Introduction
Breast cancer is one of the most prevalent types of
malignant tumor, which has a severe impact on the physical and
mental health, and can be life-threatening. In addition, the
incidence rates of breast cancer have increased by at least double,
almost triple, in the past few decades in Asian countries (1). Multidrug resistance in breast cancer
is one of the primary obstacles leading to the clinical failure of
chemotherapy (2). Paclitaxelas, a
first-line treatment with significant antitumor activity, is widely
used in the treatment of breast cancer; however, its frequent use
can lead to resistance (3). The
potential mechanisms associated with paclitaxel resistance have
been reported in several studies, and include the dysregulation of
the P-glycoprotein (P-gp) drug efflux pump, variations in tubulin
structure, altered signal transduction and inhibition of the
activation of apoptotic pathways (4–8).
However, the intricate mechanisms of drug resistance have been
associated with multiple targets and pathways in tumor cells,
therefore, it is necessary to identify novel therapeutic molecular
targets and signal transduction networks for the treatment of
breast cancer (1,2). In addition, effective chemotherapy
reversal agents, which may reduce paclitaxel resistance in breast
cancer remain to be elucidated. Traditional Chinese Medicines have
been reported to be important in sensitizing cancer cells to
chemotherapy and overcoming drug-resistance in clinical treatment
(9). Therefore, herbs used in
Traditional Chinese Medicine may offer promise in identifying
efficient multidrug resistance reversal agents with low
toxicity.
The peony phenol, 4-methoxy-2-hydroxyaceto phenone
(paeonol), is derived from Traditional Chinese Medicine and is the
primary active ingredient of cortex moutan from the root bark of
Paeoniasuffruticosa andrews and the grass of the
ricinuscommunissecco plant, XuChangqing (Pycnostel
mapaniculatum k. schum) (10).
Previous studies have demonstrated that paeonol has numerous
pharmacological properties, including antioxidant and
anti-inflammatory activities as well as the inhibition of allergic
reactions and immune regulation (11–14).
It has been reported that paeonol is also involved in defense
against tumors and the reversal of multidrug resistance in tumor
cells. Xu et al (15)
demonstrated that paeonol has a significant growth-inhibitory
effect on the human HepG2 hepatoma cell line by inducing cell
apoptosis and arresting the cell cycle in the S phase. In addition,
Kim et al (16) reported
that paeonol significantly inhibits the proliferation and migration
of tumor cells, the mechanism of which involved, a least in part,
inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt
signaling pathway and the activity of matrix metalloproteinase.
Furthermore, paeonol reverses endoplasmic reticulum stress-induced
doxorubicin resistance in human hepatocellular carcinoma cells by
targeting the cycloxygenase (COX)-2-mediated inactivation of
PI3K/Akt/CCAAT-enhancer-binding protein homologous protein
(17). These studies indicated
that, due to its significant antitumor and chemotherapy
sensitization effects, paeonol may be a novel therapeutic reversal
agent for use in the treatment of drug-resistant breast cancer.
The SET protein is a member of the nucleosome
assembly protein family, characterized by a nitrogen end structure
domain, a nucleosome assembly structure domain and a carboxylic
acid structure of domain (18).
SET, which is distributed and expressed in multiple organs and
tissues, has a wide variety of biological functions, which are
involved in controlling cell cycle, nucleosome assembly, DNA
transcription, cell apoptosis, cell migration and histone binding
(19–23). A previous study demonstrated that
the SET may be a potential molecular antitumor target. Boqun et
al (24) reported that the
overexpression of SET in polycystic ovary syndrome led to a poor
prognosis. Another study found that the expression levels of SET
were over two times higher in uterine, stomach, colon and rectal
cancer tissues compared with those in corresponding normal tissues
(25). SET contributes to
tumorigenesis, at least in part, by inhibiting endogenous protein
phosphatase 2A (PP2A), a cellular phosphatase, which negatively
regulates multiple pro-growth/prosurvival signaling pathways
associated with the progression of cancer, including Akt, β-catenin
and c-Myc (26). Taken together,
SET is important in facilitating cellular growth and proliferation,
and interacting with pathways that promote tumorigenesis and
metastasis.
In our previous study, the protein profiles between
paclitaxel-resistant MCF-7/PTX and sensitive MCF-7 cells were
analyzed using two-dimensional gel electrophoresis (2-DE) and
matrix-assisted laser desorption/ionization time of light mass
spectrometry (MALDITOF-MS), in which SET was one of the most
significantly altered proteins (27). Therefore, it was hypothesized that
SET may be important in the occurrence of drug-resistance in the
development of breast cancer. The present study aimed to detect
whether the SET protein was associated with drug resistance in
paclitaxel-resistant MCF-7/PTX human breast carcinoma cells. In
addition, whether paeonol partially reversed drug resistance in
MCF-7/PTX cells, and the reversal mechanism by which this may
proceed, was examined to determine the potential use of SET
inhibitors to sensitize breast cancer to therapeutic drugs.
Materials and methods
Materials
Paclitaxel was purchased from Nanjing Luye Sike
Pharmaceutical Co., Ltd (Nanjing, China). Paeonol was obtained from
Ningbo Tianzhen Pharmaceutical Co., Ltd (Zhejiang, China).
Verapamil was obtained from China Pharmaceutical Biological
Products Analysis Institute (Beijing, China). Verapamil is a
non-specific P-gp inhibitor, which acts an an efficient reversal
agent for overcoming drug resistance (28). Verapamil has been used as a
positive control in numerous studies regarding chemoresistance
(28–30). Furthermore, in our previous study
verapamil was able to overcome paclitaxel resistance in MCF-7/PTX
cells (31). Therefore, verapamil
was used as a positive control in the present study. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
and dimethyl sulphoxide (DMSO) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). A Lipofectamine 2000™ transfection reagent
kit and annexin-V FITC staining kit was purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). Rabbit polyclonal primary
antibodies against Akt (cat. no. 9272; 1:1,000 dilution),
phophorylated (p)-Akt (cat. no. 9271; 1:1,000 dilution), B cell
lymphoma (Bcl-2)-associated X protein (Bax; cat. no. 2772; 1:2,500
dilution), Bcl-2 (cat. no. 1876; 1:2,500 dilution), caspase 9 (cat.
no. 9501; 1:2,000 dilution), caspase 3 (cat. no. 9661; 1:2,000
dilution) and anti-poly adenosine diphosphate-ribose polymerase
(PARP; cat. no. 9542; 1:2,000 dilution) were obtained from Cell
Signaling Technology (Beverly, MA, USA). Primary rabbit polyclonal
β-actin (cat. no. bs-0061R; 1:800 dilution) antibody was purchased
from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China).
Rabbit polyclonal breast cancer resistance protein (BCRP; cat. no.
sc-25822; 1:500 dilution) and multidrug resistance-associated
protein 1 (MRP1; cat. no. sc-13960; 1:500 dilution) antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit polyclonal SET (cat. no. CTX106342; 1:2,000 dilution), P-gp
(cat. no. GTX108370; 1:500 dilution) and PP2A (cat. no. GTX101690;
1:5,000 dilution) antibodies were obtained from GeneTex (Irvine,
CA, USA). Horseradish-peroxidase-conjugated goat anti-rabbit
secondary antibody (cat. no. CW0103; 1:20,000 dilution) was
purchased from CW biotech (Beijing, China).
Cell culture generation
The human MCF-7 breast carcinoma cell line was
obtained from the Chinese Academy of Science (Shanghai, China). The
paclitaxel-resistant MCF-7/PTX cells were established, as
previously described (32).
Briefly, the MCF-7/PTX cell line was established after a continuous
induction from 2 to 30 nM paclitaxel in a stepwise escalating
concentration manner. The half maximal inhibitory concentration
(IC50) values of paclitaxel for MCF-7/S and MCF-7/PTX
cells were 20±0.085 nM and 2291±125 nM, respectively. The reversal
fold (RF) was 115. The MCF-7 cells were cultured in 4 ml RPMI-1640
medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Gibco-BRL) and 1%
penicillin/streptomycin (Qilu Pharmaceutical Co., Ltd., Jinan,
China) at 37°C under a humidified atmosphere of 5% CO2.
Furthermore, 100 μl culture medium was added to the control
wells, and each group included four replicates. The culture
conditions for the MCF-7/PTX cells were the same to those of the
MCF-7 cell line, with the exception of the addition of 30 nM
paclitaxel.
MTT cell viability assay
In order to determine cell viability, the cells were
plated at 1×104 cells per well in 96-well plates in
volumes of 100 μl RPMI-1640 medium. Following culture for 24
h at 37°C and 5% CO2, the medium was removed and 100
μl culture medium containing a series of concentrations of
paeonol (15, 30, 60, 120, 250, 320 and 400 μM) or paclitaxel
(0.75, 1.5, 2.0, 2.5, 3.0, 3.5and 4.0 μM) was added to each
well. A total of 100 μl culture medium was added to the
control wells and each group included four replicates. Following
incubation for 48 h, 20 μl (0.5 mg/ml) MTT was added to each
well for an additional 4 h. The blue MTT formazan precipitate was
then dissolved in 100 μl DMSO and the culture plates were
gently agitated for 15 min. Subsequently, the density of formazan
was measured using a plate reader (ELx808; BioTek, Winooski, VT,
USA) at a wavelength of 492 nm in each well. The IC50
values were determined using GraphPad Prism5.0 software (GraphPad
Sotware, Inc., La Jolla, CA, USA). The RF values, which indicated
the potency of reversal, were calculated as the IC50 of
the cytotoxic drug / IC50 of the cytotoxic drug with
test-drug pretreatment.
Small interference RNA (siRNA) synthesis
and transient transfection of cells
Double strand siRNA oligonucleotides encoding human
SET (SET siRNA1, siRNA2 and siRNA3) were designed by
Shanghai GenePharma Co., Ltd (Shanghai, China). The negative
control was scrambled siRNA, and the transfection efficiency was
compared to that of siRNA nucleotides targeting β-actin. For
transient transfection, the MCF-7/PTX cells were seeded into a
six-well plate at a density of 6×105 cells per well for
24 h at 37°C. After 24 h, the cells were transfected with the
siRNAs targeting SET for 48 h at a final concentration with
Lipofectamine 2000™ reagent, according to the manufacturer’s
instructions. The scrambled siRNA was used as a negative control in
the transfection assay. After 48 h, the mRNA and protein expression
levels of SET were confirmed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
immunoblotting with cellular extracts, and the cells were seeded
for proliferation assays.
RT-qPCR analysis
Total RNA was isolated using an RNA Fast 2000 kit
(Shanghai Fastagen Biotechnology Co., Ltd., Shanghai, China).
RT-qPCR was performed using a Prime Script RT Master Mix Perfect
Real Time kit (cat. no. DRR036A; Takara Bio, Inc., Dalian, China)
and SYBR Premix Ex Taq II (Takara Bio, Inc.), according to the
manufacturer’s instructions. The primer sequences and product
lengths are listed in Table I. The
cycle conditions for RT-qPCR were as follows: 40 cycles of 95°C for
30 sec, 95°C for 5 sec, various annealing temperatures for 30 sec,
depending on the target gene (58°C for SET; 60°C for
MDR1; 58°C for MRP1 and 58°C for BCRP),
followed by 60°C for 30 sec for cooling. The mRNA expression levels
in each sample were normalized to that of β-actin.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product size
(bp) |
|---|
| SET |
GGAGGAAGATGAAGAGGCAT |
TGGCTTTATTCTGCGTTTGAC | 242 |
| MDR1 |
GAGCCCATCCTGTTTGACTG |
GCTGCCCTCACAATCTCTTC | 92 |
| BCRP |
AGCAGGGACGAACAATCATC |
GCCAATAAGGTGAGGCTATCA | 82 |
| MRP1 |
AAGGTGGACGAGAACCAGAA |
AACAGGGCAGCAAACAGAAC | 110 |
| β-actin |
TGACGTGGACATCCGCAAAG |
CTGGAAGGTGGACAGCGAGG | 205 |
Western blot analysis
The cells from each treatment group were collected,
at a density of 2×105 cells/ml, and lysed in 120
μl radioimmunoprecipation assay lysis buffer (Beyotime
Institute of Biotechnolgy, Haimen, China), and the protein
concentrations were determined using bicinchoninic acid reagent
(Beyotime Institute of Biotechnolgy). Subsequently, the lysates
were subjected to 10% sodium dodecylsulfate-polyacrylamide gel
electrophoresis (Beyotime Institute of Biotechnolgy) and
transferred onto polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). Prior to incubation with specific antibodies
overnight at 4°C, the blots were blocked with 5% non-fat milk for 4
h at room temperature. The blots were then labeled with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibodies
(1:20,000 dilution), visualized using BeyoECL Plus Detection system
(Beyotime Institute of Biotechnology). All experiments were
performed independently at least three times.
Flow cytometry assay
The cells from each treatment group were double
stained with annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) in the dark for 30 min at room temperature
using an annexin V-FITC/PI apoptosis detection kit, according to
the manufacturer’s instructions. Any cells, which were annexin
V+/PI− were in early apoptosis, whereas cells
in the late apoptotic stage were Annexin
V+/PI+. Analyses of the apoptosis profiles
were performed using Coulter Elite 4.5 Multi cycle software
(Beckman Coulter, Brea, CA, USA). Experiments were performed
independently in triplicate.
Statistical analyses
The values are expressed as the mean ± standard
deviation, unless otherwise indicated. Statistical analyses were
performed using a one-way analysis of variance with SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
SET/PP2A/Akt pathway is significantly
activated in MCF-7/PTX cells
According to a previous study, the activation of the
PI3K/Akt pathway and drug resistance are closely associated
(33). As a key upstream negative
regulator of the PI3K/Akt pathway, PP2A reduces PI3K/Akt pathway
activity and inhibits apoptosis in numerous types of cancer cell
(26). In addition, SET is a
potent physiological inhibitor of PP2A (19). In order to clarify whether the SET,
PP2A and PI3K/Akt signaling pathways are activated in MCF-7/PTX
cells with the development of acquired resistance to paclitaxel.
Western blot analyses were performed to detect the protein
expression levels of the SET, PP2A, PI3K/Akt signaling pathway and
the downstream apoptosis-associated factors of Akt, including Bax
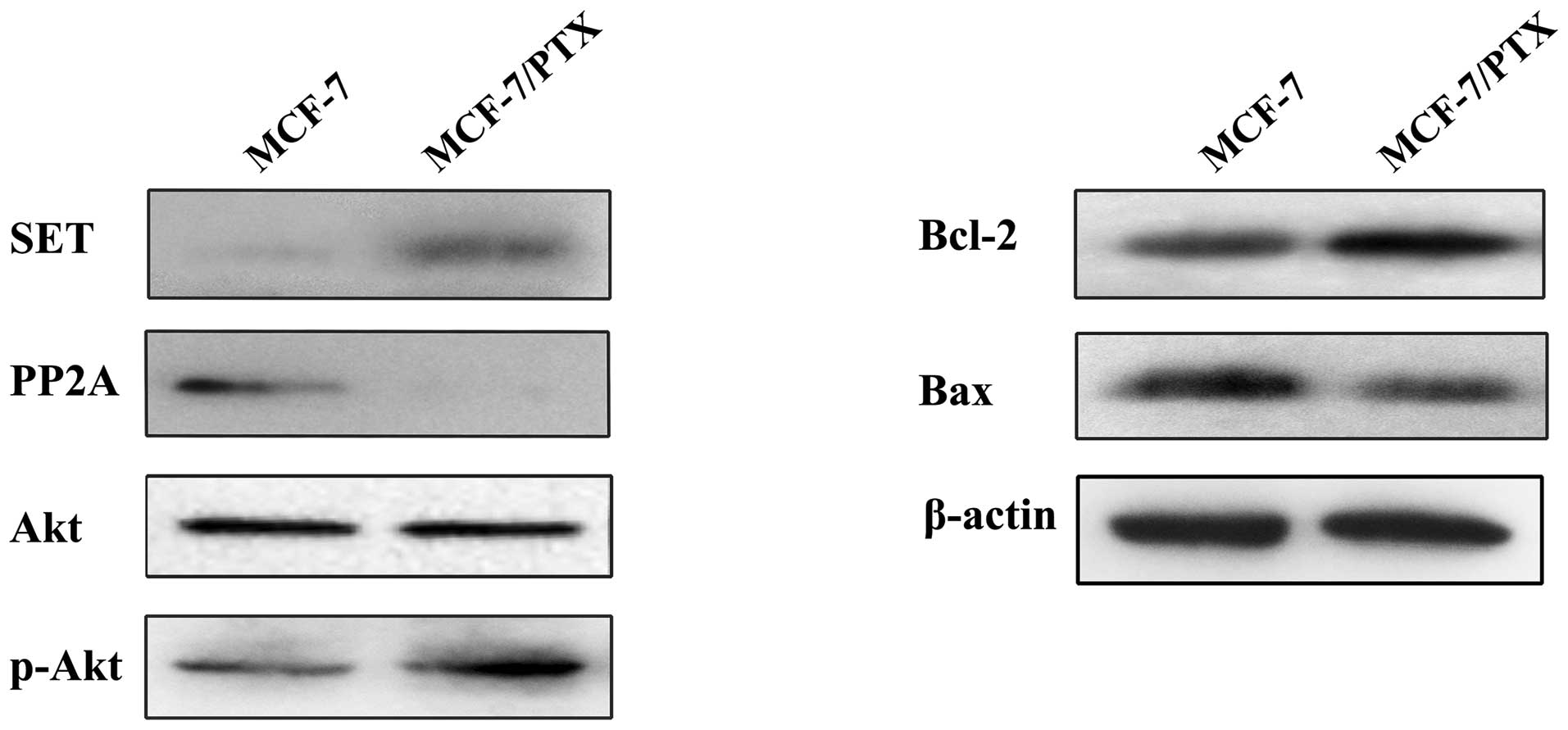
and Bcl-2. As shown in Fig. 1, the
protein expression levels of SET, p-Akt and Bcl-2 were markedly
increased in the MCF-7/PTX cells compared with that of normal MCF-7
cells, whereas the protein expression levels of PP2A and Bax were
reduced. The expression of total Akt was not altered between the
two cell lines (Fig. 1). These
results suggested that the SET/PP2A/Akt pathway may be involved in
paclitaxel resistance in breast cancer.
SET knockdown using siRNAs significantly
attenuates paclitaxel resistance in MCF-7/PTX cells
SET may be associated with paclitaxel resistance in
breast cancer. In order to detect whether the knockdown of
SET affected the sensitivity of MCF-7/PTX cells to
paclitaxel, siRNAs targeting SET were transfected into the
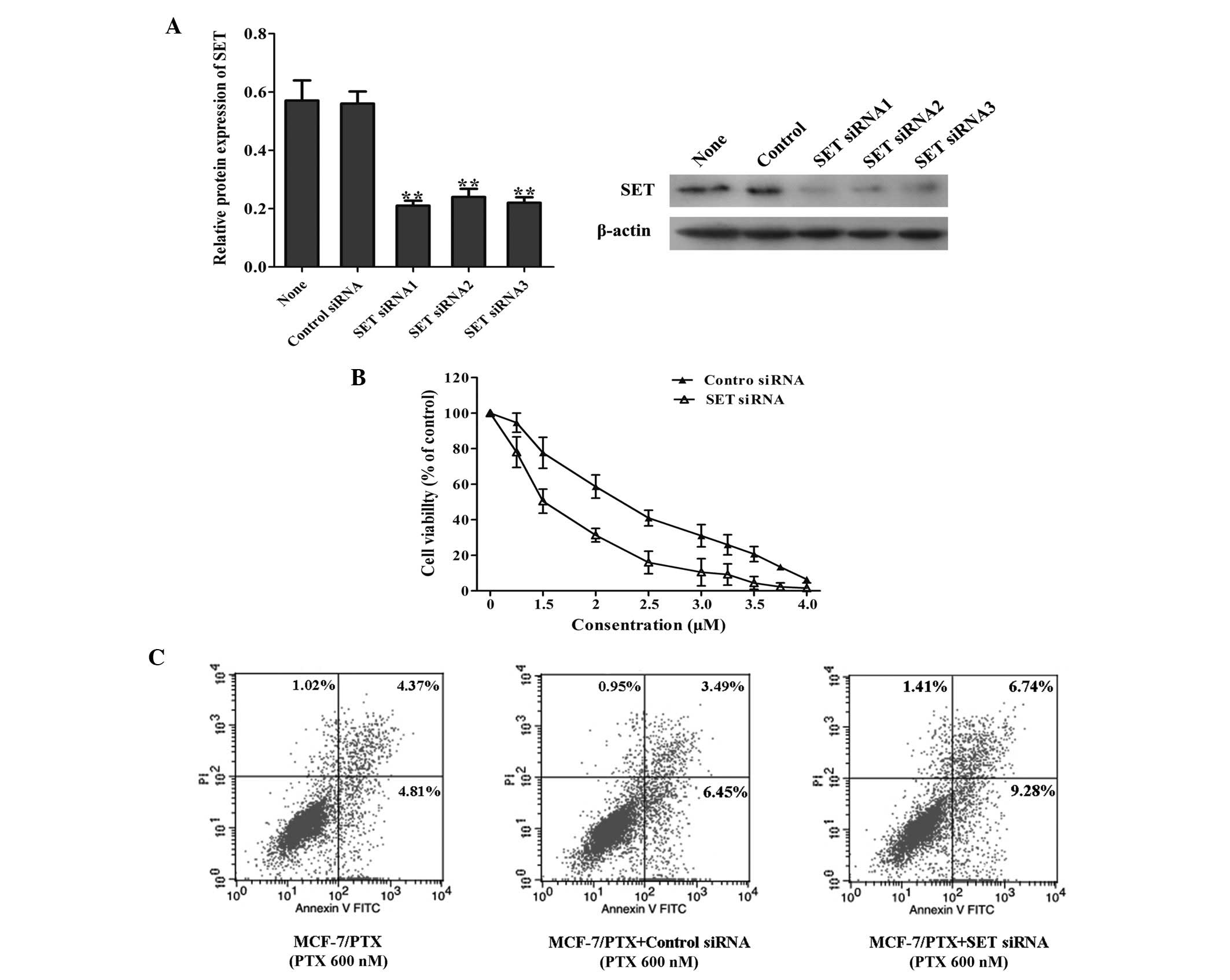
MCF-7/PTX cells, and the transient transfection efficiencies were
quantified using RT-qPCR. As shown in Fig. 2A, at 48 h post-transfection, the
mRNA expression of SET was decreased significantly. In addition, 72
h post-transfection, western blot analysis was used to detect the
protein expression of SET, which was markedly reduced compared with
that of the untransfected and siRNA control-transfected MCF-7 cells
(Fig. 2A). Growth inhibition was
determined using an MTT assay; the results of which revealed that,
following 48 h paclitaxel treatment, knockdown of SET in the
MCF-7/PTX cells sensitized the cells to paclitaxel (Fig. 2B).
The effects of SET knockdown on cell
apoptosis in the MCF-7/PTX cells were evaluated by flow cytometric
analysis. As shown in Fig. 2C,
following 48 h treatment with 600 nM paclitaxel, the apoptosis
rates were 9.18% in the parental MCF-7/PTX cells and 9.94% in the
MCF-7/PTX cells transfected with control siRNA (P>0.05), whereas
the apoptotic cells were 16.02% in MCF-7/PTX SET siRNA cells
(P<0.05). This result demonstrated that MCF-7/PTX cells with
downregulation of SET were more sensitive to paclitaxel compared
with the control group. However, compared with parental MCF-7/PTX
cells, the number of apoptotic cells was markedly increased in the
SET-knockdown MCF-7/PTX cells (Fig. 2C). Overall, these results suggested
that the knockdown of SET contributed to the sensitization
of MCF-7/PTX cells to paclitaxel.
SET knockdown markedly suppresses the
PI3K/Akt signaling pathway
In order to further examine the potential mechanisms
underlying SET knockdown-induced paclitaxel resistance
reversal in MCF-7/PTX cells, western blot analysis was used to
detect the expression levels of ABC transporter proteins and the
activity of the PI3K/Akt signaling pathway in MCF-7/PTX cells
transfected with SET siRNA. The results revealed that the
mRNA and protein levels of classic multidrug resistance proteins,
P-gp, BCRP and MRP1 in the SET-knockdown MCF-7/PTX cells
were significantly reduced compared with those in the untransfected
or control siRNA-transfected MCF-7/PTX cells (Fig. 3A). As shown in Fig. 3B, the SET-knockdown
MCF-7/PTX cells had significantly increased protein expression of
PP2A. These results demonstrated that knockdown of SET led
to the reversal of paclitaxel resistance, which was closely
associated with the expression of PP2A. As an important downstream
factor of PP2A, Akt is important in tumor cell survival and drug
resistance (26). Western blot
analysis revealed that, in the SET-knockdown MCF-7/PTX
cells, the protein expression of p-Akt was significantly reduced
(Fig. 3B). In addition, the
protein expression of Bax was significantly increased, whereas that
of Bcl-2 was decreased (Fig. 3B).
These results suggested that SET mediated cellular apoptosis
through the activation of the PI3K/Akt signaling pathway in the
MCF-7/PTX cells.
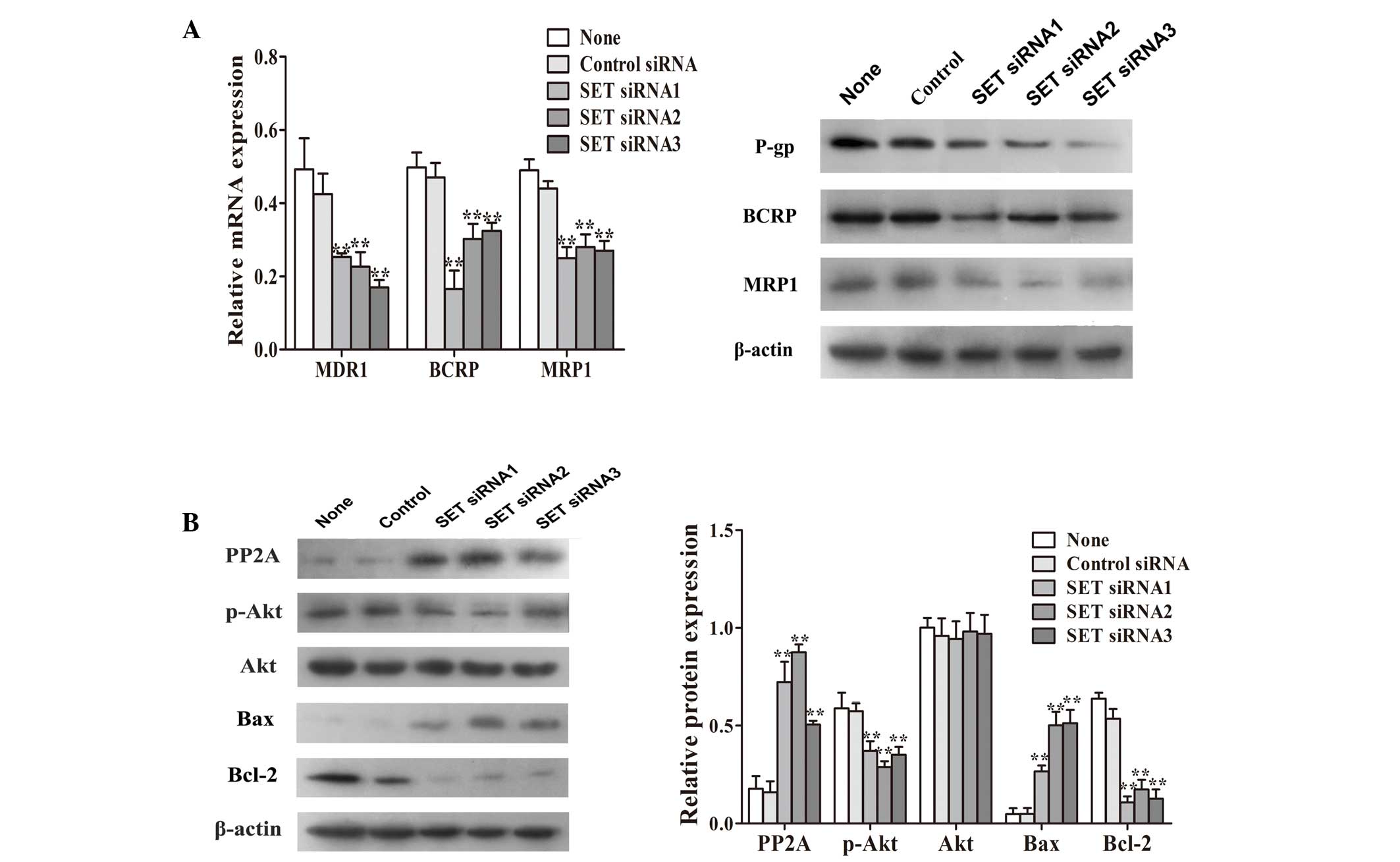 | Figure 3Adenosine triphosphate binding
cassette transporter and PI3K/Akt pathway expression levels were
evaluated using RT-qPCR and western blot analysis. (A) RT-qPCR and
western blot analysis of the gene and protein expression levels of
P-gp, BCRP and MRP1, repsectively in MCF-7/PTX cells transfected
with either SET siRNA or control siRNA. (B) Protein
expression levels of PP2A, p-Akt, Akt, Bax and Bcl-2 in MCF-7/PTX
cells transfected with either SET siRNA or control siRNA
were determined using western blot analysis. β-actin was used as an
internal control. Results are presented as the mean ± standard
deviation of three independent experiments. *P<0.05
and **P<0.01 vs. untransfected cells. PI3K,
phosphatidylinositol 3-kinase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; P-gp,
P-glycoprotein; BCRP, breast cancer resistance protein; MRP1,
multidrug resistance-associated protein 1; siRNA, small
interference RNA; PP2A, protein phosphatase 2A; p−, phosphorylated;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
MCF-7/PTX, paclitaxel-resistant MCF-7 human breast carcinoma
cells. |
Intrinsic cytotoxicity of paeonol in the
MCF-7 and MCF-7/PTX cells
The MCF-7 and MCF-7/PTX cells were treated with
various concentrations of paeonol (15, 30, 60, 120, 250, 320 and
400 μM) for 48 h, and the intrinsic cytotoxicity of paeonol
was determined using an MTT assay. As shown in Fig. 4, paeonol inhibited the growth of
MCF-7 and MCF-7/PTX cells in a dose-dependent manner. According to
the cell viability curves (Fig.
4A), three doses of Paeonol were identified (15, 30 and 60
μM), which had the lowest cytotoxic effects on the MCF-7/PTX
cells and the inhibitory concentration was <5%. Therefore, to
investigate the effect of paeonol on reversal efficiency, with
minimal effects on cell vitality, the concentrations of 15, 30 and
60 μM were selected for use. Verapamil was used as a
positive control. MTT assays were performed and RF values were
determined to examine whether paeonol reversed the resistance of
MCF-7/PTX cells to paclitaxel. As shown in Table II, after 48 h of treatment with
paeonol (15, 30 and 60 μM), the RF values were 3.3, 5.9 and
8.2, respectively. RF>1 indicated that the drug sensitized the
MCF-7/PTX cells to paclitaxel; RF=1 indicated no reversal effect;
and RF<1 indicated that paeonol desensitized the cells to
paclitaxel. The results demonstrated that paeonol significantly
reduced the concentration of paclitaxel required to obtain 50%
growth inhibition, and reversed paclitaxel resistance in the
MCF-7/PTX cells.
 | Table IIEffects of paeonol on the
cytotoxicity of paclitaxel on MCF-7/PTX cells. |
Table II
Effects of paeonol on the
cytotoxicity of paclitaxel on MCF-7/PTX cells.
| Group | Paeonol
(μM) | IC50 of
paclitaxel (nM) | RF |
|---|
| Control | 0 | 2290.87±125.2 | – |
| Paeonol | 15 | 688.90±5.13 | 3.32 |
| 30 | 389.15±2.64 | 5.88 |
| 60 | 280.13±4.15 | 8.18 |
| Verapamil | 10 | 225.28±2.24 | 10.17 |
Paeonol potentiates apoptosis in
MCF-7/PTX cells
In order to investigate the mechanisms of
sensitization induced by paeonol in the MCF-7/PTX cells, the
expression levels of apoptosis-associated proteins were
investigated following paeonol treatment. As shown in Fig. 4B, the results indicated that
paeonol markedly increased the cleavage of full length caspase-9,
caspase-3 and PARP in the MCF-7/PTX cells 72 h after treatment with
paeonol compared with the untreated cells, and this occurred in a
dose-dependent manner. These results suggested that paeonol
promoted cell apoptosis in the MCF-7/PTX cells.
Paeonol suppresses the actvity of the
PI3K/Akt signaling pathway through inhibition of SET
In order to detect whether SET and its downstream
targets were modulated by paeonol, the MCF-7/PTX cells were treated
with 15, 30 and 60 μM paeonol for 48 h. RT-qPCR and western
blot analysis revealed that paeonol significantly decreased the
mRNA and protein expression of SET in the MCF-7/PTX cells, in a
dose-dependent manner (Fig. 5A).
In addition, the mRNA and protein levels of P-gp, MRP1 and BCRP,
which were previously found to be overexpressed in the MCF-7/PTX
cells (Fig. 3A), were also
significantly reduced (Fig. 5A).
To further investigate the potential reversal mechanism of paeonol,
the protein levels of PP2A, p-Akt and Akt were detected by western
blot analysis, following treatment with paeonol in the MCF-7/PTX
cells. The results demonstrated that, 72 h after treatment with 15,
30 and 60 μM paeonol in the MCF-7/PTX cells, the protein
expression of PP2A was significantly increased and those of p-Akt
were significantly decreased in a dose-dependent manner (Fig. 5B). Furthermore, following treatment
with increasing concentrations of paeonol, the protein expression
of Bax was significantly increased and that of Bcl-2 was
significantly decreased (Fig. 5B).
These results demonstrated that paeonol inhibited the PI3K/Akt
pathway, enhancing the sensitivity to paclitaxel, possibly through
down-regulating SET in MCF-7/PTX cells.
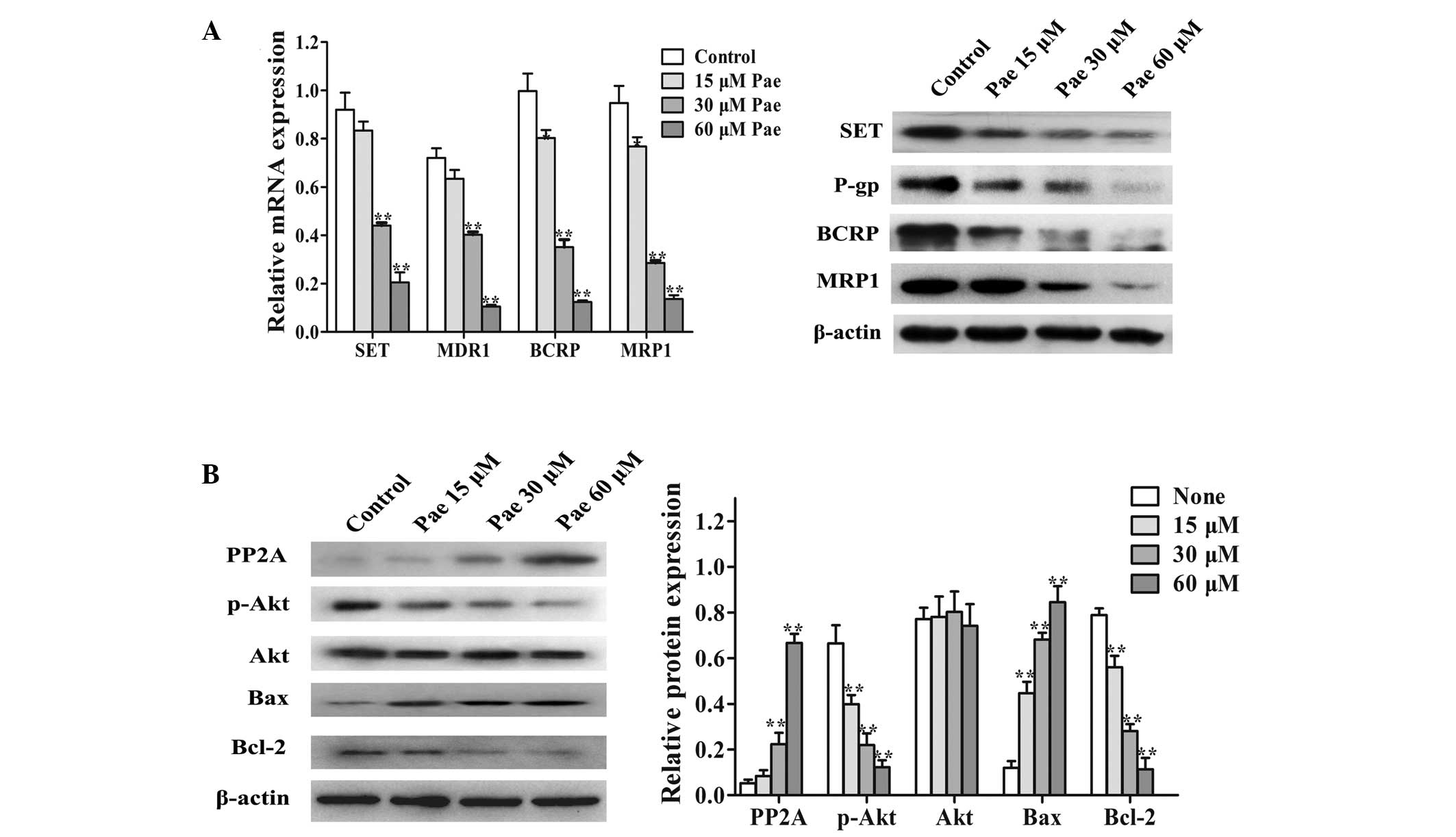 | Figure 5Effects of paeonol on the levels of
SET, adenosine triphosphate binding cassette transporters and
phosphatidylinositol 3-kinase/Akt pathway proteins in MCF-7/PTX
cells. (A) Reverse transcription-quantitative polymerase chain
reaction and western blot analysis of the gene and protein
expression, respectively, of SET, P-gp, BCRP and MRP1 in the
MCF-7/PTX cells treated with paeonol (15, 30 and 60 μM). (B)
Protein expression of PP2A, p-Akt, Akt, Bax and Bcl-2 in MCF-7/PTX
cells treated with various concentration of paeonol for 72 h were
determined by western blot analysis. Values are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05 and **P<0.01 vs. control. Pae,
paeonol, P-gp, P-glycoprotein; BCRP, breast cancer resistance
protein; MDRI, multidrug resistance gene 1; MRP1, multidrug
resistance-associated protein 1; PP2A, protein phosphatase 2A; p−,
phosphorylated; Bcl-2, B cell lymphoma 2; Bax, Bcl-2-associated X
protein; MCF-7/PTX, paclitaxel-resistant MCF-7 human breast
carcinoma cells. |
Discussion
As a novel anticancer drug, paclitaxel is widely
used for chemotherapy in the treatment of breast cancer. However,
drug resistance is one of the primary obstacles leading to the
failure of chemotherapy in breast cancer (34). Previous studies have reported
numerous mechanisms, which may be involved in paclitaxel
resistance, including differences in the expression of drug efflux
pump ABC transporter proteins, tubulin mutation and inhibition of
the apoptotic pathway (4–8). The SET gene was first
identified in patients with acute undifferentiated leukemia, and
its biological function involves histone acetylation, apoptosis,
transcription regulation, nucleosome assembly and other
post-translational modifications (18). In head and neck squamous cell
carcinoma, SET inhibits the expression of its downstream tumor
suppressor factor, PP2A, and activates the PI3K/Akt signaling
pathway (35). Activation of the
PI3K/Akt pathway inhibits cell apoptosis by mediating the
endogenous expression of Bcl-2 and Bax, which are mediators of
apoptosis and are are the most frequently targeted genes regulating
apoptosis in cells (36). In
addition, activation of the PI3K/Akt pathway reduces the expression
levels of P-gp, BCRP, MRP1 and other members of the ABC transporter
superfamily (37–40). These two aspects of the PI3K/Akt
pathway, at least in part, induce the development of drug
resistance.
In the present study, siRNAs were used to knockdown
the expression of SET in MCF-7/PTX cells. This resulted in a
significant increase in the sensitivity of MCF-7/PTX cells to
paclitaxel, including the promotion of apoptosis, decreased
expression of ABC transporter proteins and Bcl-2, and increased
expression of Bax to attenuation chemoresistance in breast cancer
cells. In further mechanistic investigations, the knockdown of
SET increased the expression of downstream PP2A and
significantly reduced the phosphorylation of Akt. These results
suggested that the dysregulation of SET mediated cell apoptosis and
the expression of ABC transporter proteins, eventually leading to
drug resistance by promoting the activity of the PP2A/PI3K/Akt
pathway. In contrast to previous studies on SET, which were only
performed in tumor cells, the present study revealed the expression
patterns of SET in drug-resistant cells, using paclitaxel-resistant
breast cancer cells as a model to elucidate the mechanism of
SET-induced drug resistance. However, whether SET affects the
activation of Akt signaling pathway and induces paclitaxel
resistance in breast cancer primarily by inhibiting PP2A rather
than other downstream factors, including tumor metastasis
suppressor (nm-23-H1) or Ras-related C3botulinum toxin
substrate 1, requires further investigation (26).
Several previous studies have investigated the
antitumor and drug-resistance-reversing effects of paeonol. A study
demonstrated that paeonol induces apoptosis in ovarian cancer cells
by promoting the activation of caspase-3 and inhibiting the protein
expression of suvivin (41).
Paeonol also inhibits tumor cell proliferation and migration
through inhibition of the classic Akt and mitogen-activated protein
kinase signaling transduction pathways (16). Furthermore, paeonol regulates
expression of pro-apoptotic transcription factor
CCAAT-enhancer-binding protein homologous protein in HepG2 cells
(17), and paeonol significantly
regulates the expression of Bax and Bcl-2 in various types of
cancer cells (42). However, no
previous studies have investigated whether the antitumor effects of
paeonol involve SET, or whether paeonol can be applied in the
reversal of paclitaxel resistance in breast cancer.
The present study used RT-qPCR and western blot
analysis to demonstrate that paeonol significantly upregulated the
activated Akt downstream targets, cleaved-caspase 9,
cleaved-caspase 3 and cleaved-PARP, promoting their function in
inducing cell apoptosis. In addition, paeonol decreased the
expression levels of SET and ABC transporters in a dose-dependent
manner, promoting the expression of Bax and suppressing the
expression of Bcl-2, which reversed paclitaxel resistance in breast
cancer cells. In examining the potential reversal mechanism of
paeonol, paeonol treatment led to increased expression of PP2A and
attenuated the phosphorylation of Akt in MCF-7/PTX cells, in a
dose-dependent manner. These results suggested that, by inhibiting
the SET/PP2A/Akt signaling pathway, paeonol induced cell apoptosis
and reduced the expression of ABC transporters, which eventually
reversed paclitaxel resistance in the breast cancer cells. In
contrast with previous antitumor studies, the present study
introduced the potential application of paeonol in the reversal of
paclitaxel resistance in breast cancer cells, and discussed the
reversal mechanism underlying paclitaxel resistance. However, it is
possible that other mechanisms are also involved in
paeonol-regulated apoptosis in drug-resistant cells. There are
numerous key downstream targets of the PI3K/Akt pathway, including
mammalian target of rapamycin and p70S6 kinase, which are also
important in regulating apoptosis (43). Therefore future studies are
required to further investigate the reversal mechanisms of
paeonol.
In conclusion, the present study provided the first
evidence, to the best of our knowledge of SET protein as a
potential molecular target in MCF-7/PTX cells, and confirmed that
SET regulated the PP2A and Akt tumor-suppresor signaling pathways,
including the expression of downstream apoptosis-associated
proteins and ABC transporter proteins. In addition, paeonol
reversed paclitaxel resistance in breast cancer cells by inhibiting
the expression of the SET-mediated PI3K/Akt signaling pathway
proteins in the paclitaxel-resistant cells. Therefore, paeonol may
have potential as a novel reversal agent in the treatment of
paclitaxel-resistant breast cancer. However, the present study was
performed at the cellular level, and animal models and human
clinical trials have yet to be performed.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30973673 and
30973578).
References
|
1
|
Bhoo-Pathy N, Yip CH, Hartman M, et al:
Breast cancer research in Asia: adopt or adapt Western knowledge?
Eur J Cancer. 49:703–709. 2013. View Article : Google Scholar
|
|
2
|
Kim H, Park GS, Lee JE and Kim JH: A
leukotriene B4 receptor-2 is associated with paclitaxel resistance
in MCF-7/DOX breast cancer cells. Br J Cancer. 109:351–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajabnoor GM, Crook T and Coley HM:
Paclitaxel resistance is associated with switch from apoptotic to
autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis.
3:e2602012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carrara L, Guzzo F, Roque DM, et al:
Differential in vitro sensitivity to patupilone versus paclitaxel
in uterine and ovarian carcinosarcoma cell lines is linked to
tubulin-beta-III expression. Gynecol Oncol. 125:231–236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin S, Zeng C, Hari M and Cabral F: Random
mutagenesis of beta-tubulin defines a set of dispersed mutations
that confer paclitaxel resistance. Pharm Res. 29:2994–3006. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Zhao J, Zhang W, et al:
Establishment of paclitaxel-resistant cell line and the underlying
mechanism on drug resistance. Int J Gynecol Cancer. 22:1450–1456.
2012.PubMed/NCBI
|
|
7
|
Bhattacharya R and Cabral F: Molecular
basis for class V beta-tubulin effects on microtubule assembly and
paclitaxel resistance. J Biol Chem. 284:13023–13032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller AV, Hicks MA, Nakajima W,
Richardson AC, Windle JJ and Harada H: Paclitaxel-induced apoptosis
is BAK-dependent, but BAX and BIM-independent in breast tumor. PLoS
One. 8:e606852013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Youns M, Hoheisel JD and Efferth T:
Traditional Chinese medicines (TCMs) for molecular targeted
therapies of tumours. Curr Drug Discov Technol. 7:37–45. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee H, Lee G, Kim H and Bae H: Paeonol, a
major compound of moutan cortex, attenuates Cisplatin-induced
nephrotoxicity in mice. Evid Based Complement Alternat Med.
2013:3109892013.PubMed/NCBI
|
|
11
|
Huang H, Chang EJ, Lee Y, Kim JS, Kang SS
and Kim HH: A genome-wide microarray analysis reveals
anti-inflammatory target genes of paeonol in macrophages. Inflamm
Res. 57:189–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee B, Shin YW, Bae EA, et al:
Antiallergic effect of the root of Paeonia lactiflora and its
constituents paeoniflorin and paeonol. Arch Pharm Res. 31:445–450.
2008. View Article : Google Scholar
|
|
13
|
Ishiguro K, Ando T, Maeda O, et al:
Paeonol attenuates TNBS-induced colitis by inhibiting NF-kappaB and
STAT1 transactivation. Toxicol Appl Pharmacol. 217:35–42. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Zhou L, Hou D, Tang J, Sun J and
Bondy SC: Paeonol increases levels of cortical cytochrome oxidase
and vascular actin and improves behavior in a rat model of
Alzheimer’s disease. Brain Res. 1388:141–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu SP, Sun GP, Shen YX, Peng WR, Wang H
and Wei W: Synergistic effect of combining paeonol and cisplatin on
apoptotic induction of human hepatoma cell lines. Acta Pharmacol
Sin. 28:869–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SA, Lee HJ, Ahn KS, et al: Paeonol
exerts anti-angiogenic and anti-metastatic activities through
downmodulation of Akt activation and inactivation of matrix
metalloproteinases. Biol Pharm Bull. 32:1142–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan L, Song B, Sun G, Ma T, Zhong F and
Wei W: Endoplasmic reticulum stress-induced resistance to
Doxorubicin is reversed by paeonol treatment in human
hepatocellular carcinoma cells. PLoS One. 8:e626272013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
von Lindern M, van Baal S, Wiegant J, Raap
A, Hagemeijer A and Grosveld G: Can, a putative oncogene associated
with myeloid leukemogenesis, may be activated by fusion of its 3′
half to different genes: characterization of the set gene. Mol Cell
Biol. 12:3346–3355. 1992.PubMed/NCBI
|
|
19
|
Canela N, Rodriguez-Vilarrupla A, Estanyol
JM, et al: The SET protein regulates G2/M transition by modulating
cyclin B-cyclin-dependent kinase 1 activity. J Biol Chem.
278:1158–1164. 2003. View Article : Google Scholar
|
|
20
|
Zhang P, Compagnone NA, Fiore C, et al:
Developmental gonadal expression of the transcription factor SET
and its target gene, P450c17 (17alpha-hydroxylase/c17,20 lyase).
DNA Cell Biol. 20:613–624. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wagner S, Weber S, Kleinschmidt MA, Nagata
K and Bauer UM: SET-mediated promoter hypoacetylation is a
prerequisite for coactivation of the estrogen-responsive pS2 gene
by PRMT1. J Biol Chem. 281:27242–27250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madeira A, Pommet JM, Prochiantz A and
Allinquant B: SET protein (TAF1beta, I2PP2A) is involved in
neuronal apoptosis induced by an amyloid precursor protein
cytoplasmic subdomain. Faseb J. 19:1905–1907. 2005.PubMed/NCBI
|
|
23
|
Almeida LO, Goto RN, Pestana CR, et al:
SET overexpression decreases cell detoxification efficiency: ALDH2
and GSTP1 are downregulated, DDR is impaired and DNA damage
accumulates. FEBS J. 279:4615–4628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boqun X, Xiaonan D, Yugui C, et al:
Expression of SET protein in the ovaries of patients with
polycystic ovary syndrome. Int J Endocrinol. 2013:3679562013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cervoni N, Detich N, Seo SB, Chakravarti D
and Szyf M: The onco-protein Set/TAF-1beta, an inhibitor of histone
acetyltransferase, inhibits active demethylation of DNA,
integrating DNA methylation and transcriptional silencing. J Biol
Chem. 277:25026–25031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Switzer CH, Cheng RY, Vitek TM,
Christensen DJ, Wink DA and Vitek MP: Targeting SET/I (2) PP2A
oncoprotein functions as a multi-pathway strategy for cancer
therapy. Oncogene. 30:2504–2513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Dong Q, Hu S, et al: Proteomic
analysis of the proteins that are associated with the resistance to
paclitaxel in human breast cancer cells. Mol Biosyst. 10:294–303.
2014. View Article : Google Scholar
|
|
28
|
Chen LM, Liang YJ, Ruan JW, et al:
Reversal of P-gp mediated multidrug resistance in-vitro and in-vivo
by FG020318. J Pharm Pharmacol. 56:1061–1066. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XD, Sun H and Liu GT:
5-Bromotetrandrine enhances the sensitivity of doxorubicin-induced
apoptosis in intrinsic resistant human hepatic cancer Bel7402
cells. Cancer Lett. 292:24–31. 2010. View Article : Google Scholar
|
|
30
|
Fazly BB, Iranshahi M, Naderinasab M,
Hajian S, Sabeti Z and Masumi E: Evaluation of the effects of
galbanic acid from Ferula szowitsiana and conferol from F.
badrakema, as modulators of multi-drug resistance in clinical
isolates of Escherichia coli and Staphylococcus aureus. Res Pharm
Sci. 5:21–28. 2010.
|
|
31
|
Cai J, Chen S, Zhang W, et al: Salvianolic
acid A reverses paclitaxel resistance in human breast cancer MCF-7
cells via targeting the expression of transgelin 2 and attenuating
PI3 K/Akt pathway. Phytomedicine. 21:1725–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen SY, Hu SS, Dong Q, et al:
Establishment of paclitaxel-resistant breast cancer cell line and
nude mice models and underlying multidrug resistance mechanisms in
vitro and in vivo. Asian Pac J Cancer Prev. 14:6135–6140. 2013.
View Article : Google Scholar
|
|
33
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar
|
|
34
|
Li Z, Tian T, Hu X, et al: Six1 mediates
resistance to paclitaxel in breast cancer cells. Biochem Biophys
Res Commun. 441:538–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leopoldino AM, Squarize CH, Garcia CB, et
al: SET protein accumulates in HNSCC and contributes to cell
survival: antioxidant defense, Akt phosphorylation and AVOs
acidification. Oral Oncol. 48:1106–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rooswinkel RW, van de Kooij B, de Vries E,
et al: Anti-apoptotic potency of Bcl-2 proteins primarily relies on
their stability, not binding selectivity. Blood. 123:2806–2815.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao Z, Zhou J, Luan J, Sheng W, Shen X and
Dong X: Tamoxifen reduces P-gp-mediated multidrug resistance via
inhibiting the PI3K/Akt signaling pathway in ER-negative human
gastric cancer cells. Biomed Pharmacother. 68:179–183. 2014.
View Article : Google Scholar
|
|
38
|
Kazi AA, Gilani RA, Schech AJ, et al:
Nonhypoxic regulation and role of hypoxia-inducible factor 1 in
aromatase inhibitor resistant breast cancer. Breast Cancer Res.
16:R152014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng L, Luo S, Jin C, Ma H, Zhou H and
Jia L: FUT family mediates the multidrug resistance of human
hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell
Death Dis. 4:e9232013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pick A and Wiese M: Tyrosine kinase
inhibitors influence ABCG2 expression in EGFR-positive MDCK BCRP
cells via the PI3K/Akt signaling pathway. Chem Med Chem. 7:650–662.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yin J, Wu N, Zeng F, Cheng C, Kang K and
Yang H: Paeonol induces apoptosis in human ovarian cancer cells.
Acta Histochem. 115:835–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bao MH, Zhang YW and Zhou HH: Paeonol
suppresses oxidized low-density lipoprotein induced endothelial
cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway. J
Ethnopharmacol. 146:543–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ponnurangam S, Standing D, Rangarajan P
and Subramaniam D: Tandutinib inhibits the Akt/mTOR signaling
pathway to inhibit colon cancer growth. Mol Cancer Ther.
12:598–609. 2013. View Article : Google Scholar : PubMed/NCBI
|