 21 | 16 |
| Isolated colonic | 27 | 13 |
| Isolated
ileocolonic | 42 | 17 |
| Isolated
anoperineal | 9 | 20 |
| Anoperineal
associated with another site | 35 | 28 |
| Ileal, colonic or
ileocolonic associated with another non-anoperineal site | 5 | 7 |
The clinical activity was determined using the
Crohn’s Disease Activity Index (CDAI). A CDAI ≥ 150 was defined as
the active phase of the disease and a CDAI < 150 was defined as
remission. Written informed consent was obtained from all the
individuals and all procedures involving human subjects were
approved by the Institutional Review Boards and ethics committee of
Qianfoshan Hospital of Shandong Province.
Determination of serum PTX3 levels
Human serum PTX3 levels were detected using the
Human Pentraxin 3/TSG-14 Quantikine enzyme-linked immunosorbent
assay (ELISA) kit (R&D Systems, Inc., Minneapolis, MN, USA).
The ELISAs were performed in triplicate, according to the
manufacturer’s instructions.
Western blot analysis
The colonic tissue samples, which were frozen in
liquid nitrogen (Qingdao Ruifeng Gas Co., Ltd., Qingdao, China),
were lysed in cell lysis buffer (Sigma-Aldrich, St. Louis, MO, USA)
containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
Na2EDTA, 1 mM ethylene glycol tetraacetic acid, 1%
Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 μg/ml leupeptin. The
samples were homogenized using a polytron tissue grinder (CH-6010;
Kinematica, Luzern, Switzerland) at 4°C and then cleared by
centrifugation at 15,000 × g for 30 min at 4°C and the protein
concentration was determined using a Bicinchonic Acid kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). The total proteins from
each sample (50 μg) were heated at 95°C for 5 min subsequent
to mixing with an equal volume of 2x SDS loading buffer (Cyagen
Bioscience Inc., Guangzhou, China). The samples were separated
using 12% SDS-polyacrylamide gel electrophoresis gels (Cyagen
Bioscience Inc.) and electrotransferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membrane was
blocked in Tris-buffered saline with Tween 20 (TBS-T; Cyagen
Bioscience Inc.) containing 5% skimmed milk (Cyagen Bioscience
Inc.) at room temperature for 2 h. The membranes were then
incubated with the anti-mouse monoclonal anti-PTX3 antibody
(1:2,000; sc-358922; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) in TBS-T overnight at 4°C. Subsequent to washing with TBS-T,
the membranes were incubated in 5% skimmed milk in TBS-T buffer
containing a rabbit anti-mouse monoclonal immunoglobulin G
secondary antibody (1:5,000; sc-365062Santa Cruz Biotechnology,
Inc.) for 60 min at room temperature with agitation (B11-1,
Shanghai Sile Co., Shanghai, China). PTX3 was detected using a
Chemiluminescent Reagent Plus kit (Perkin-Elmer Life Sciences,
Santa Clara, CA, USA). Normalization of the protein loading was
performed using the mouse monoclonal anti-GAPDH housekeeping
control antibody (1:5,000; sc-365062; Santa Cruz Biotechnology,
Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA) and the
Kolmogorov-Smirnov test was used to analyze the data for normality.
The data are presented as either the mean ± standard deviation or
the median ± interquartile range if continuous and as ‘n’ if
categorical. Differences between two groups were analyzed using the
Mann-Whitney U test. Spearman’s/Pearson correlation analysis was
used to measure the correlation between the serum PTX3 levels and
the CDAI. Receiver operating characteristic (ROC) curves and the
area under the ROC curve (AUC) were calculated. P<0.05 was
considered to indicate a statistically significant difference.
Results
Increased expression of PTX3 in patients
with active CD
Serum PTX3 levels were evaluated using ELISA and
were significantly increased in patients with active CD compared
with the control patients with UC (621.98±178.09, vs. 410.40±106.34
ng/ml; P<0.001). A significant difference was also observed
between the serum PTX3 levels in patients with active CD compared
with those with CD in remission (621.98±178.09 vs. 379.72±93.21
ng/ml; P<0.001; Fig. 1). To
further confirm these results, the serum levels of PTX3 were
determined in 80 healthy controls. The serum PTX3 levels were
significantly increased in the patients with active CD compared
with the healthy controls (621.98±178.09 vs. 362.9 7±94.32 ng/ml;
P<0.001; Fig. 1).
In addition, the expression levels of PTX3 were
examined in 35 recently resected inflamed colonic tissues vs.
uninflamed tissues from patients with CD using western blot
analysis. This confirmed that the expression of PTX3 was
significantly higher in the inflamed colonic tissues than in
uninflamed sites (Fig. 2). These
results suggested that PTX3 was upregulated in active CD intestinal
inflammation.
Serum PTX3 levels are correlated with
disease activity in patients with CD
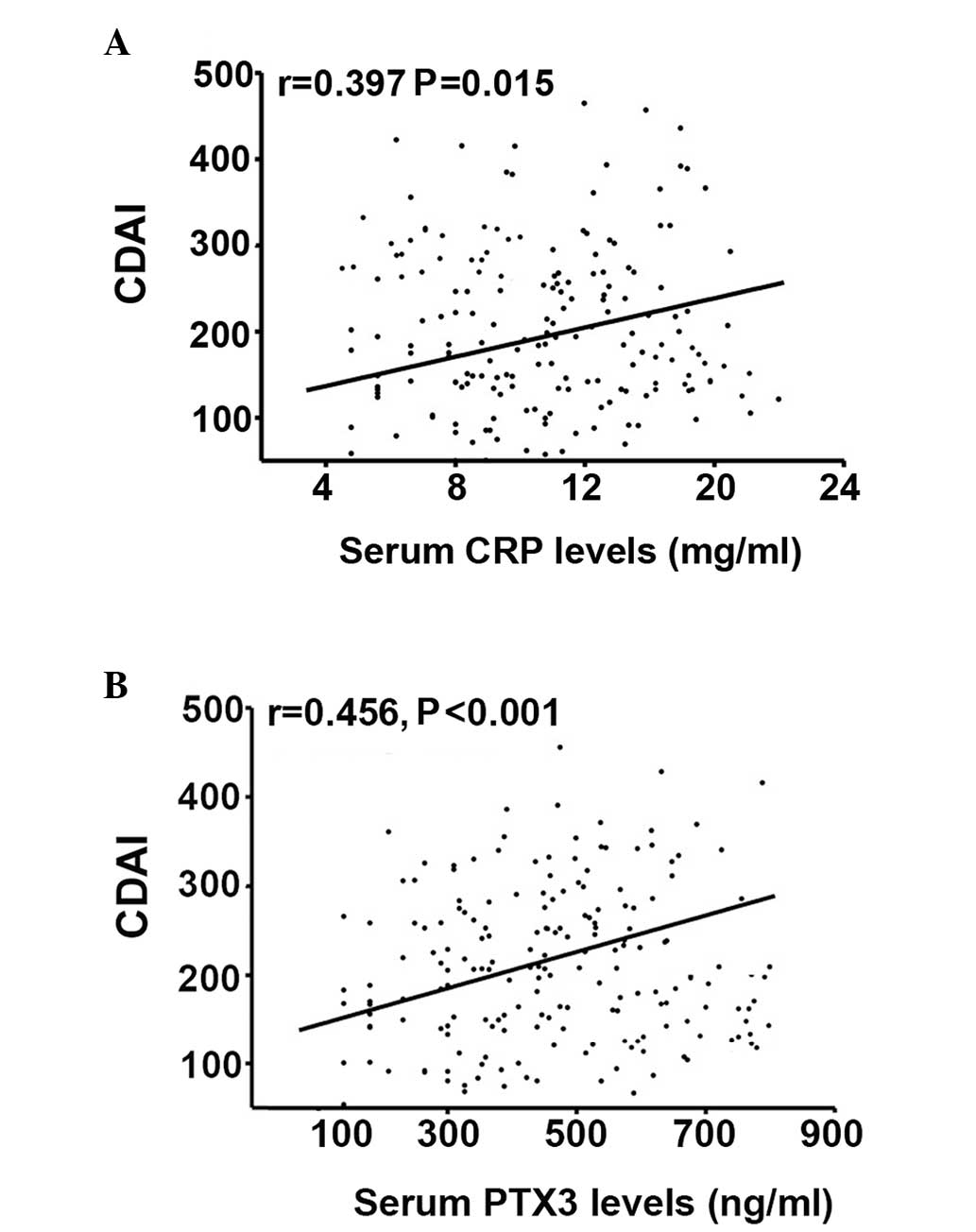
The present study also investigated the correlation
between serum PTX3 levels and the CDAI in patients with CD. A
positive correlation was observed between the levels of CRP and the
CDAI (r=0.397; P=0.015; Fig. 3A)
and between the levels of serum PTX3 and the CDAI (r=0.456;
P<0.001; Fig. 3B). The serum
levels of PTX3 were inversely correlated with the CRP levels in
patients with CD (r=−0.62; P<0.0001), however, no correlation
was observed when a CRP-negative subgroup (CRP cutoff value <4.2
mg/ml) was analyzed (r=0.13; P=0.48); data not shown). In this
CRP-negative group, the serum PTX3 levels remained significantly
correlated with CDAI (r=0.416; P=0.026), however, no significant
correlation was identified between CRP and the CDAI in this
subgroup (r=0.085; P=0.44). These observations indicated a more
marked correlation between the CDAI and serum PTX3 than serum CRP
in CD.
Evaluation of serum PTX3 levels as an
activity index in patients with CD
By generating an ROC curve, the sensitivity and
specificity of serum PTX3, for active CD and CD in remission, as a
CDAI were determined (Fig. 4).
Cutoff points were determined by the maximum sum of the sensitivity
and specificity. The cutoff value of serum PTX3 levels was 506.74
ng/ml (sensitivity and specificity values of 79.6 and 87.3%,
respectively). By contrast, when the cutoff value of CRP levels was
set at 4.2 mg/ml, the sensitivity was 61.6% and the specificity was
68.3%. The AUC for serum PTX3 levels was 0.85, whereas the AUC for
CRP levels was 0.64. These results highlight the potential of using
serum PTX3 levels as a marker for the evaluation of disease
activity in patients with CD.
Discussion
In clinical practice, currently used inflammatory
markers, including CRP and ESR do not provide sufficient accuracy
to predict and quantify mucosal inflammation and lesions in CD
(10). The present study aimed to
evaluate the expression levels and function of PTX3 in CD. The
expression levels of serum and colonic PTX3 were found to be
significantly upregulated in patients with active CD compared with
the patients with UC and the healthy controls. Additionally,
significant correlation was observed between the expression levels
of serum PTX3 with disease activity in CD and PTX3 was superior to
CRP as a marker for disease activity. These results demonstrated
that serum PTX3 is a potential indicator for disease activity in
patients with CD.
PTX3 has been identified as an early marker of
innate immunity and inflammatory responses and is structurally
linked to CRP (11,12). PTX3 belongs to the acute phase
class of proteins, of which CRP is also a member, and its
concentration has been reported to increase significantly in the
early stages of inflammation (13). PTX3 is an essential component of
the humoral arm of innate immunity and independently associates
with the risk of developing vascular inflammation (14). The plasma PTX3 concentration has
been observed to increase significantly during hemodialysis as a
rapid and sensitive marker of hemodialysis-induced inflammation
(15). A previous study also
observed that PTX3 correlated with the severity of sepsis and
peaked earlier than CRP in patients with ventilator-associated lung
inflammation, demonstrating superior prognostic value in the
prediction of mortality (16).
Changes in the concentration of PTX3 in the early phase of acute
pancreatitis are similar to that of interleukin-6 and its levels
peak at an earlier stage compared with CRP, suggesting that PTX3
may be of value in the early evaluation and in predicting the
severity of acute pancreatitis (13). In addition, data from a previous
study demonstrated that PTX3 may be a more sensitive marker of the
local inflammatory response resulting from vessel injury compared
with CRP, and lower levels of PTX3 may reflect potent
anti-inflammatory properties (17). In the present study, it was also
observed that the correlation between serum PTX3 and CDAI in CD was
more marked than that observed with serum CRP.
In conclusion, the present study demonstrated that
the expression levels of serum and colonic PTX3 were increased in
patients with active CD. These findings suggested that PTX3 may be
a more effective potential biomarker of inflammation compared with
CRP to predict CD activity. Further investigation is required to
clarify the role of PTX3 as an alternative biomarker to CRP in
CD.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Shandong Province (no. ZR2011HQ054).
References
|
1
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewis JD: The utility of biomarkers in the
diagnosis and therapy of inflammatory bowel disease.
Gastroenterology. 140:1817–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang YF, Ouyang Q and Hu RW: Progression
of inflammatory bowel disease in China. J Dig Dis. 11:76–82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lahiff C, Safaie P, Awais A, et al: The
Crohn’s disease activity index (CDAI) is similarly elevated in
patients with Crohn’s disease and in patients with irritable bowel
syndrome. Aliment Pharmacol Ther. 37:786–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alles VV, Bottazzi B, Peri G, Golay J,
Introna M and Mantovani A: Inducible expression of PTX3, a new
member of the pentraxin family, in human mononuclear phagocytes.
Blood. 84:3483–3493. 1994.PubMed/NCBI
|
|
6
|
Klouche M, Peri G, Knabbe C, Eckstein HH,
Schmid FX, Schmitz G and Mantovani A: Modified atherogenic
lipoproteins induce expression of pentraxin-3 by human vascular
smooth muscle cells. Atherosclerosis. 175:221–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fazzini F, Peri G, Doni A, et al: PTX3 in
small-vessel vasculitides: an independent indicator of disease
activity produced at sites of inflammation. Arthritis Rheum.
44:2841–2850. 2001. View Article : Google Scholar
|
|
8
|
Baldini M, Maugeri N, Ramirez GA, et al:
Selective up-regulation of the soluble pattern-recognition receptor
pentraxin 3 and of vascular endothelial growth factor in giant cell
arteritis: relevance for recent optic nerve ischemia. Arthritis
Rheum. 64:854–865. 2012. View Article : Google Scholar
|
|
9
|
Lennard-Jones JE: Classification of
inflammatory bowel disease. Scand J Gastroenterol Suppl. 170:2–6.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benitez JM, Meuwis MA, Reenaers C, Van
Kemseke C, Meunier P and Louis E: Role of endoscopy,
cross-sectional imaging and biomarkers in Crohn’s disease
monitoring. Gut. 62:1806–1816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Valentino S, Gentile S,
Inforzato A, Bottazzi B and Garlanda C: The long pentraxin PTX3: a
paradigm for humoral pattern recognition molecules. Ann NY Acad
Sci. 1285:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunes P, Holubcova Z, Kolackova M and
Krejsek J: Pentraxin 3(PTX 3): an endogenous modulator of the
inflammatory response. Mediators Inflamm. 2012:9205172012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kusnierz-Cabala B, Gurda-Duda A, Dumnicka
P, Panek J, Pawlica-Gosiewska D, Kulig J and Solnica B: Plasma
pentraxin 3 concentrations in patients with acute pancreatitis.
Clin Lab. 59:1003–1008. 2013.PubMed/NCBI
|
|
14
|
Bottazzi B, Doni A, Garlanda C and
Mantovani A: An integrated view of humoral innate immunity:
pentraxins as a paradigm. Annu Rev Immunol. 28:157–183. 2010.
View Article : Google Scholar
|
|
15
|
Sjöberg B, Qureshi AR, Anderstam B,
Alvestrand A and Bárány P: Pentraxin 3, a sensitive early marker of
hemodialysis-induced inflammation. Blood Purif. 34:290–297. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin Q, Fu F, Shen L and Zhu B: Pentraxin 3
in the assessment of ventilator-associated pneumonia: an early
marker of severity. Heart Lung. 42:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hudzik B, Szkodzinski J, Pietka-Rzycka A,
et al: Plasma pentraxin 3 may be a more sensitive marker of
inflammatory response than high-sensitivity C-reactive protein
after bare-metal stent compared to drug-eluting stent implantation.
J Interferon Cytokine Res. 33:280–284. 2013. View Article : Google Scholar : PubMed/NCBI
|