Introduction
Malignant glioma is the most common type of primary
brain tumor (1), which is
responsible for ~1/3 of central nervous system intrinsic neoplasms
in adults and children (2–4). Gliomas are aggressive tumors that
possess a tendency to invade the surrounding brain tissue. In
addition, glioma cells are proliferate rapidly and are often
resistant to common forms of treatment, including surgical
resection, chemotherapy and radiotherapy (5,6).
However, the factors that govern the progression and invasion of
glioma are currently not well understood.
N-myc downstream-regulated gene 1 (NDRG1) was
initially identified as gene that was upregulated in N-myc knockout
mouse embryos, and it was able to be repressed by N-myc and c-myc
(7,8). Since its initial identification,
NDRG1 has been isolated by numerous laboratories under various
physiological conditions (9,10).
NDRG1 is a 43-kD protein, which is comprised of 394 amino acids and
is known to be highly conserved among multicellular organisms.
NDRG1 is predominantly cytosolic and is ubiquitously expressed in
tissues in response to cellular stress signals (11,12).
There are currently known to be four members of the human NDRG
family: NDRG1, NDRG2, NDRG3 and NDRG4. The amino acid homology
among each member is ~57–65% (13,14).
mRNA and protein expression of NDRG1 was found to be
decreased in primary cancer and metastatic cells, including colon
(15,16), prostate (17,18),
breast (18) and esophageal
squamous cancer (19), as well as
glioma (20), as compared with
that in normal cells. In addition, NDRG1 expression has been shown
to be upregulated in mouse skin carcinomas and in hyperplastic skin
epithelium, as compared with that in normal mouse skin (13). Numerous studies have demonstrated
that NDRG1 is associated with cellular growth (21–23),
differentiation (13,24), tumorigenesis (25), metastasis and poor clinical outcome
(26–28) in certain tumors.
Phosphoinositide 3-kinase (PI3K)/Akt signaling is an
important survival/proliferative pathway involving various growth
factors, cytokines, and activation of receptors (29). Akt is upregulated in numerous types
of human cancer, including glioma, and links to oncogenesis to
alter cellular functions (29).
For example, Akt promotes tumor proliferation by inhibiting
apoptosis (29); Akt is involved
in cell cycle regulation by preventing degradation of cyclin D1
(30), and by negatively
regulating p27 (31) and p21
(32). The present study aimed to
determine whether NDRG1 could inhibit proliferation and invasion of
glioma through the PI3K/Akt signaling pathway.
In a previous study, Sun et al (20) demonstrated that NDRG1 expression
was downregulated in tissue specimens from high-grade gliomas, as
compared with that in tissue from low-grade gliomas and normal
brain tissue. These results suggested that NDRG1 may be an
intrinsic regulator of gliomagenesis. In addition, NDRG1 was shown
to negatively regulate myc protein (7). However, the role of NDRG1 in human
glioma has yet to be fully elucidated. The present study aimed to
determine the expression and pathological roles of NDRG1 in human
glioma, and to investigate whether NDRG1 could serve as a potential
target for the treatment of glioma.
Materials and methods
Cell culture
The U87 MG and SHG-44 human malignant glioma cell
lines, and the normal human astrocyte cell line 1800 were obtained
from the Cell Library of the Chinese Academy of Sciences (Shanghai,
China). The U87 MG and SHG-44 cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS), 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin (all
Gibco-BRL) at 37°C in an atmosphere containing 5% CO2.
The normal astroctyes (1800) were cultured in modified RPMI-1640
(HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10%
FBS, 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin, at
37°C in an atmosphere containing 5% CO2. The medium was
replaced every 3–4 days, and the cultures were split using 0.25%
trypsin (Gibco-BRL).
Transfections
Small interfering (si) RNA targeting human NDRG1
(siNDRG1) (sense 5′-GCUGAAGCUCGUCAGUU CACCAUCC-3′ and anti-sense
5′-GGAUGGUGAACUGACGAGCUUCAGCAC-3′), and negative control si RNA (si
NC) (sense 5′-UUCUCCGAACGUGUCACGU-3′ and antisense
5′-ACGUGACACGUUCGGAGAA-3′), were purchased from Biomics
Biotechnologies Co., Ltd. (Nantong, China). The siRNA were
transfected into SHG-44 cells using Lipofectamine®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. Human pLPCX-NDRG1 and
pLPCX were purchased from Biowot Technologies (Shenzhen, China). To
generate a retrovirus, the packaging line gp2–293 (Cell Library of
the Chinese Academy of Sciences, Shanghai, China) was
co-transfected with pCMV-VSVG (Adgene, Cambridge, MA, USA), and
either pLPCX or pLPCX-NDRG1, using FuGENE® 6
transfection reagent (Roche Diagnostics Corp., Indianapolis, IN,
USA). Retrovirus-containing conditioned medium was harvested,
filtered through a 0.45-μm syringe filter unit (EMD
Millipore, Billerica, MA, USA), and used to trans-duce U87 MG
cells, according to standard procedures (33). Following retroviral infection,
single-cell clonal isolates were selected in the presence of
puromycin (Sigma-Aldrich, St. Louis, MO, USA).
MTT assay
Normal untransfected cells and transfected cells (48
h post-transfection) were seeded in 96-well plates at a density of
2×103 cells/well. After 24, 48 and 72 h, the medium was
replaced with 200 μl DMEM supplemented with 10% FBS and 0.5
mg/ml MTT (Sigma-Aldrich), and the cells were incubated at 37°C in
a 5% CO2 incubator for 4 h. Subsequently, the medium was
removed, and the reduced MTT was solubilized in 100 μl/well
dimethyl sulfoxide (Sigma-Aldrich). The absorbance was measured at
a wavelength of 570 nm using an iMark microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell cycle and apoptosis analysis
Cell cycle and apop-tosis assays were performed as
described previously (34).
Annexin V-fluorescein isothiocyanate apoptosis kit and cell cycle
analysis kit (both from BD Biosciences, Franklin Lakes, NJ, USA)
were used according to the manufacturer’s instructions. The cells
were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Cell invasion assay
Invasion was measured using 24-well BioCoat cell
culture inserts (BD Biosciences) with an 8-μm-porosity
polyethylene terephthalate membrane coated with Matrigel Basement
Membrane Matrix (BD Biosciences). The invasion assay was performed
as previously described (35).
Immunofluorescence
Briefly, 2×105 cells were seeded onto
coverslips, fixed with 4% (w/v) paraformaldehyde (Sigma-Aldrich)
for 10 min and permeabilized with 0.1% (v/v) Triton X-100
(Sigma-Aldrich) for 5 min at room temperature. The cells were
subsequently incubated at 4°C overnight with the following primary
antibodies: Rabbit polyclonal anti-human N-cadherin (1:100; cat.
no. ab18203; Abcam, Cambridge, MA, USA) and mouse monoclonal
anti-human vimentin (1:100; cat. no. ab8978; Abcam). The cells were
then incubated with polyclonal Alexa Fluor®
555-conjugated goat anti-mouse (1:200; cat. no. A21422; Invitrogen
Life Technologies) and polyclonal Alexa Fluor®
488-conjugated goat anti-rabbit (1:200; cat. no. A11008; Invitrogen
Life Technologies) immunoglobulin G (IgG) secondary antibodies for
1 h at room temperature. The coverslips were then washed with
phosphate-buffered saline (PBS; Sigma-Aldrich), and were mounted
using an anti-fade mounting solution containing DAPI (Vector
Laboratories, Inc., Burlingame, CA, USA). The images were
visualized and captured using a fluorescent microscope (Eclipse
Ni-E; Nikon Corporation, Tokyo, Japan).
Western blot analysis
Western blot analysis was performed as described
previously (34). Whole cell
protein lysates were extracted using lysis buffer (Invitrogen Life
Technologies) supplemented with a proteinase inhibitor mixture
(Sigma-Aldrich) and PhosSTOP (Roche Diagnostics Corp.). Protein
concentrations were determined using a bicinchoninic acid protein
assay (Beyotime Institute of Biotechnology, Jiangsu, China).
Protein lysates were mixed with 6X protein sample buffer [0.35
mol/l Tris-HCl (pH 6.8; Sigma-Aldrich), 30% glycerol
(Sigma-Aldrich), 21.4% β-mercaptoethanol (Sigma-Aldrich), 10% SDS]
and boiled for 5 min. A total of 25 μg protein was then
loaded onto 10 or 12% SDS-polyacrylamide gels for electrophoresis,
and transferred onto polyvinylidene difluoride membranes (EMD
Millpore), at a constant voltage of 100 V. The membranes were then
blocked with 10% nonfat milk (Wuhan Boster Biological Technology
Ltd., Wuhan, China) at room temperature for 1 h and washed with a
large volume of trisbuffered saline containing Tween [20 mmol/l
Tris-HCl, 137 mmol/l NaCl (Sigma-Aldrich), 0.1% Tween 20
(Sigma-Aldrich)].
Monoclonal rabbit anti-human NDRG1 (dilution
1:1,000; cat. no. ab124689), monoclonal mouse anti-human cyclin D1
(dilution 1:1,000; cat. no. ab101430), polyclonal rabbit anti-human
cyclin E (dilution 1:1,000; cat. no. ab7959), monoclonal mouse
anti-human PCNA (dilution 1:2,000; cat. no. ab29) and monoclonal
mouse anti-human Ki-67 (dilution 1:1,000; cat. no. ab6526) primary
antibodies were purchased from Abcam. Monoclonal mouse anti-human
AKT (dilution 1:2,000; cat. no. 2920), monoclonal rabbit anti-human
p-AKT (Ser473) (dilution 1:1,000; cat. no. 4060), monoclonal rabbit
anti-human Bcl-xL (dilution 1:1,000; cat. no. 2764), polyclonal
rabbit anti-human Bcl-2 (dilution 1:1,000; cat. no. 2876),
polyclonal rabbit anti-human Bax (dilution 1:1,000; cat. no. 2774),
monoclonal rabbit anti-human cleaved-PARP (dilution 1:1,000; cat.
no. 5625) and monoclonal rabbit anti-human cleaved-caspase-3
(dilution 1:1,000; cat. no. 9664) antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Polyclonal
rabbit anti-human N-cadherin (dilution 1:1,000, cat. no. sc-7939),
polyclonal rabbit anti-human E-cadherin (dilution 1:1,000; cat. no.
sc-7870), monoclonal mouse anti-human vimentin (dilution 1:1,000;
cat. no. sc-6260) and monoclonal mouse anti-human β-actin (dilution
1:3,000; cat. no. sc-47778) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, Texas, USA). The membranes were
incubated with the antibodies targeting NDRD1, cyclin D1, cyclin E,
p-AKT (Ser473), Bcl-xL, Bcl-2, cleaved-PARP, cleaved-caspase-3,
N-cadherin, E-cadherin and vimentin overnight at 4°C, and with the
antibodies targeting PCNA, Ki-67, AKT, Bax and β-actin for 1 h at
room temperature. Subsequently, the membranes were incubated with
the secondary antibodies for 1 h at room temperature. The following
secondary antibodies were used: Goat anti-mouse IgG-horseradish
peroxidase (HRP) (dilution 1:2,000; cat. no. sc-2005) and goat
anti-rabbit IgG-HRP (dilution 1:2,000; cat. no. sc-2004) from Santa
Cruz Biotechnology, Inc. The blots were then assessed using a
Pierce ECL western blotting substrate (Thermo Scientific, Rockford,
IL, USA). The resulting bands were evaluated by densitometric
measurement using ImageJ 1.46r (National Institutes of Health,
Bethesda, MD, USA).
Nude mouse xenograft studies
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Harbin Medical University (Harbin,
China). A total of 14 female athymic nude mice (BALBc nu/nu;
average weight 20 g; 6 weeks-old; Experimental Animal Laboratories,
Shanghai, China) were used in all experiments (n=7/group). The mice
were maintained in a specific pathogen-free environment and had
ad libitum access to autoclaved food and water. The mice
were maintained in a room at 20–22°C under a 12-hour light/dark
cycle. Each mouse was injected subcutaneously with stably
transfected U87 MG cells and control cells (1×106).
Tumor size was measured using a vernier caliper, and tumor volume
(mm3) was calculated using the following standard
formula: Tumor volume = length × width × height × 0.5236. All mice
were sacrificed by CO2 inhalation six weeks after
implantation, the tumor tissues were frozen immediately in liquid
nitrogen and paraffin-embedded tumor tissue blocks were obtained
for further analysis.
Immunohistochemistry
Immunohistochemistry was performed using mouse
monoclonal anti-Ki-67 (dilution 1:200; cat. no. ab6526; Abcam),
rabbit monoclonal anti-cleaved-caspase-3 (dilution 1:200; cat. no.
9664; Cell Signaling Technology, Inc.) and rabbit polyclonal
anti-CD31 (dilution 1:100; cat. no. ab28364; Abcam) antibodies.
Briefly, tissue sections were deparaffinized in xylene
(Sigma-Aldrich) and rehydrated with ethanol (Sigma-Aldrich). The
tissue sections were subsequently incubated with 10% normal goat
serum (Vector Laboratories, Inc., Burlingame, CA, USA in PBS (pH
7.5), followed by an overnight incubation at 4°C with the primary
antibodies. The tissue sections were then stained with biotinylated
secondary antibody (Vector Laboratories, Inc.) for 1 h at room
temperature, followed by an incubation with Vectastain Elite
avidin-biotin complex reagent (Vector Laboratories, Inc.) for 30
min. The peroxidase reaction was developed using diaminobenzidine
(DAB kit; Vector Laboratories, Inc.) and the slides were
counterstained with hematoxylin (Sigma-Aldrich).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 4.02 software (GraphPad Software Inc., La Jolla, CA,
USA) or SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Values
are expressed as the mean ± standard deviation. Comparisons between
multiple groups were made using a one-way analysis of variance,
followed by Dunnet’s t-test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
NDRG1 is lowly expressed in glioma
cells
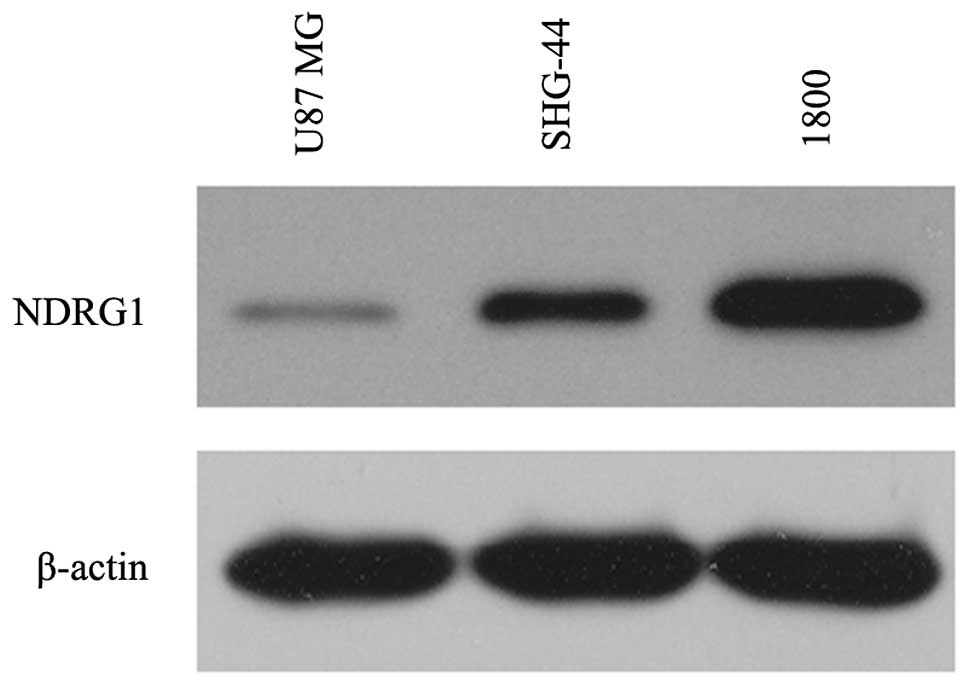
The present study examined the expression levels of
NDRG1 in the established human glioma cell lines U87 MG and SHG-44,
and in the normal astroglial cell line 1800. NDRG1 protein
expression levels were low in the U87 MG and SHG-44 glioma cells,
whereas high expression levels of NDRG1 were observed in the normal
astroglial cell line (1800), as determined by western blotting.
Furthermore, NDRG1 expression was lower in the U87 MG cells as
compared with that in the SHG-44 cells (Fig. 1).
NDRG1 inhibits cell proliferation in
glioma cells
To determine whether NDRG1 expression had an effect
on glioma cell progression, the present study used siNDRG1 to
specifically knockdown NDRG1 expression, and used retroviral
constructs expressing NDRG1 (RV-NDRG1) to enforce NDRG1
overexpression. NDRG1 expression levels were lowest in the U87 MG
cells and were highest in the SHG-44 cells (Fig. 1). siNDRG1 was transfected into the
SHG-44 cells in order to downregulate NDRG1 expression, and
RV-NDRG1 was transfected into the U87 MG cells to enhance NDRG1
expression. Transfection with siNDRG1 knocked down NDRG1 expression
levels in SHG-44 cells by ~80% within two days of transfection,
whereas no decrease in NDRG1 expression was observed in the cells
transfected with the siNC, as determined by western blot analysis
(Fig. 2A). Furthermore, U87 MG
cells transfected with RV-NDRG1 exhibited increased protein
expression levels of NDRG1, as determined by western blotting
(Fig. 2A).
 | Figure 2Effects of NDRG1 on cell
proliferation, cell cycle-associated protein expression and cell
cycle progression in SHG-44 and U87 MG glioma cells. (A) Western
blot analysis showed that protein expression levels of NDRG1 were
affected by transfection with siNDRG1 and RV-NDRG1. β-actin was
used as the internal control. (B) Effects of NDRG1 knockdown and
overexpression on the proliferation of glioma cells. Cell
proliferation of untransfected cells and cells transfected with
siNDRG1, siNC, RV-NDRG1 or RV-control was assessed by MTT assay.
Values are expressed as the mean ± standard deviation of three
independent experiments. (C and D) Cell cycle analysis of glioma
cells transfected with siNDRG1 or RV-NDRG1 demonstrated an increase
in the number of siNDRG1-transfected SHG-44 cells in S phase, and
an increase in the number of RV-NDRG1-transfected U87 MG cells in
G0/G1 phase. The percentage of cells in each
phase of the cell cycle is presented as the mean ± standard
deviation from three independent experiments. (E) Changes in
protein expression levels of proliferation indices: Ki-67, PCNA,
cyclin D1 and cyclin E following transfection with siNDRG1 or
RV-NDRG1. β-actin was used as the internal control. NDRG1, N-myc
downstream-regulated gene 1; si, small interfering RNA; RV,
retrovirus; NC, negative control; PCNA, proliferating cell nuclear
antigen. |
To determine whether there was an association
between NDRG1 expression and glioma cell proliferation, an MTT
assay was performed. SHG-44 cells transfected with siNDRG1
exhibited a marked increase in cell proliferation as compared with
that in the siNC and normal groups (P<0.001; Fig. 2B). In addition, there was a
significant decrease in cell proliferation in the U87 MG cells
transfected with RV-NDRG1 (P<0.001; Fig. 2B).
Flow cytometry revealed an increase in the number of
siNDRG1-transfected SHG-44 cells in S phase at 72 h, as compared
with that in the siNC group (P<0.001; Fig. 2C and D). Conversely, there was an
increase in the number of RV-NDRG1-transfected U87 MG cells in
G0/G1 phase (P<0.001; Fig. 2C and D). Concurrently, the
expression levels of cell cycle arrest-associated proteins were
examined by western blot analysis (Fig. 2E). The protein expression levels of
cyclin D1, cyclin E, Ki-67 and PCNA were higher in the
siNDRG1-transfected SGH-44 cells, as compared with those in the
siNC-transfected and untransfected cells. Conversely, the
expression levels were lower in the RV-NDRG1-transfected U87 MG
cells as compared with those in untransfected cells, which was
concordant with the results from the MTT and cell cycle assays.
These results suggested that NDRG1 may inhibit cell proliferation
and induce G0/G1 cell cycle arrest in glioma
cells.
NDRG1 increases the percentage of
apoptotic glioma cells
The number of apoptotic glioma cells was measured
using an Annexin V/PI Apoptosis Detection kit and flow cytometry at
three days post-transfection. RV-NDRG1-transfected U87 MG cells
exhibited a relatively high rate of cell apoptosis as compared with
that in the cells transfected with the scrambled control or the
untransfected cells (P<0.001; Fig.
3A and B). Conversely, SHG-44 cells transfected with siNDRG1
exhibited a relatively low rate of apoptosis as compared with that
of the untransfected cells (P<0.001; Fig. 3A and B).
 | Figure 3NDRG1 induces cell death in a
caspase-dependent manner. NDRG1 affects constitutive and inducible
p-AKT expression in SHG-44 and U87 MG glioma cells, and
overexpression of NDRG1 downregulates the expression of
anti-apoptotic proteins. (A and B) Flow cytometry results of
annexin V-PI staining of glioma cells following transfection with
siNDRG1 or RV-NDRG1. An increase in the percentage of apoptotic
cells following transfection with RV-NDRG1 is shown. Values are
expressed as the mean ± standard deviation of three independent
experiments. (C) Changes in protein expression levels of anti- or
pro-apoptotic proteins following transfection with siNDRG1 or
RV-NDRG1. β-actin was used as the internal control. NDRG1, N-myc
downstream-regulated gene 1; p, phosphorylated; si, small
interfering RNA; RV, retrovirus; NC, negative control; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; xL, extra
large; PARP; poly(ADP ribose) polymerase; PI, propidium iodide. |
In addition, western blot analysis was performed to
determine the expression levels of the following
apoptosis-associated proteins in glioma cells: Bcl-2, Bax, Bcl-xL,
cleaved-PARP, cleaved-caspase-3, AKT and p-AKT. At three days
post-transfection, the expression levels of Bcl-2 and Bcl-xL were
significantly decreased in the RV-NDRG1-transfected U87 MG cells as
compared with the cells transfected with the RV-control, whereas
the expression levels of Bax, cleaved-PARP and cleaved-caspase-3
were increased. In addition, RV-NDRG1-transfected U87 MG cells
exhibited lower expression levels of p-AKT, whereas the expression
levels of total AKT were unaffected (Fig. 3C). In accordance with the data from
the cells overexpressing NDRG1, three days post-transfection,
Bcl-2, Bcl-xL and p-AKT expression were upregulated, and the
expression levels of Bax, cleaved-PARP and cleaved-caspase-3 were
decreased in the siNDRG1-trans-fected SHG-44 cells, but not in the
control group (Fig. 3C). These
results indicated that the NDRG1-dependent increase in the rate of
glioma cell apoptosis may be partially mediated by regulation of
Bcl-2, Bcl-xL, Bax, cleaved-PARP, cleaved-caspase-3 and p-AKT.
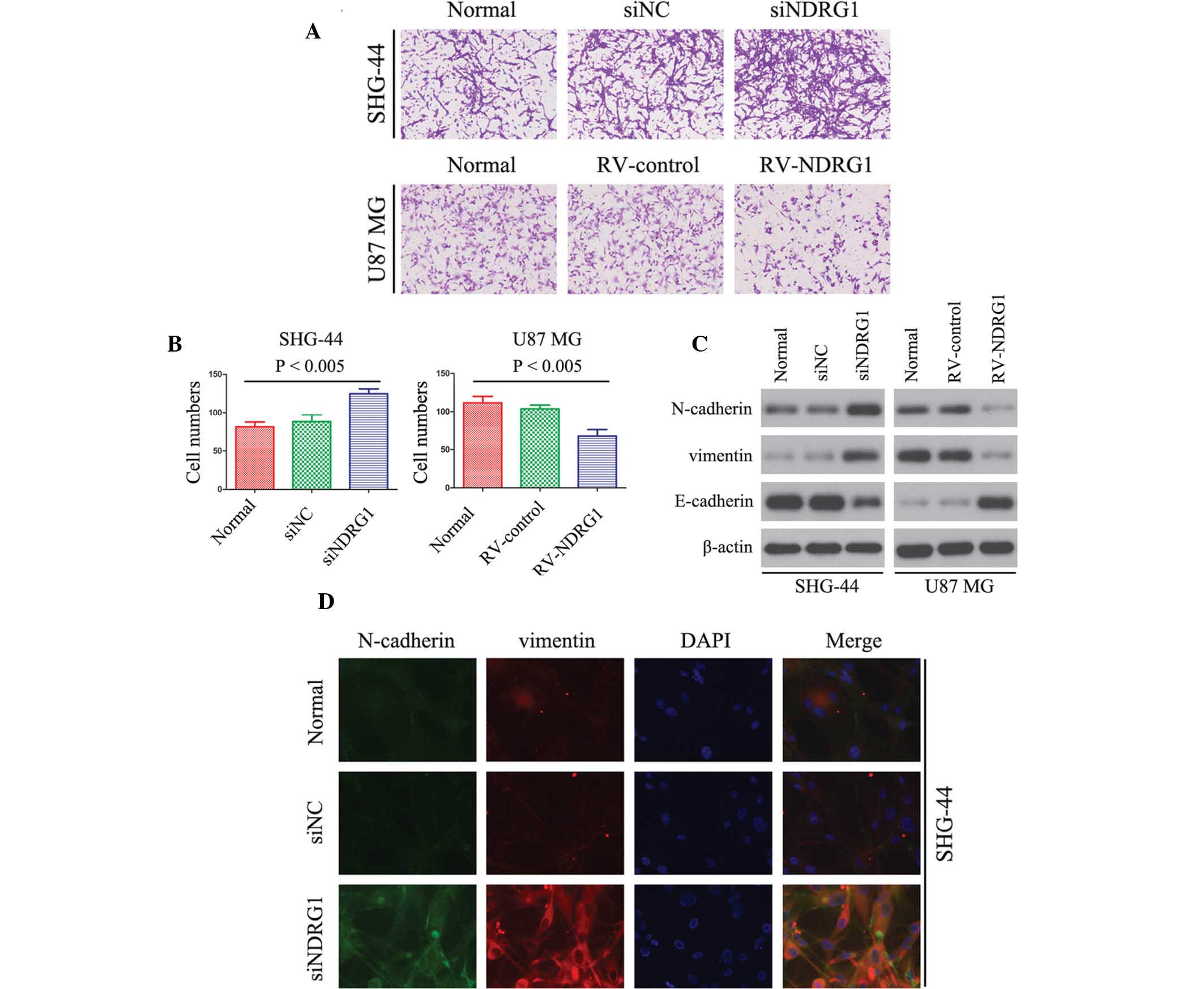
NDRG1 inhibits glioma cell invasion in
vitro
The association between NDRG1 expression and glioma
cell invasion was detected using a Matrigel invasion assay. The
number of invaded RV-NDRG1-transfected U87 MG cells was
significantly lower as compared with that in the cells in the
RV-control and untransfected groups (P<0.005; Fig. 4A and B), thus suggesting that the
percentage of invaded cells decreased in cells overexpressing
NDRG1. Furthermore, the invasiveness of siNDRG1-transfected SHG-44
cells was increased as compared with that of untransfected cells
(P<0.005; Fig. 4A and B).
Vimentin, N-cadherin and E-cadherin have essential
roles in the invasion of tumor cells (36). Therefore, the present study
examined the expression levels of vimentin, N-cadherin and
E-cadherin in glioma cells by western blotting. The protein
expression levels of vimentin and N-cadherin were downregulated in
RV-NDRG1-transfected U87 MG cells as compared with those in the
control groups, and E-cadherin expression levels were upregulated
(Fig. 4C). In addition, in
siNDRG1-transfected SHG-44 cells, the expression levels of vimentin
and N-cadherin were significantly increased, and expression levels
of E-cadherin were significantly decreased (Fig. 4C). As shown by immunofluorescence
(Fig. 4D), transfection with
siNDRG1 markedly increased N-cadherin and vimentin expression in
SHG-44 cells, which was in concordance with the results of the
western blot analysis. These results suggested that NDRG1 may
inhibit glioma cell invasion in vitro.
NDRG1 overexpression inhibits glioma
tumor growth in vivo
The present study further examined the effects of
NDRG1 on glioma growth by establishing a U87 MG xenograft nude
mouse glioma model. Mice injected with glioma cells over-expressing
NDRG1 exhibited significantly smaller tumors as compared with those
in the control group (P<0.005; Fig.
5A). Immunohistochemical analysis was used to stain Ki-67 to
determine cell proliferation; cleaved-caspase-3 to detect apoptotic
cells; and CD31 to detect tumor microvessels. There were fewer
Ki-67 positive cells, more apoptotic cells and fewer CD31-stained
vessels in tumors overexpressing NDRG1, as compared with the
control tumors (Fig. 5B). In
addition, the expression levels of the same proteins were assessed
in vitro by western blotting (Fig. 5C). The protein expression levels of
p-AKT (ser473), cyclin D1, cyclin E, PCNA, Ki-67, Bcl-2 and Bcl-xL
were decreased in the NDRG1 overexpressing cells, as compared with
the control cells (Fig. 5C). In
addition, the expression levels of Bax, cleaved-PARP and
cleaved-caspase-3 were increased in the cells overexpressing NDRG1.
Fig. 5D shows the quantification
of the immunohistochemical analyses (P<0.005). The
quantification revealed a 2-fold decrease in the number of Ki-67
positive cells in the RV-NDRG1 group, a >3-fold increase in the
number of apoptotic cells in the RV-NDRG1 group and a 3-fold
decrease in microvessel formation in the RV-NDRG1 group
(P<0.005). These results indicate the functional significance of
NDRG1, and its high propensity to inhibit proliferation in
glioma.
 | Figure 5NDRG1 overexpression inhibits
proliferation of glioma in vivo. (A) Photomicrographs were
taken of U87 MG xenograft tumors grown in nude mice. Representative
images of one mouse from each group are presented. Tumor volumes in
the mice with tumors overexpressing NDRG1 were smaller, as compared
with those in the control mice. Values are expressed as the mean ±
standard deviation of experiments performed in triplicate. (B)
Tumors from the different groups were immunostained for cleaved
caspase-3, CD31 and Ki-67. Images are representative of three
independent experiments. (C) Western blotting was performed to
detect the protein expression levels of the indicated molecules
from tumor samples. β-actin was used as the internal control. (D)
Quantification of immunostaining in B. CD31-stained microvessels
were counted to record microvessel density, apoptotic cells were
counted to give the apoptosis index and cells expressing Ki-67 were
counted to calculate the proliferation index. NDRG1, N-myc
downstream-regulated gene 1; RV, retrovirus; Bcl-2, B-cell lymphoma
2; Bax, Bcl-2-associated X protein; xL, extra large; PARP; poly ADP
ribose polymerase; PCNA, proliferating cell nuclear antigen; p,
phosphorylated; RV, retrovirus. |
Discussion
NDRG1 has been associated with numerous cellular
processes, including cell cycle, apoptosis and differentiation
(12,24,37).
Differentiation of various cancer cell lines in vitro has
been shown to induce the expression of NDRG1 (38–40).
In addition, the expression of NDRG1 in tumors has been shown to be
associated with an improved outcome (41). Previous studies have suggested an
important role for NDRG1 expression in numerous types of cancer
(13,15,16,18).
In addition, NDRG1 has often been shown to be downregulated in
numerous types of human malignancy, including prostate (18), breast (26) and colon cancer (15). These observations suggested that
NDRG1 may potentially act as a tumor suppressor in certain types of
cancer.
The roles of NDRG1 are beginning to be elucidated in
multiple malignancies; however, its function in brain
tumori-genesis remained to be fully elucidated. Therefore, the
present study aimed to investigate whether the expression of NDRG1
was associated with the progression of malignant glioma. A previous
study demonstrated that the downregulation of NDRG1 is
significantly associated with a higher World Health Organization
tumor grade, and a worse overall survival rate (20). However, the mechanisms underlying
the effects of NDRG1 on glioma tumorigenesis had yet to be
elucidated. Therefore, it is required to study the role of NDRG1 in
the regulation of glioma cell growth, survival and invasion.
In order to elucidate the role of NDRG1 in glioma,
RV-NDRG1 was transiently transfected into U87 MG glioma cells. In
the present study MTT, flow cytometric and invasive assays
demonstrated that upregulation of NDRG1, following transfection of
cells with RV-NDRG1, resulted in the inhibition of cell
proliferation, induction of apoptosis and suppression of
invasiveness. These results suggested that NDRG1 has an important
role in the regulation of gliomagenesis. Further evidence regarding
this finding was obtained from glioma cells with NDRG1 knockdown.
Transfection of SHG-44 cells with siNDRG1 was used to silence NDRG1
expression, which resulted in increased cell proliferation and
invasiveness, as well as decreased levels of apoptosis.
Furthermore, in a subcutaneous mouse tumor model, NDRG1
overexpression significantly reduced subcutaneous tumor growth.
To provide further evidence regarding the mechanisms
underlying the effects of NDRG1 on glioma cells, the present study
examined the expression levels of proteins associated with glioma
cell proliferation (cyclin D1, cyclin E, Ki-67 and PCNA), apoptosis
(Bcl-2/Bax, Bcl-xL, cleaved-PARP, cleaved-caspase-3, p-AKT and
AKT), and invasion (N-cadherin, vimentin and E-cadherin) by western
blot analysis. The protein expression levels of Ki-67, a biological
tumor marker that indicates changes in tumor proliferation
(42), were reduced in the
RV-NDRG1-transfected U87 MG cells, and increased in the
siNDRG1-transfected SHG-44 cells. In addition, changes in the
expression levels of PCNA, another well-known proliferation marker
(43), were similar to those of
Ki-67 in the RV-NDRG1-transfected U87 MG and the
siNDRG1-transfected SHG-44 cells.
The protein expression levels of Bcl-2 have
previously been shown to be associated with apoptosis in numerous
cell types, including glioma cells (44). The results of the present study
demonstrated that the downregulation of NDRG1 induced an increase
in the protein expression levels of Bcl-2, and also significantly
decreased the expression levels of Bax. The PI3K/Akt pathway is
important in gliomas (45).
Numerous studies have been performed to demonstrate that the p-Akt
expression levels were elevated in gliomas in vitro and
in vivo, and this expression was revealed to be correlated
with the loss of phosphatase and tensin homolog (46,47).
In glioma tumor samples, elevated p-Akt has been demonstrated to be
associated with a worse prognosis (48). The present study revealed that
NDRG1 knockdown also induced AKT phosphorylation, whereas it did
not affect the expression of total AKT. Therefore, the increase in
p-AKT, together with the increased expression of Bcl-2, may explain
why cells with low NDRG1 expression are resistant to
chemotherapy-induced apoptosis (49).
Another key characteristic of glioma cells, besides
rapid proliferation and resistance to apoptosis, is their invasion
into the surrounding healthy brain tissue (50). It has previously been suggested
that vimentin, N-cadherin and the invasive marker E-cadherin are
present at markedly elevated levels in numerous glioma cell lines
and surgically resected specimens (51). The present study demonstrated a
negative correlation between vimentin, N-cadherin and NDRG1
expression, and a positive correlation between E-cadherin and NDRG1
expression. These results indicated that the overexpression of
NDRG1 may inhibit the invasiveness of glioma cells through
modulation of vimentin, N-cadherin and E-cadherin. Poor
histological differentiation is a property of malignancy.
The results of the nude mouse xenograft studies also
confirmed that overexpression of NDRG1 was able to significantly
inhibit proliferation and microvessel formation in vivo.
Immunohistochemical analysis demonstrated that the expression of
Ki-67 and CD31 were decreased in the tumor tissue, whereas the
expression of cleaved-caspase-3 was increased in the RV-NDRG1
group. Western blotting also demonstrated that overexpression of
NDRG1 resulted in increased expression levels of Bax, cleaved-PARP,
cleaved-caspase-3 and decreased the expression levels of p-AKT (ser
473), cyclin D1, cyclin E, PCNA, Ki-67, Bcl-2 and Bcl-xL. The
present study demonstrated that NDRG1 is important in gliomas.
However, it remains to be elucidated why NDRG1 has a low level of
expression in glioma. Specific signaling pathways may inhibit the
expression of NDRG1, or NDRG1 may be degraded by certain microRNAs.
Therefore, further studies are required to elucidate these
questions.
In conclusion, the present study demonstrated that
NDRG1 is lowly expressed in glioma cells. In addition, the results
demonstrated that NDRG1 may have an important role in the
regulation of growth and invasion of glioma cells. These findings
indicated that NDRG1 may serve as a potential diagnostic or
prognostic marker, and a novel therapeutic target in the treatment
of glioma.
Acknowledgments
The present study was supported by the International
Science and Technology Cooperation Project of the Ministry of
Science and Technology (grant no. 2014DFA31630).
References
|
1
|
Komotar RJ, Otten ML, Moise G and Connolly
ES Jr: Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma-a critical review. Clin Med Oncol. 2:421–422.
2008.PubMed/NCBI
|
|
2
|
Kleihues P, Burger PC and Scheithauer BW:
The new WHO classification of brain tumours. Brain Pathol.
3:255–268. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakimoto H, Aoyagi M, Nakayama T, et al:
Prognostic significance of Ki-67 labeling indices obtained using
MIB-1 monoclonal antibody in patients with supratentorial
astrocytomas. Cancer. 77:373–380. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pollack IF: Brain tumors in children. N
Engl J Med. 331:1500–1507. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soffietti R, Leoncini B and Rudà R: New
developments in the treatment of malignant gliomas. Expert Rev
Neurother. 7:1313–1326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimono A, Okuda T and Kondoh H:
N-myc-dependent repression of ndr1, a gene identified by direct
subtraction of whole mouse embryo cDNAs between wild type and N-myc
mutant. Mech Dev. 83:39–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okuda T and Kondoh H: Identification of
new genes ndr2 and ndr3 which are related to Ndr1/RTP/Drg1 but show
distinct tissue specificity and response to N-myc. Biochem Biophys
Res Commun. 266:208–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin TM and Chang C: Cloning and
characterization of TDD5, an androgen target gene that is
differentially repressed by testosterone and dihydrotestosterone.
Proc Natl Acad Sci USA. 94:4988–4993. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou D, Salnikow K and Costa M: Cap43, a
novel gene specifically induced by Ni2+ compounds. Cancer Res.
58:2182–2189. 1998.PubMed/NCBI
|
|
11
|
Kokame K, Kato H and Miyata T:
Homocysteine-respondent genes in vascular endothelial cells
identified by differential display analysis. GRP78/BiP and novel
genes. J Biol Chem. 271:29659–29665. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurdistani SK, Arizti P, Reimer CL, Sugrue
MM, Aaronson SA and Lee SW: Inhibition of tumor cell growth by
RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res.
58:4439–4444. 1998.PubMed/NCBI
|
|
13
|
Qu X, Zhai Y, Wei H, et al:
Characterization and expression of three novel
differentiation-related genes belong to the human NDRG gene family.
Mol Cell Biochem. 229:35–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou RH, Kokame K, Tsukamoto Y, Yutani C,
Kato H and Miyata T: Characterization of the human NDRG gene
family: A newly identified member, NDRG4, is specifically expressed
in brain and heart. Genomics. 73:86–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM and
Pardee AB: Drg-1 as a differentiation-related, putative metastatic
suppressor gene in human colon cancer. Cancer Res. 60:749–755.
2000.PubMed/NCBI
|
|
16
|
van Belzen N, Dinjens WN, Diesveld MP, et
al: A novel gene which is up-regulated during colon epithelial cell
differentiation and down-regulated in colorectal neoplasms. Lab
Invest. 77:85–92. 1997.PubMed/NCBI
|
|
17
|
Bandyopadhyay S, Pai SK, Hirota S, et al:
PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in
prostate and breast cancer. Cancer Res. 64:7655–7660. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bandyopadhyay S, Pai SK, Gross SC, et al:
The Drg-1 gene suppresses tumor metastasis in prostate cancer.
Cancer Res. 63:1731–1736. 2003.PubMed/NCBI
|
|
19
|
Ando T, Ishiguro H, Kimura M, et al:
Decreased expression of NDRG1 is correlated with tumor progression
and poor prognosis in patients with esophageal squamous cell
carcinoma. Dis Esophagus. 19:454–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun B, Chu D, Li W, et al: Decreased
expression of NDRG1 in glioma is related to tumor progression and
survival of patients. J Neurooncol. 94:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kokame K, Kato H and Miyata T:
Nonradioactive differential display cloning of genes induced by
homocysteine in vascular endothelial cells. Methods. 16:434–443.
1998. View Article : Google Scholar
|
|
22
|
Taketomi Y, Sugiki T, Saito T, et al:
Identification of NDRG1 as an early inducible gene during in vitro
maturation of cultured mast cells. Biochem Biophys Res Commun.
306:339–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwala KL, Kokame K, Kato H and Miyata
T: Phosphorylation of RTP, an ER stress-responsive cytoplasmic
protein. Biochem Biophys Res Commun. 272:641–647. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piquemal D, Joulia D, Balaguer P, Basset
A, Marti J and Commes T: Differential expression of the
RTP/Drg1/Ndr1 gene product in proliferating and growth arrested
cells. Biochim Biophys Acta. 1450:364–373. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gómez-Casero E, Navarro M,
Rodríguez-Puebla ML, et al: Regulation of the
differentiation-related gene Drg-1 during mouse skin
carcinogenesis. Mol Carcinog. 32:100–109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bandyopadhyay S, Pai SK, Hirota S, et al:
Role of the putative tumor metastasis suppressor gene Drg-1 in
breast cancer progression. Oncogene. 23:5675–5681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shah MA, Kemeny N, Hummer A, et al: Drg1
expression in 131 colorectal liver metastases: Correlation with
clinical variables and patient outcomes. Clin Cancer Res.
11:3296–3302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J and Kretzner L: The growth-inhibitory
Ndrg1 gene is a Myc negative target in human neuroblastomas and
other cell types with overexpressed N- or c-myc. Mol Cell Biochem.
250:91–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hajduch E, Litherland GJ and Hundal HS:
Protein kinase B (PKB/Akt) - a key regulator of glucose transport?
FEBS Lett. 492:199–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gesbert F, Sellers WR, Signoretti S, Loda
M and Griffin JD: BCR/ABL regulates expression of the
cyclin-dependent kinase inhibitor p27Kip1 through the
phosphatidylinositol 3-kinase/AKT pathway. J Biol Chem.
275:39223–39230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sciaudone M, Gazzerro E, Priest L, Delany
AM and Canalis E: Notch 1 impairs osteoblastic cell
differentiation. Endocrinology. 144:5631–5639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Ma Y, Jiang H, et al:
Overexpression of von Hippel- Lindau protein synergizes with
doxorubicin to suppress hepatocellular carcinoma in mice. J
Hepatol. 55:359–368. 2011. View Article : Google Scholar
|
|
35
|
Li X, Yang Q, Yu H, et al: LIF promotes
tumorigenesis and metastasis of breast cancer through the AKT-mTOR
pathway. Oncotarget. 5:788–801. 2014.PubMed/NCBI
|
|
36
|
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic
Z and Richardson DR: The iron chelators Dp44mT and DFO inhibit
TGF-β-induced epithelial-mesenchymal transition via up-regulation
of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem.
287:17016–17028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stein S, Thomas EK, Herzog B, et al: NDRG1
is necessary for p53-dependent apoptosis. J Biol Chem.
279:48930–48940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu B, Lin L and Rote NS: Identification of
a stress-induced protein during human trophoblast differentiation
by differential display analysis. Biol Reprod. 61:681–686. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Floryk D and Thompson TC:
Antiproliferative effects of AVN944, a novel inosine
5-monophosphate dehydrogenase inhibitor, in prostate cancer cells.
Int J Cancer. 123:2294–2302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ulrix W, Swinnen JV, Heyns W and Verhoeven
G: The differentiation-related gene 1, Drg1, is markedly
upregulated by androgens in LNCaP prostatic adenocarcinoma cells.
FEBS Lett. 455:23–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Melotte V, Qu X, Ongenaert M, et al: The
N-myc downstream regulated gene (NDRG) family: Diverse functions,
multiple applications. FASEB J. 24:4153–4166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Niikura N, Iwamoto T, Masuda S, et al:
Immunohistochemical Ki67 labeling index has similar proliferation
predictive power to various gene signatures in breast cancer.
Cancer Sci. 103:1508–1512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takawa M, Cho HS, Hayami S, et al: Histone
lysine methyltrans-ferase SETD8 promotes carcinogenesis by
deregulating PCNA expression. Cancer Res. 72:3217–3227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Afshar G, Jelluma N, Yang X, et al:
Radiation-induced caspase-8 mediates p53-independent apoptosis in
glioma cells. Cancer Res. 66:4223–4232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McDowell KA, Riggins GJ and Gallia GL:
Targeting the AKT pathway in glioblastoma. Curr Pharm Des.
17:2411–2420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Haas-Kogan D, Shalev N, Wong M, Mills G,
Yount G and Stokoe D: Protein kinase B (PKB/Akt) activity is
elevated in glioblastoma cells due to mutation of the tumor
suppressor PTEN/MMAC. Curr Biol. 8:1195–1198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Choe G, Horvath S, Cloughesy TF, Crosby K,
Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL and Mischel PS:
Analysis of the phosphatidylinositol 3′-kinase signaling pathway in
glioblastoma patients in vivo. Cancer Res. 63:2742–2746.
2003.PubMed/NCBI
|
|
48
|
Suzuki Y, Shirai K, Oka K, Mobaraki A,
Yoshida Y, Noda SE, Okamoto M, Suzuki Y, Itoh J, Itoh H, et al:
Higher pAkt expression predicts a significant worse prognosis in
glioblastomas. J Radiat Res (Tokyo). 51:343–348. 2010. View Article : Google Scholar
|
|
49
|
Motwani M, Sirotnak FM, She Y, Commes T
and Schwartz GK: Drg1, a novel target for modulating sensitivity to
CPT-11 in colon cancer cells. Cancer Res. 62:3950–3955.
2002.PubMed/NCBI
|
|
50
|
Nakada M, Okada Y and Yamashita J: The
role of matrix metal-loproteinases in glioma invasion. Front
Biosci. 8:e261–e269. 2003. View
Article : Google Scholar
|
|
51
|
Bolteus AJ, Berens ME and Pilkington GJ:
Migration and invasion in brain neoplasms. Curr Neurol Neurosci
Rep. 1:225–232. 2001. View Article : Google Scholar
|