Introduction
More than 500,000 hand, foot and mouth disease
(HFMD) cases caused by human enterovirus 71 (EV71) are reported in
the People’s Republic of China annually, including 176 fatal cases
since March 2008 (1). One study
has shown that EV71 and Coxsackie virus A16 (CVA16) are the two
predominant causative agents of HFMD, accounting for >70% of
recent outbreaks (2). EV71
infection is more frequently associated with serious neurological
diseases, such as aseptic meningitis, encephalitis and acute
flaccid paralysis, while CVA16-associated HFMD has a milder outcome
(1). Although the pathogens that
cause HFMD have been confirmed, the association between host cells
and the HFMD viruses, and the mechanism by which the HFMD virus
induces host cell apoptosis remains unclear. An increasing number
of studies have revealed that numerous viral infections, including
hepatitis B virus, hepatitis C virus, human immunodeficiency virus
and sarcoma-associated herpes virus, among others, are closely
associated with the regulation of miRNAs (miRNAs) (3–6).
miRNAs are a class of naturally occurring single-stranded short
21–23 nt non-coding RNAs (7,8) that
exist in a wide range of eukaryotic organisms (7–12).
Each mammalian miRNA prevents the translation of a number of
downstream target mRNAs and ultimately results in the inhibition of
target gene expression (13,14).
Therefore, a shift away from the manipulation of crucial target
genes towards miRNA interference techniques may improve the
effectiveness of current gene-based diagnostic and therapeutic
strategies (9). Although the
majority of miRNA studies focus on the growth and differentiation
of stem cells (15–17), tumorigenesis (18,19)
and other pathological processes (13,14),
few studies have focused on the role of miRNAs in the interaction
between EV71 and human neurons. Thus far, certain studies have
reported that miRNAs are involved in the host response to EV71
infection. Cui et al (1)
used a deep sequencing approach to determine that 64 miRNAs in host
cells exhibited >2-fold expression level changes in response to
EV71 infection. Wen et al (20) found that miRNA-23b in host cells
inhibited EV71 replication through downregulation of the EV71 viral
capsid protein (VPl). Zheng et al (21) showed that miRNA-296-5p suppressed
EV71 replication in host cells by inhibiting two potential targets
(2,115-2,135 nt and 2,896-2,920 nt) located in the EV71 genome.
Furthermore, Li et al (22)
demonstrated that the members of the miRNA-548 family, including
miR-548b-5p, miR-548c-5p, miR-548i, miR-548j and miR-548n,
downregulate the host antiviral response during EV71 or vesicular
stomatitis virus infection via direct targeting of interferon-λ1.
In addition, Cui et al (2)
compared host serum miRNA levels in patients with HFMD caused by
EV71 and CA16 as well as in healthy individuals. In the sera of
patients with the enteroviral infections, 102 miRNAs were
upregulated and 26 miRNAs were downregulated. Therefore, altered
circulating miRNA profiles have been observed in patients with
microbial infections. These results enhance the understanding of
miRNA involvement resulting from EV71 infection in HFMD and offer
insight into potential prevention and treatment approaches.
Let-7 is a well-known miRNA known to regulate cell
cycle and development, that is underexpressed in various types of
cancer (23). Restoration of
normal let-7 expression levels has been demonstrated to inhibit
cancer growth by targeting various oncogenes and inhibiting the key
regulators of several mitogenic signaling pathways (23–26).
Yu et al (26) found that
let-7 suppressed self-renewal and tumorigenicity in breast cancer
cells by reducing H-RAS and high-mobility group AT-hook (HMGA) 2
expression levels. Furthermore, Schultz et al (24) reported that let-7b, a member of the
let-7 miRNA family, interfered with the proliferation and growth of
primary malignant melanoma cells by targeting and suppressing
important cell cycle molecules, such as cyclin D (CCND1). In
addition, Dangi-Garimella et al (25) revealed that elevated let-7
expression levels inhibited HMGA2 expression and suppressed
metastasis in breast cancer cells. In view of this evidence,
whether EV71 stimulates endogenous miRNA let-7 expression to
inhibit growth and proliferation, and induce apoptosis in host
cells was investigated in the present study.
Materials and methods
Cell culture and viral infection
The SH-SY5Y human neuroblastoma cell line, which was
purchased from the Cell Resources Center of Shanghai Institute of
Life Science, Chinese Academy of Sciences (Shanghai, China) was
grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum (FBS), penicillin (100 U/ml),
streptomycin (100 U/ml) and 2 mM L-glutamine (all purchased from
Hyclone, Logan, USA). The SH-SY5Y cells were at 37°C in a
humidified atmosphere of air containing 5% CO2. The
prototype EV71 was donated by Dr Weihao Li (Handan Municipal Center
for Disease Prevention and Control, Hubei, China). The SH-SY5Y
cells were infected with EV71 virus as previously described
(1,27). Briefly, SH-SY5Y cells were grown to
80% confluence prior to infection. For virus absorption, the cells
were infected for 60 min with EV71 at a multiplicity of infection
(MOI) of 1, 50% tissue culture infectious doses, in serum-free
medium. Following infection, the cells were washed with
phosphate-buffered saline (PBS) and maintained at 37°C in DMEM
medium with 2% FBS.
Reverse transcription (RT) and
quantitative polymerase chain reaction (qPCR) analysis
Total RNA was isolated from each cell type using
TRIzol® Reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The RNA
samples were subsequently treated with DNase I (Sigma-Aldrich, St.
Louis, MO, USA), quantified and reverse-transcribed to cDNA using
the ReverTra Ace-α First Strand cDNA Synthesis kit (Toyobo Co.,
Ltd., Osaka, Japan). The qPCR was conducted using a RealPlex4
real-time PCR detection system (Eppendorf, Hamburg, Germany) with
SYBR Green Realtime PCR Master mix (Toyobo Co., Ltd.). The qPCR
amplification was performed over 40 cycles of denaturation at 95°C
for 15 sec and annealing at 58°C for 45 sec, and target cDNA was
measured using the relative quantification method. The comparative
threshold cycle (Ct) calculation was used to determine the relative
gene expression levels, normalized to 18S rRNA. For each sample,
the Ct values were normalized using the formula: ΔCt = Ct_genes -
Ct_18S RNA. The relative expression levels were calculated using
the formula: ΔΔCt = ΔCt_all_groups - ΔCt_blank control_group. The
values used to plot relative gene expression levels were calculated
using 2−ΔΔCt. The primers used for the cDNA
amplification were as previously described (15).
Transmission electron microscopy (TEM)
analysis
TEM analysis was conducted as previously described
(28). Briefly, each group of
cells was fixed in 1% glutaraldehyde 1 h prior to post-fixing in 1%
osmium tetroxide for 1 h, then the cells were dehydrated in an
acetone dilution series and embedded in resin 12 (Ted Pella, Inc.,
Redding, CA, USA). Transverse sections (900 nm) were stained with
toluidine blue (Sigma-Aldrich, St. Louis, MO, USA) and examined
using a Nikon Eclipse 80i microscope (Nikon Instruments, Inc.,
Melville, NY, USA). Ultra-thin sections (70 nm) were stained with
1% uranyl acetate and 1% lead citrate, and examined using a
JEM-1230 (JEOL, Tokyo, Japan) transmission electron microscope.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
TUNEL assay was performed as previously described
(27,29). Briefly, each group of cells treated
as indicated was fixed with 4% paraformaldehyde, rinsed with PBS,
then permeabilized with 0.1% Triton X-100 for fluorescein
isothiocyanate (FITC)-end-labeling the fragmented DNA of apoptotic
SH-SY5Y cells using a TUNEL cell apoptosis detection kit (Beyotime
Institute of Biotechnology, Shanghai, China). The FITC-labeled
TUNEL-positive cells were imaged under a fluorescent microscope
(DMI3000; Leica, Allendale, NJ, USA) using 488 nm excitation and
530 nm emission.
Northern blotting
Northern blotting was conducted as previously
described (17). For all cell
treatment groups, total RNA was isolated from each cell type using
TRIzol reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. The RNA samples were subsequently
treated with DNase I (Sigma-Aldrich), and 20 μg total RNA
was analyzed on a 7.5 M urea, 12% polyacrylamide denaturing gel,
then transferred to a Hybond N+ nylon membrane (Amersham, Freiburg,
Germany). The membranes were cross-linked using ultraviolet light
for 30 s at 1,200 mJ/cm2 and hybridized to the let-7b
antisense starfire probe (GenScript, Piscataway, NJ, USA), for the
detection of 21 nt let-7b fragments, according to the
manufacturer’s instructions. Following washing, the membranes were
exposed to Kodak XAR-5 film for 20–40 h (Sigma-Aldrich Chemie GmbH,
Steinheim, Germany). A human U6 snRNA probe, 5′-G
CAGGGGCCATGCTAATCTTCTCTGTATCG-3′, served as a positive control. The
exposure time was 15–30 min.
Western blotting
Total proteins extracts of each cell treatment group
were resolved by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride (Millipore, Billerica, MA, USA) membranes. The membranes
were blocked with Tris-buffered saline containing 0.3% Tween-20
(TBST) and 5% normal goat serum at 37°C for 60 min. Subsequent to
blocking, the membranes were washed four times for 15 min with TBST
at room temperature and then incubated with the following primary
polyclonal antibodies: Rabbit anti-human EV71 (1:1,000; Millipore),
rabbit anti-human CDK4, rabbit anti-human caspase-3, rabbit
anti-human active caspase-3, rabbit anti-human Bcl-2, rabbit
anti-human BAX and rabbit anti-GAPDH (1:1,000; all Cell Signaling
Technology, Inc., Danvers, MA, USA). The membranes were washed four
times for 15 min with TBST at room temperature. Following washing,
the membranes were incubated at room temperature with
peroxidase-linked goat anti-rabbit IgG secondary antibody (1:1,000;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h.
Protein bands were visualized by autoradiography, using an enhanced
chemiluminescence kit (Pierce Biotechnology, Inc., Rockford, IL,
USA).
Flow cytometric (FCM) analysis of the
cell cycle by propidium iodide (PI) staining
A total of 3×105 cells per well were
seeded in 6-well plates and cultured until 85% confluent. Each
group of cells was washed with PBS three times, then collected by
centrifugation (Allegra X-22R; Beckman Coulter, Miami, FL, USA) at
1,000 × g for 5 min. The cell pellets were subsequently resuspended
in 1 ml PBS, fixed in 70% ice-cold ethanol and stored in a freezer
for >48 h at −20°C. Prior to flow cytometric analysis, the fixed
cells were centrifuged, washed twice with PBS and resuspended in PI
staining solution (Sigma-Aldrich Chemie GmbH) containing 50
μl/ml PI and 250 μg/ml RNase A (Sigma-Aldrich Chemie
GmbH). The cell suspension was incubated for 30 min at 4°C in the
dark, and analyzed by FACS (FCM-500; Beckman Coulter). A total of
20,000 events were recorded for analysis using CellQuest™ software
(BD Biosciences, Franklin Lakes, NJ, USA).
MTT assay of cell proliferation
Each group of cells was seeded at 2×103
cells per well in 96-well plates and cultured in DMEM supplemented
with 10% FBS at 37°C with 5% CO2, until 85% confluent.
MTT reagent (5 mg/ml; Sigma-Aldrich Chemie GmbH) was added to the
cell medium at different time points and incubated at 37°C for an
additional 4 h. The reaction was terminated by adding 150 μl
dimethylsulfoxide (Sigma-Aldrich Chemie GmbH) per well and the
cells were lysed for 15 min, with the plates gently agitated every
5 min. The absorbance values were determined using an enzyme-linked
immunosor-bent assay reader (Model 680; Bio-Rad, Hercules, CA, USA)
at 490 nm.
2′-O-Me RNA transfected
The 2′-O-Me RNA oligonucleotide, targeting silenced
miRNA let-7b, was synthesized by Shanghai GenePharma Co.,Ltd.,
(Shanghai, China). The SH-SY5Y cells were transfected with 20
μM 2′-O-Me using Lipofectamine 2000 (Invitrogen Life
Technologies), according to the manufacturer’s instructions.
Statistical analysis
Each experiment was performed at least three times.
The data are presented as the means ± standard error of the mean,
where applicable, and the differences were evaluated using
Student’s t-test with SPSS 18.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). A P<0.05 was considered to indicate a
statistically significant difference.
Results
EV71 induces SH-SY5Y human neuroblastoma
cell apoptosis
To determine whether EV71 suppressed SH-SY5Y cell
proliferation, an MTT assay was performed at 0, 12, 24, 48 and 72 h
after infection (Fig. 1). At the
12–72 h time points, the wild-type (WT) and PBS groups were
significantly less susceptible to the proliferation inhibitory
effect of EV71 than the EV71 group (Table I). Furthermore, EV71 suppressed
SH-SY5Y proliferation in a time-dependent manner, but no
significant differences were identified between the WT and the PBS
group at any time point. In addition, cell morphology confirmed
that the original SH-SY5Y cells were healthy, and there was no
indication of apoptosis or necrosis prior to EV71 infection
(Fig. 1). However, 48 h after EV71
infection, SH-SY5Y cells exhibited features typical of apoptosis:
The cells were round and no longer adherent, and fewer cell
pseudopods were observed. In addition, TEM analysis revealed
increased numbers of intracellular vacuoles in SH-SY5Y cells 48 h
post EV71 infection, compared with the control cells at this time
point. In addition, typical EV71 virus particles within or
surrounding the SH-SY5Y cells were identified (Fig. 1). Furthermore, TUNEL assay revealed
a strong FITC hybridization signal in the EV71 infection group at
48 h, that was not evident in the non-infected cells (Fig. 1). These results demonstrate that
the EV71 virus induced SH-SY5Y apoptosis.
 | Figure 1Enterovirus (EV)71 inhibited SH-SY5Y
human neuroblastoma cell proliferation. (A) An MTT proliferation
assay was used to determine the ability of EV71 to suppress SH-SY5Y
cell proliferation at 0, 12, 24, 48 and 72 h after infection.
Between 12 and 72 h, EV71-infected cells exhibited significant
proliferation inhibition compared with control cells
(**P<0.01 and #P>0.05 vs. wild-type
(WT) group; n=3). (B) Cell morphological analysis confirmed that 48
h after EV71 virus infection, SH-SY5Y cells exhibited signs typical
of apoptosis (round, no longer adherent, fewer cell pseudopods).
Magnification, ×200. (C) Transmission electron scanning analysis
revealed EV71 virus particles within or surrounding SH-SY5Y cells
(scale bar=1 μm; magnification, ×10,000). (D) Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling assay
showed a strong fluorescein isothiocyanate hybridization signal in
the EV71-infected group at 48 h, but not in non-infected SH-SY5Y
cells (0 h; magnification, ×200). |
 | Table IAnalysis of SH-SY5Y cell proliferation
inhibition rate following EV71 infection, by MTT assay. |
Table I
Analysis of SH-SY5Y cell proliferation
inhibition rate following EV71 infection, by MTT assay.
| Time (h) | EV71 (MOI=1; %) | PBS (%) | WT (%) |
|---|
| 0 | 1.67±1.68 | 2.32±0.60 | 1.15±1.54 |
| 12 | 29.57±5.75** | 3.90±1.54 | 5.65±2.64 |
| 24 | 47.36±2.52** | 3.88±2.73 | 1.32±1.26 |
| 48 | 48.91±12.54** | 5.22±2.90 | 7.27±2.98 |
| 72 | 61.68±12.19** | 5.37±2.69 | 3.97±0.96 |
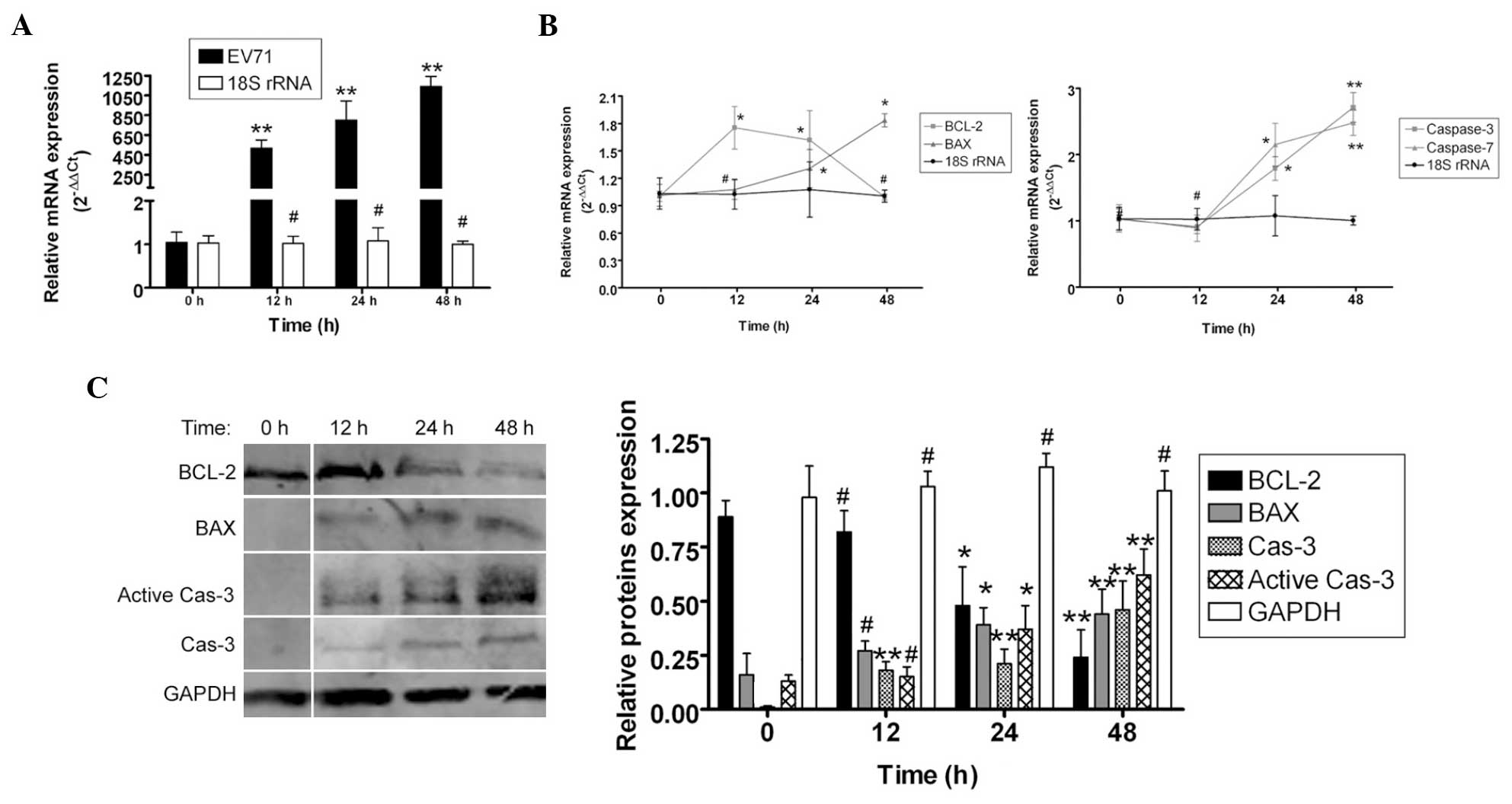
To determine how EV71 virus induced apoptosis in the
SH-SY5Y cells, qPCR and western blotting were used to measure the
expression levels of apoptosis-related factors. qPCR revealed that
the mRNA expression levels of the apoptosis inhibitor Bcl-2 were
markedly lower in the SH-SY5Y cells 12–48 h post EV71 infection,
compared with the non-infected cells (0 h). By contrast, the mRNA
expression levels of the apoptosis-promoting factors Bax, caspase-7
and caspase-3 were markedly higher in SH-SY5Y cells 12–48 h post
EV71 infection, compared with non-infected cells (0 h). In
addition, western blot analysis found that the Bcl-2 protein
expression levels were significantly reduced in SH-SY5Y cells 12–48
h post EV71 infection compared with non-infected cells (0 h).
Furthermore, the Bax, caspase-3 and active caspase-3 protein
expression levels were significantly elevated in SH-SY5Y cells
12–48 h post EV71 infection compared with non-infected cells
(Fig. 2). These data indicate that
EV71 virus stimulated the expression of apoptosis-related proteins
to induce apoptosis.
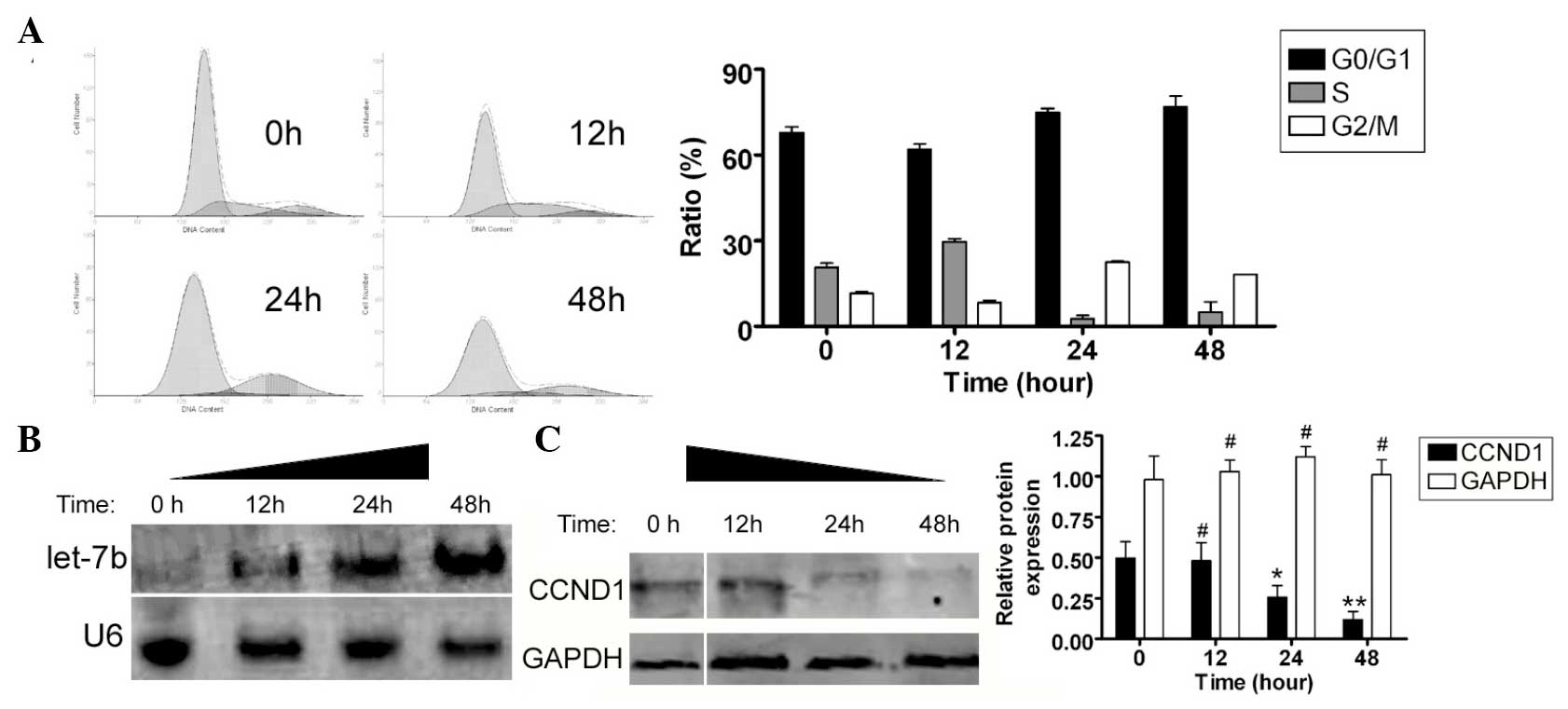
EV71 stimulates endogenous miRNA let-7b
and inhibits CCND1 expression
FCM was used to determine whether EV71 influenced
the SH-SY5Y cell cycle. Subsequent to co-culture with the EV71
virus, the SH-SY5Y cells underwent significant cell cycle arrest.
Compared with non-infected cells, a greater number of SH-SY5Y cells
were arrested in the G2/M phase and the percentage of cells in the
S phase was significantly lower (Fig.
3A). These results suggest that EV71 significantly affected
cell cycle regulation in the SH-SY5Y cells. Northern and western
blotting were used to determine whether the expression levels of
endogenous miRNA let-7b were different between EV71 virus-infected
SH-SY5Y cells and control cells. The northern blot analysis
revealed a marked let-7b hybridization signal in the EV71-infected
group, compared with non-infected SH-SY5Y cells (Fig. 3B). Furthermore, western blotting
confirmed that CCND1 protein expression levels were significantly
reduced in the EV71-infected SH-SY5Y cells at each time point
(0.481±0.192, 0.257±0.123 and 0.119±0.085, respectively), compared
with the non-infected cells (0.496±0.178; Fig 3C). These data indicate that the
expression levels of endogenous miRNA let-7b were significantly
higher and those of CCND1 protein were significantly lower in the
SH-SY5Y cells following EV71 infection.
Inhibiting endogenous miRNA let-7b
expression levels with 2′-O-Methyl-RNA maintains SH-SY5Y
proliferation
To confirm that EV71 induces host cell SH-SY5Y
apoptosis by influencing let-7b, 2′-O-Methyl-RNA was used to
inhibit endogenous let-7b expression levels. Northern blot analysis
revealed significantly increased let-7b hybridization in the
EV71-infected SH-SY5Y cells (mock group). However, a significant
reduction in let-7b hybridization signal was observed in the
EV71-infected 2′-O-Me group and in the SH-SY5Y cells without viral
infection (WT group; Fig. 4A). In
addition, western blot analysis confirmed that the protein
expression levels of CCND1 were significantly increased in the WT
and the 2′-O-Me groups, compared with the mock group (Fig. 4B). However, the protein expression
levels of caspase-3 and active caspase-3 were significantly reduced
in the WT and 2′-O-Me groups, compared with the mock group
(Fig. 4B). In addition, compared
with the mock group, the FCM results revealed that the cell cycle
of the 2′-O-Me group was modified and the percentage of cells in
G2/M phase was markedly reduced (Fig.
4C).
Discussion
Thus far, miRNAs have been demonstrated to be
important in the complicated interactions between virus and host in
HFMD. The majority of reports indicate that miRNAs inhibit EV71
replication in host cells by downregulating the expression levels
of viral core proteins (1,2,17,20–22).
However, the present study was the first, to the best of our
knowledge, to analyze the role of EV71 in stimulating the
endogenous miRNAs of host cells in order to facilitate the
induction of host cell apoptosis following infection. Typically,
EV71 transfers genetic material into host cells through cell
membrane receptors, then utilizes host cell machinery to assist
viral processing (1,2), including replication and packaging,
prior to producing viral particles (1,2).
Simultaneously, host cells gradually undergo necrosis or apoptosis
due to cell destruction by EV71. However, how the EV71 virus
induces host cell apoptosis following infection remains unclear.
Several studies have reported that host cellular miRNAs inhibit
EV71 infection and replication, and that virus mutations escape
suppression by cellular miRNAs (20–22).
These findings suggest that since inhibition of viral replication
and packaging of miRNAs occurs in host cells, certain miRNAs in
host cells are assisting viral processing. The preliminary results
of the present study suggest that when EV71 infected SH-SY5Y cells,
the expression levels of endogenous, cellular let-7b were
significantly increased. In addition, a number of studies have
demonstrated that let-7b initiates cell cycle arrest and inhibits
cell proliferation by targeting the expression of cell
cycle-related proteins (23–26).
Thus, as determined by these data, EV71 is hypothesized to inhibit
host cell growth and promote apoptosis through stimulation of host
let-7b expression.
In the present study, the SH-SY5Y cell line served
as model host cells to determine injury following EV71 infection.
EV71 infection was observed to undermine mitochondrial stability in
these cells. Simultaneously, EV71 arrested cell cycle progression
and subsequently inhibited the proliferation of host cells. EV71
stimulated the overexpression of apoptosis-related genes and
induced host cell apoptosis. Conversely, through analysis of
epigenetic regulation of EV71 in host cells, EV71 infection was
found to stimulate endogenous miRNA let-7b expression; let-7b
suppressed the expression of the target gene CCND1 and
induced normal cell cycle arrest in host cells. To further confirm
that EV71 induced cell cycle arrest through let-7b, 2′-O-Methyl-RNA
oligonucleotides were used to inhibit endogenous let-7b expression
levels. The assay results revealed that following EV71 exposure,
cell cycle arrest in the 2′-O-Methyl-RNA transfected group cell was
significantly reduced compared with that in the mock-transfected
cells. In conclusion, the present study demonstrates that EV71
inhibits growth and proliferation of host cells through stimulating
the expression of miRNA let-7b. Furthermore, the findings suggest
that miRNA let-7b is a potential candidate for antiviral therapy in
HFMD.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81202811, 31100139,
31140037), the Shanghai Municipal Health Bureau Fund (grant nos.
20124320), the National Natural Science Foundation of China (grant
no. 31140037) and the China Postdoctoral Science Foundation (no.
2014M550250).
Abbreviations:
|
HFMD
|
hand, foot and mouth disease
|
|
CVA16
|
Coxsackie virus A16
|
|
miRNA
|
microRNA
|
|
HMGA
|
H-RAS and high-mobility group
AT-hook
|
References
|
1
|
Cui L, Guo X, Qi Y, et al: Identification
of miRNAs involved in the host response to enterovirus 71 infection
by a deep sequencing approach. J Biomed Biotechnol.
2010:4259392010. View Article : Google Scholar
|
|
2
|
Cui L, Qi Y, Li H, et al: Serum miRNA
expression profile distinguishes enterovirus 71 and coxsackievirus
16 infections in patients with hand-foot-and-mouth disease. PLoS
One. 6:e270712011. View Article : Google Scholar
|
|
3
|
Hu W, Wang X, Ding X, et al: MiRNA-141
represses HBV replication by targeting PPARA. PLoS One.
7:e341652012. View Article : Google Scholar
|
|
4
|
Houzet L, Klase Z, Yeung ML, et al: The
extent of sequence complementarity correlates with the potency of
cellular miRNA-mediated restriction of HIV-1. Nucleic Acids Res.
40:11684–11696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Narbus CM, Israelow B, Sourisseau M, et
al: HepG2 cells expressing miRNA miR-122 support the entire
hepatitis C virus life cycle. J Virol. 85:12087–12092. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Umbach JL and Cullen BR: In-depth analysis
of Kaposi’s sarcoma-associated herpesvirus miRNA expression
provides insights into the mammalian miRNA-processing machinery. J
Virol. 84:695–703. 2009. View Article : Google Scholar
|
|
7
|
Sumazin P, Yang X, Chiu HS, et al: An
extensive miRNA-mediated network of RNA-RNA interactions regulates
established oncogenic pathways in glioblastoma. Cell. 147:370–381.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poulton JS, Huang YC, Smith L, et al: The
miRNA pathway regulates the temporal pattern of Notch signaling in
Drosophila follicle cells. Development. 138:1737–1745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei P, Li Y, Chen X, Yang S and Zhang J:
Microarray based analysis of miRNA expression in rat cerebral
cortex after traumatic brain injury. Brain Res. 1284:191–201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MiRNAs: genomics, biogenesis,
mechanism, and function. Cell. 116:281–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoo AS, Sun AX, Li L, et al:
MiRNA-mediated conversion of human fibroblasts to neurons. Nature.
476:228–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai Y, Diao Z, Sun H, et al: MiRNA-155 is
involved in the remodelling of human-trophoblast-derived
HTR-8/SVneo cells induced by lipopolysaccharides. Hum Reprod.
26:1882–1891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He L and Hannon GJ: MiRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El Ouaamari A, Baroukh N, Martens GA, et
al: miR-375 targets 3′-phosphoinositide-dependent protein kinase-1
and regulates glucose-induced biological responses in pancreatic
beta-cells. Diabetes. 57:2708–2717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu T, Shen D, Xing S, et al: Attenuation
of exogenous angiotensin II stress-induced damage and apoptosis in
human vascular endothelial cells via miRNA-155 expression. Int J
Mol Med. 31:188–196. 2013.
|
|
16
|
Liu T, Cheng W, Huang Y, et al: Human
amniotic epithelial cell feeder layers maintain human iPS cell
pluripotency via inhibited endogenous miRNA-145 and increased Sox2
expression. Exp Cell Res. 318:424–434. 2011. View Article : Google Scholar
|
|
17
|
Liu T, Huang Y, Liu J, et al: MiRNA-122
influences the development of sperm abnormalities from human
induced pluripotent stem cells by regulating TNP2 expression. Stem
Cells Dev. 22:1839–1850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng W, Liu T, Wan X, Gao Y and Wang H:
MiRNA-199a targets CD44 to suppress the tumorigenicity and
multidrug resistance of ovarian cancer-initiating cells. FEBS J.
279:2047–2059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin W, Ren Q, Liu T, Huang Y and Wang J:
MiRNA-155 is a novel suppressor of ovarian cancer-initiating cells
that targets CLDN1. FEBS Lett. 587:1434–1439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen BP, Dai HJ, Yang YH, Zhuang Y and
Sheng R: MiRNA-23b inhibits enterovirus 71 replication through
downregulation of EV71 VPl protein. Intervirology. 56:195–200.
2013. View Article : Google Scholar
|
|
21
|
Zheng Z, Ke X, Wang M, et al: Human miRNA
hsa-miR-296-5p suppresses enterovirus 71 replication by targeting
the viral genome. J Virol. 87:5645–5656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Xie J, Xu X, et al: MiRNA-548
down-regulates host antiviral response via direct targeting of
IFN-lambda1. Protein Cell. 4:130–141. 2013. View Article : Google Scholar
|
|
23
|
Barh D, Malhotra R, Ravi B and Sindhurani
P: MiRNA let-7: an emerging next-generation cancer therapeutic.
Curr Oncol. 17:70–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schultz J, Lorenz P, Gross G, Ibrahim S
and Kunz M: MiRNA let-7b targets important cell cycle molecules in
malignant melanoma cells and interferes with anchorage-independent
growth. Cell Res. 18:549–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dangi-Garimella S, Yun J, Eves EM, et al:
Raf kinase inhibitory protein suppresses a metastasis signalling
cascade involving LIN28 and let-7. EMBO J. 28:347–358. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu F, Yao H, Zhu P, et al: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen TC, Lai YK, Yu CK and Juang JL:
Enterovirus 71 triggering of neuronal apoptosis through activation
of Abl-Cdk5 signalling. Cell Microbiol. 9:2676–2688. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng Z, Sun X, Wang G, Liu H and Zhu J:
LBD29 regulates the cell cycle progression in response to auxin
during lateral root formation in Arabidopsis thaliana. Ann Bot.
110:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Tang X, Li Y, et al:
20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in
pulmonary artery smooth muscle cells. Eur J Pharmacol. 588:9–17.
2008. View Article : Google Scholar : PubMed/NCBI
|