Introduction
Gallbladder cancer (GBC) is the most common cancer
of the biliary tract and has a particularly high incidence in
humans (1). According to the
United States Centers for Disease Control and Prevention, GBC is
the sixth most common type of gastrointestinal cancer in the USA
and >10,000 novel cases of gastric cancer are diagnosed each
year. In addition, the incidence of GBC appears to increase with
age and females are affected 2-6 fold more frequently than males
(2). GBC is characterized by local
invasion, extensive regional lymph node metastasis, vascular
encasement and distant metastases. The five-year survival rates are
5–10% for patients with a GBC diagnosis, and this poor prognosis is
associated with the commonly advanced stage at diagnosis due to the
lack of effective screening programs and non-specific symptoms
(3).
At present, complete surgical resection is the only
potentially curative treatment for GBC. However, few patients are
eligible for this surgery due to the advanced stage at diagnosis
(4). Although there have been
significant advances in the management of GBC over the previous
decade, the majority of treatments are palliative and aimed at
improving quality of life through relief of pain and jaundice and
by potentially prolonging survival time (5). In order to improve treatment options
for GBC, an improved understanding of the molecular mechanisms
underlying carcinogenesis in the gall bladder is required.
MicroRNAs (miRNAs) are a class of small,
single-stranded noncoding RNAs, consisting of 19–24 nucleotides,
which regulate gene expression by silencing messenger RNAs (mRNAs)
via binding to their 3′-untranslated region (3′UTR) (6). miRNAs are involved in a variety of
biological processes, including cellular differentiation,
apoptosis, metabolism and proliferation, by targeting different
genes (7). Accumulating lines of
evidence have indicated that miRNAs exhibit aberrant expression
patterns and functional abnormalities in numerous types of cancer,
including GBC. Dysregulated miRNAs are common in GBC and contribute
to gallbladder carcinogenesis via the alteration of cell growth,
cell cycle, apoptosis and cell migration (8–10).
Human miR-146b-5p is located within 10q24-26
(104186259−104186331+), an area in which genetic material has been
observed to be frequently deleted in cancer cells (11). Previous evidence has also indicated
that miR-146b-5p is able to function as a tumor suppressor in
pancreatic and breast cancer (12,13).
To date, despite the recent findings regarding miR-146b-5p and its
important roles in carcinogenesis, no studies investigating an
association between miR-146b-5p and GBC have been performed, to the
best of our knowledge.
The present study aimed to examine the expression
levels of miR-146b-5p in order to evaluate the clinical
characteristics of miR-146b-5p expression in GBC and to evaluate
the effects of aberrant miR-146b-5p expression on GBC cell
lines.
Patients and methods
Patients and tissue samples
Human GBC samples (n=46) were obtained from a random
sample of patients who had undergone curative resection at the
Department of General Surgery, East Hospital Tongi University
School of Medicine (Shanghai, China), between October 2012 and
October 2013. All samples were immediately frozen in liquid
nitrogen. None of the patients had been previously treated with
radiotherapy or chemotherapy prior to the surgery. The GBC tissues
from the surgical resections of the 46 patients were all verified
by pathologists in the hospital. All samples were obtained with
informed consent of the patients or their families, and the study
protocol was approved by the Ethics Committee of the East
Hospital.
RNA extraction and miRNA expression
assay
For the analysis of miRNA in tissue samples using
reverse transcription quantitative polymerase chain reaction
(RT-qPCR) assay, total RNA was isolated using a mirVana™ miRNA
isolation kit (Applied Biosystems, Foster City, CA, USA). RT and
PCR amplification were performed using a TaqMan MicroRNA assay
(Applied Biosystems) according to manufacturer’s instructions. The
primer sequences were synthesized by Applied Biosystems, and the
PCR cycles comprised 40 cycles of 15 or 30 sec at 98°C, 90 sec at
58°C and 30 sec at 72°C; with a final extension at 72°C for 10 min.
All cycles were performed on an Eppendorf real time PCR machine
5331 (Eppendorf, Hamburg, Germany). All reactions, including those
for the blank controls, were assessed for amplification success on
a 1.5% agarose gel (Sigma-Aldrich, St. Louis, MO, USA) and
visualized using a SYBR®Safe (Invitrogen Life
Technologies, Carslbad, CA, USA The relative quantification of
miR-146b-5p was calculated using the 2−∆∆Ct method
normalized with RNU6B as the internal control and relative to a
calibrator sample as the external control.
Cell culture and generation of stably
transfected cell lines
The SGC-996 human GBC cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA) and grown
in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand Island, NY,
USA) with 10% fetal bovine serum (Hyclone, Logan, UT, USA) in a
cell culture incubator at 37°C and with 5% CO2. To
generate the stable miR-146b-5p-transfected cell line, the
miR-146b-5p gene was amplified from human genomic DNA using
Accuprime Taq polymerase (Invitrogen Life Technologies) and cloned
into pMSCV-PIG vectors (Clontech Laboratories, Mountain View, CA,
USA). SGC-996 cells were infected with retro-viruses generated in
Phoenix cells (American Type Culture Collection), as described
previously (14). After 72 h,the
cells were selected with 2 μg/ml puromycin (Sigma-Aldrich).
The primer sequences used to amplify the miR-146b-5p gene were as
follows: Forward, 5′-TGACCCATCCTGGGCCTCAA-3′ and reverse,
5′-CCAGTGGGCAAGATGTGGGCC-3′.
Cell proliferation assay
The cells were plated onto 96-well m icroplates
(104 cells/well) and cell viability was monitored using
an MTT assay (Sigma-Aldrich), as described previously (15). The absorbance value (A) at 570 nm
was read using a Bio-Rad 3550 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Three independent experi ments were performed
and mean values were calculated as the final result for
comparison
Cell cycle analysis
For cell cycle analysis, flow cytometry was used.
Briefly, the cells were harvested by trypsinization, washed with
cold phosphate-buffered saline (PBS) and fixed with 70% cold
ethanol (Sigma-Aldrich) at 4°C overnight. Subsequently, the fixed
cells were collected, washed in PBS and stained with propidium
iodide (PI; Sigma-Aldrich) in the presence of RNAse A
(Sigma-Aldrich). Cell cycle analysis was performed using a
FACS/Calibur flow cytometer using the CellQuest or ModFit 3.0
software packages (BD Bioscienciences, Franklin Lakes, NJ,
USA).
Apoptotic assay
To evaluate apoptosis, an Annexin V-fluorescein
isothiocyanate (FITC) apoptosis kit (Cell Signaling Technology,
Inc., Boston, MA, USA) was used. Briefly, the cells were harvested
and washed with cold PBS and resuspended in binding buffer,
followed by incubation with Annexin-V/FITC and PI buffers for 15
min at 4°C in the dark. Annexin-V/FITC and PI signals were detected
using flow cytometry.
Xenograft model
For xenograft experiments, 5–6-week-old male BALB/c
and nude mice (on a BALB/c background), free of specific pathogens,
were purchased from the Animal Laboratory of Tongji University
School of Medicine. The mice were bred under accredited specific
pathogen-free conditions in separate filter-top cages, and were
acclimated for at least 1 week prior to treatment. The Balb/c nude
mice (5–6 weeks old, male, ~20 g) were inoculated subcutaneously
into the flank with 1×107 cells suspended in 0.1 ml PBS.
The tumor volumes were measured every week using a Vernier caliper
(Fisher, Pittsburgh, PA, USA) to measure the maximal tumor diameter
(L) and transverse diameter (W). The total tumor volume was
calculated as: (L × W2)/2. After 6 weeks, the mice were
anesthetized with 2% sodium pentobarbital (10 μl/g body
weight) and then sacrificed via cervical dislocation. The tumors
were removed and weighed. All procedures were performed in
accordance with the Guidelines of the Chinese Association for
Laboratory Animal Science.
Western blot analysis
Cells were lysed on ice in radioimmunoprecipitation
assay lysis buffer (Sigma-Aldrich), containing 150 mM NaCl, 1%
NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris (pH 7.9), 10
mM NaF, phenylmethylsulfonyl fluoride and 1X protease inhibitors.
The protein concentrations were measured with a bicinchoninic acid
protein assay reagent kit (Pierce, Rockford, IL, USA). The protein
extracts (30 μg) were separated by 8% SDS-PAGE (Beyotime
Institute of Biotechnology, Haimen, China) and then transferred
onto polyvinylidene fluoride membranes. Human monoclonal epidermal
growth factor receptor (EGFR) was used as the primary antibody
(1:1,000; Cell Signaling Technology; Cat. no. #2646) at 4°C
overnight. A monoclonal β-actin antibody (1:1,000; Cell Signaling
Technology, Inc.; cat. no. #8457), at 4°C overnight, was used as a
control. The bands were detected with an enhanced chemiluminiscence
kit (GE Healthcare, Little Chalfont, UK) and visualized with the
ChemiDoc XRS system (Bio-Rad Laboaratories, Inc.).
Plasmid construction
To generate reporter constructs for luciferase
assays, the full-length 3′UTR of EGFR, as well as the mutant (Mut)
sequence of EGFR, were synthesized using PCR. The used primers
contained the following restriction sites: EGFR, 3′UTR forward,
5′-GGGG TACCCCACGGAGGATAGTATGAGCCC-3′ and reverse,
5′-GAAGATCTTCAGAGTGGAAATGAATATAGTT-3′; and Mut Rab23 3UTR forward,
5′-GTTTGTGTTACTTCTAAA AGATAGTTTTCT-3′ and reverse,
5′-AGAAAACTATCTTTT AGAAGTAACACAAAC-3′.
The PCR product of the EGFR 3′UTR was cloned into
the KpnΙ and BglΙΙ restriction sites downstream of
the open reading frame of luciferase in a pGL3-promoter luciferase
vector (Invitrogen Life Technologies).
The 3UTR deletion of EGFR was amplified via PCR with
the following primer sequences: EGFR forward,
5′-GCAGCGATGCGACCCTCCGGGACGGCC-3′ and reverse,
5′-CAGTGAATTTATTGGAGCATGACCAC-3′. The resulting PCR amplicons of
EGFR were cloned into the T vector (Promega, Madison, WI, USA). The
correct clones were confirmed by sequencing.
Luciferase assays
SGC-996 cells were seeded onto a 24-well plate
(1.5×105 per well) and co-transfected with miRNA mimics
(50 nM; Dharmacon, Lafayette, CO, USA) and plasmid (200 ng) using
Lipofectamine™ 2000 reagent (Invitrogen Life Technologies)
according to the manu facturer’s instructions. The luciferase
activity was analyzed using dual luciferase assays (Promega) after
48 h of co-transfection and normalized against Renilla
luciferase gene activity.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Values are expressed as the mean
± standard deviation of at least three repeated individual
experiments for each group. Significant differences were determined
using Student’s t-test or χ2 analysis for comparisons
between two groups and one-way analysis of variance or the
non-parametric Kruskal-Wallis H test was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Elevated miR-146b-5p expression inhibits
GBC growth
To investigate the pathogenicity of miR-146b-5p in
the development and progression of GBC, 46 GBC tissue samples were
analyzed using TaqMan RT-qPCR. The clinicopathological data for 46
patients are shown in Table I.
Based on the overall expression levels of miR-146b-5p, the GBC
specimens were divided into two groups (a high miR-146b-5p
expression group and a low expression group). Elevated miR-146b-5p
expression was observed in patients with a smaller tumor size and
well-differentiated tumors. No significant difference was observed
when comparing the groups with any other clinicopathological
feature, including gender, age, tumor-node-metastasis stage and
metastasis. Subsequently, the anti-tumorigenic function of
miR-146b-5p in human GBC cell lines was examined by generating
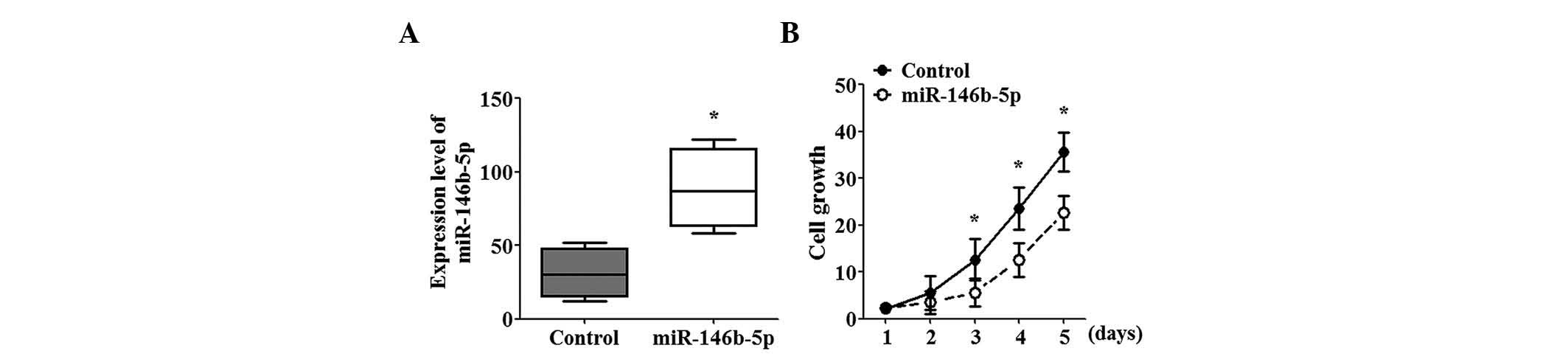
stable cell lines expressing miR-146b-5p. SGC-996 cells
overexpressing miR-146b-5p exhibited a 2.5-fold increase in
miR-146b-5p expression compared with that in the control cells
(Fig. 1A). Functional studies
revealed that miR-146b-5p overexpression significantly inhibited
the growth of SGC-996 cells and this effect increased with time
(Fig. 1B).
 | Table IAssociation between miR-146b-5p
expression in gallbladder cancer and clinical characteristics. |
Table I
Association between miR-146b-5p
expression in gallbladder cancer and clinical characteristics.
| Characteristic | Cases (n) | Relative miR-146b-5p
expression
| P-value |
|---|
| Low (n) | High (n) |
|---|
| Gender | | | | 0.7672 |
| Male | 23 | 12 | 11 | |
| Female | 23 | 13 | 10 | |
| Age (years) | | | | 0.3447 |
| >60 | 25 | 16 | 9 | |
| ≦60 | 21 | 10 | 11 | |
| Size of carcinoma
(cm) | | | | 0.0083 |
| >3 | 26 | 18 | 8 | |
| ≦3 | 20 | 6 | 14 | |
| Degree of
differentiation | | | | 0.0191 |
| Well and moderately
differentiated | 21 | 7 | 14 | |
| Poorly
differentiated | 25 | 17 | 8 | |
| TNM stage | | | | 0.3514 |
| I–II | 20 | 8 | 12 | |
| III–IV | 26 | 14 | 12 | |
| Lymph node
metastasis | | | | 0.2515 |
| Negative | 27 | 11 | 16 | |
| Positive | 19 | 11 | 8 | |
| Distant
metastasis | | | | 0.5949 |
| Negative | 22 | 9 | 13 | |
| Positive | 24 | 8 | 16 | |
miR-146b-5p induces G1 arrest
and apoptosis in SGC-996 cells
To further elucidate the growth inhibition mechanism
of miR-146b-5p in SGC-996 cells, the cell cycle stage and apoptotic
levels were analyzed. The overexpression of miR-146b-5p in SGC-996
cells significantly increased the percentage of cells in G0/G1
phase compared with that in the control group (Fig. 2A). In addition, compared with the
that in the control group, the apoptotic rate of SGC-996 cells in
miR-146b-5p-overexpressing cells was markedly increased (Fig. 2B).
miR-146b-5p inhibits tumor growth in
vivo
To confirm the effect of miR-146b-5p in vivo,
a GBC-bearing nude mouse model was generated.
miR-146b-5p-overexpressing and control cells were injected
subcutaneously into the flanks of nude mice. miR-146b-5p suppressed
the growth of the tumor, as indicated by decreased tumor weight and
volume as compared with that in the controls. Differences between
the miR-146b and control group were significant and increased with
time (Fig. 3A–C).
EGFR is a direct target of miR-146b-5p in
SGC-996 cells
As miRNA functions primarily by inhibiting target
genes, the target of miR-146b-5p in GBC was examined. miR-146b-5p
has recently been proposed to suppress EGFR expression by binding
to the 3′UTR of EGFR in glioma (16). To investigate whether miR-146b-5p
decreased EGFR expression in GBC, SGC-996 cells were transfected
with miR-146b-5p mimics. Western blot analysis also confirmed that
transfection with miR-146b-5p mimics significantly suppressed the
expression of EGFR protein in SGC-996 cells (Fig. 4A). To confirm that the inverse
correlation between miR-146b-5p and EGFR expression in SGC-996
cells was due to a direct interaction, the potential seed sequence
for miR-146b-5p was analyzed in the 3′UTR region of EGFR, and the
wild-type and mutant EGFR 3′UTR fragments were cloned into a
luciferase reporter gene system (Fig.
4B). miR-146b-5p mimics and luciferase reporter plasmids were
co-transfected into SGC-996 cells. Of note, it was identified that
the activity of a luciferase reporter gene linked to the wild-type
EGFR 3′UTR fragment decreased in response to transfection with
miR-146b-5p mimics (Fig. 4C). By
contrast, miR-146b-5p had no effect on the activity of the
luciferase reporter gene cloned into the mutant EGFR 3′UTR region
(Fig. 4C).
Ectopic expression of EGFR is able to
reverse the growth inhibition caused by miR-146b-5p
EGFR was recently hypothesized to have a crucial
role in GBC growth (17). To
demonstrate whether miR-146b-5p functions were mediated through
EGFR, expression vectors for EGFR lacking the respective 3′UTR or
empty vector were transfected into miR-146b-5p-overexpressing
cells. Western blot analysis demonstrated an increased expression
of EGFR proteins in SGC-996 cells transfected with miR-146b-5p and
EGFR compared with those transfected with the empty vector
(Fig. 5A). Cells overexpressing
miR-146b-5p exhibited an apparent rescue of SGC-996 cell
proliferation, as revealed using an MTT assay, following recovery
of EGFR expression and no significant differences were observed
compared with the control (Fig.
5B). The cell cycle and apoptosis assays revealed that EGFR
overexpression decreased the percentage of cells in
G0/G1 phase, decreased the rate of apoptosis
and reversed the growth inhibitory effect of miR-146b-5p (Fig. 5C and D).
Discussion
As one of the most frequent malignant neoplasms of
the biliary tract system, GBC is characterized by late clinical
presentation and diagnosis, with limited treatment options and poor
prognosis (4). Although the
incidence of GBC has appeared to increase, the molecular mechanisms
that cause GBC carcinogenesis remain to be elucidated, and the
available treatment methods have not significantly improved the
survival rates of affected patients.
In the present study, it was identified that the
expression of miR-146b-5p was associated with tumor size and
differentiation stage in patients, and that tumors of patients with
lower levels of miR-146b-5p tended to be larger and poorly
differentiated. However, no difference in miR-146b-5p expression
was observed regarding the presence or absence of metastasis,
indicating that miR-146b-5p is not involved in the regulation of
GBC metastasis. These results indicated that miR-146b-5p expression
may be an important indicator for GBC carcinogenesis and
growth.
EGFR belongs to the ErbB family of receptor tyrosine
kinases, which are activated by several ligands, including EGF,
transforming growth factor-α, amphiregulin, heparin-binding EGF,
betacellulin and epiregulin (18).
The EGFR signaling pathway exerts its biological effects via
multiple signaling cascades and is crucial in tumor growth
(19–21). Previous studies have demonstrated
that EGFR overexpression is common in GBC and that inhibiting EGFR
signaling results in considerable anti-proliferative effects on
in vitro models of GBC (22,23).
In the present study, a significant inverse correlation was
observed between miR-146b-5p expression levels and EGFR protein in
human GBC cell lines. In addition, it was demonstrated that EGFR
was negatively regulated by miR-146b-5p via binding to the 3′UTR of
EGFR mRNA in vitro and the inhibitory effect of miR-146b-5p
on proliferation may be reversed by overexpression of EGFR. The
present study demonstrated that miR-146b-5p inhibited GBC growth,
with concomitant suppression of EGFR.
Despite numerous studies aiming to elucidate the
mechanisms involved in GBC, the treatment of GBC remains a major
challenge in oncology. Molecularly targeted agents, which inhibit
EGFR pathways are being assessed in clinical trials and appear to
be a potent therapeutic target for patients with GBC. The findings
of the present study, regarding miR-146b-5p and EGFR in GBC are in
accordance with those of previous studies, which demonstrated that
miR-146b-5p decreased EGFR expression in the MDA-MB-231 breast
cancer cell line and the U87-MG glioma cell line (12,16,24),
elucidating the function of miR-146b-5p in GBC. It was demonstrated
in the present study that miR-146b-5p is an essential miRNA, which
regulates GBC growth and relies on the EGFR signaling pathway. The
manipulation of miR-146b-5p may be an effective therapeutic target
for GBC patients in the future.
Acknowledgments
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
Y2110335).
References
|
1
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hueman MT, Vollmer CM Jr and Pawlik TM:
Evolving treatment strategies for gallbladder cancer. Ann Surg
Oncol. 16:2101–2115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Groen PC, Gores GJ, LaRusso NF,
Gunderson LL and Nagorney DM: Biliary tract cancers. N Engl J Med.
341:1368–1378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang LQ, Zhang XD, Xu J, et al: Potential
therapeutic targets for the primary gallbladder carcinoma: estrogen
receptors. Asian Pac J Cancer Prev. 14:2185–2190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McNamara MG, Metran-Nascente C and Knox
JJ: State-of-the-art in the management of locally advanced and
metastatic gallbladder cancer. Curr Opin Oncol. 25:425–431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farh KK, Grimson A, Jan C, et al: The
widespread impact of mammalian MicroRNAs on mRNA repression and
evolution. Science. 310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu WK, Lee CW, Cho CH, et al: MicroRNA
dysregulation in gastric cancer: a new player enters the game.
Oncogene. 29:5761–5771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng HH, Zhang YD, Gong LS, Liu WD and
Zhang Y: Increased expression of microRNA-335 predicts a favorable
prognosis in primary gallbladder carcinoma. Onco Targets Ther.
6:1625–1630. 2013.PubMed/NCBI
|
|
9
|
Kono H, Nakamura M, Ohtsuka T, et al: High
expression of microRNA-155 is associated with the aggressive
malignant behavior of gallbladder carcinoma. Oncol Rep. 30:17–24.
2013.PubMed/NCBI
|
|
10
|
Chang Y, Liu C, Yang J, et al: MiR-20a
triggers metastasis of gallbladder carcinoma. J Hepatol.
59:518–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rasheed BK, Fuller GN, Friedman AH, Bigner
DD and Bigner SH: Loss of heterozygosity for 10q loci in human
gliomas. Genes Chromosomes Cancer. 5:75–82. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hurst DR, Edmonds MD, Scott GK, Benz CC,
Vaidya KS and Welch DR: Breast cancer metastasis suppressor 1
up-regulates miR-146, which suppresses breast cancer metastasis.
Cancer Res. 69:1279–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin F, Wang X, Jie Z, et al: Inhibitory
effects of miR-146b-5p on cell migration and invasion of pancreatic
cancer by targeting MMP16. J Huazhong Univ Sci Technolog Med Sci.
31:509–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Datta J, Majumder S, Kutay H, et al:
Metallothionein expression is suppressed in primary human
hepatocellular carcinomas and is mediated through inactivation of
CCAAT/enhancer binding protein alpha by phosphatidylinositol
3-kinase signaling cascade. Cancer Res. 67:2736–2746. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasser MW, Datta J, Nuovo G, et al:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katakowski M, Zheng X, Jiang F, Rogers T,
Szalad A and Chopp M: MiR-146b-5p suppresses EGFR expression and
reduces in vitro migration and invasion of glioma. Cancer Invest.
28:1024–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harder J, Waiz O, Otto F, et al: EGFR and
HER2 expression in advanced biliary tract cancer. World J
Gastroenterol. 15:4511–4517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harris RC, Chung E and Coffey RJ: EGF
receptor ligands. Exp Cell Res. 284:2–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ménard S, Casalini P, Campiglio M, Pupa SM
and Tagliabue E: Role of HER2/neu in tumor progression and therapy.
Cell Mol Life Sci. 61:2965–2978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aaronson SA: Growth factors and cancer.
Science. 254:1146–1153. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harder J, Waiz O, Otto F, et al: EGFR and
HER2 expression in advanced biliary tract cancer. World J
Gastroenterol. 15:4511–4517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pignochino Y, Sarotto I, Peraldo-Neia C,
et al: Targeting EGFR/HER2 pathways enhances the antiproliferative
effect of gemcitabine in biliary tract and gallbladder carcinomas.
BMC Cancer. 10:6312010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|