Introduction
Renal cell carcinoma (RCC) is the most common
neoplasm of the kidney, with ~80% of patients with RCC diagnosed
worldwide with the clear cell RCC subtype (1). It has been reported that the
prognosis of advanced RCC is poor, with a 5-year survival rate of
5–10% (2,3). At diagnosis, ~30% of patients with
RCC exhibit metastatic disease, and radical nephrectomy remains the
primary treatment option for patients with RCC, due to resistance
of the tumors to radiation and chemotherapy (4,5).
Although several environmental and genetic factors have been found
to be associated with RCC, the molecular mechanisms underlying the
initiation and progression of RCC remain to be fully elucidated
(6). Therefore, there is an urgent
requirement for the identification of sensitive and reliable
biomarkers for the development of novel targeted therapies for
RCC.
MicroRNA (miRNA), are small non-coding RNA of ~20–22
nucleotides, which regulate gene expression by targeting messenger
RNA (mRNA) through translational repression or mRNA degradation
(7). To date, ~2,000 human miRNAs
have been identified, which are involved in the regulation of
biological processes, including proliferation, apoptosis, migration
and differentiation (8). Several
miRNAs have been implicated as oncogenes or as tumor suppressors,
and are aberrantly expressed in various types of cancer, including
RCC (9–12). Previous studies have demonstrated,
through transfection with pre-miR-509-3p in vitro, that
miR-509-3p is downregulated in RCC, which is associated with cell
proliferation and migration in 786-O RCC cells. However, the
mechanism of action of miR-509-3p in RCC remains to be elucidated.
Therefore, there is a requirement to further investigate the
functional significance of miR-509-3p, and to identify novel
targets regulated by miR-509-3p in 786-O and ACHN RCC cells.
Mitogen-activated protein kinase kinase kinase 8
(MAP3K8), also termed COT, is an oncogene encoding a member of the
serine/threonine protein kinase family (13). The encoded protein localizes to the
cytoplasm and activates the MAP kinase and c-Jun N-terminal kinase
(JNK) kinase pathways (13).
Previous studies have demonstrated that over-expression of MAP3K8
correlates with the development of breast cancer (14), prostate cancer (15) and endometrioid carcinoma (16). In addition, increased expression of
MAP3K8 is a significant prognostic factor of poor survival rates in
patients with colorectal cancer (CRC), which suggests that MAP3K8
is important in carcinogenesis (17). Bioinformatics has revealed that the
3′UTR of MAP3K8 contains a putative binding site for miR-509-3p.
However, the regulation of miR-509-3p in RCC, and its association
with MAP3K8, have not been reported.
In the present study, the expression pattern of
miR-509-3p in RCC cells was examined, and the effects of miR-509-3p
on the proliferation and migration of two RCC cell lines were
assessed. To improve understanding of the underlying regulatory
mechanism, a target MAP3K8 gene was identified using a luciferase
reporter assay in the RCC cell lines. The present study aimed to
establish whether overexpression of miR-509-3p affected the mRNA
and protein expression levels of MAP3K8, and whether knockdown of
MAP3K8 affected the migration and proliferation of the RCC
cells.
Materials and methods
Tissue samples
The present study was approved by the Institutional
Review Board and Ethical Committee of Peking University Shenzhen
Hospital (Shenzhen, China). All patients involved provided written
informed consent. A total of 10 normal specimens were obtained from
10 patients diagnosed with RCC following nephrectomy at Peking
University Shenzhen Hospital in 2013. The normal specimens were
located 2.0 cm away from visible RCC lesions. All tissue samples
were reviewed and classified with hematoxylin & eosin staining
(Beijing Disinbio Science & Technology Co., Ltd., Beijing,
China), and the disease Tumor Node Metastasis stages of the
patients were classified according to the 2009 American Joint
Committee on Cancer staging system (18). The clinical and pathological
characteristics of the 10 patients with RCC included in the present
study are shown in Table I. The
fresh tissue samples were immediately immersed in RNAlater (Qiagen,
Hilden, Germany), following surgical resection, were stored at 4°C
overnight and were subsequently frozen in liquid nitrogen for
storage at −80°C until analysis. A pathologist confirmed that all
the specimens were derived from normal tissues.
 | Table IClinical characteristics of the
patients with ccRCC. |
Table I
Clinical characteristics of the
patients with ccRCC.
| Patient | Gender | Age (years) | TNM | Histological
type |
|---|
| 1 | M | 32 | T1N0M0 | ccRCC |
| 2 | M | 36 | T2N0M0 | ccRCC |
| 3 | M | 41 | T2N0M0 | ccRCC |
| 4 | M | 40 | T1N0M0 | ccRCC |
| 5 | M | 27 | T1N0M0 | ccRCC |
| 6 | F | 52 | T2N0M0 | ccRCC |
| 7 | F | 40 | T1N0M0 | ccRCC |
| 8 | M | 58 | T2N0M0 | ccRCC |
| 9 | F | 48 | T2N0M0 | ccRCC |
| 10 | M | 52 | T4N0M0 | ccRCC |
Cell culture and cell transfection
The 786-O and ACHN cell lines used in the present
study were obtained from the American Type Culture Collection
(Manassas, VA, USA. These cell lines were maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS;
Shanghai ExCell Biology, Inc., Shanghai, China) and 1% antibiotics
(100 U/ml penicillin and 100 mg/ml streptomycin sulfates;
BioWiseTech Co., Ltd., Shanghai, China) and were cultured in a
humidified air atmosphere of 5% CO2 at 37°C.
Small interfering (si)RNA (siRNA-MAP3K8; si-MAP3K8)
were designed to target MAP3K8. The miR-509-3p mimics, si-MAP3K8
and negative control were transfected into the 786-O and ACHN human
RCC cells lines. The sequences are listed in Table II. The RNA oligoribonucleotides
were purchased from GenePharma (Shanghai, China). Prior to
transfection, 3×105 cells were seeded into six-well
plates and cultured for 24 h at 37°C. Once the cells had reached
60–80% confluence, they were transfected with RNA using
Lipofectamine 2000® reagent (Invitrogen Life
Technologies), according to the manufacturer’s instructions.
 | Table IIPrimer sequences used in the present
study. |
Table II
Primer sequences used in the present
study.
| Name | Primer | Sequence
(5′–3′) |
|---|
| miR-509-3p
mimics | Forward |
TGATTGGTACGTCTGTGGGTAG |
| Reverse |
ACCCACAGACGTACCAATCATT |
| si-MAP3K8 | Forward |
GCGCCTTTGGAAAGGTATATT |
| Reverse |
TATACCTTTCCAAAGGCGCTT |
| Negative
control | Forward |
TTCTCCGAACGTGTCACGTTT |
| Reverse |
ACGTGACACGTTCGGAGATT |
| miR-509-3p | Forward |
TGATTGGTACGTCTGTGGGTAG |
| Reverse | Universal primers
(miScript SYBR Green PCR kit) |
| U6 | Forward |
CTCGCTTCGGCAGCACA |
| Reverse |
ACGCTTCACGAATTTGCGT |
| MAP3K8 | Forward |
ATGGAGTACATGAGCACTGGA |
| Reverse |
GCTGGCTCTTCACTTGCATAAAG |
| GAPDH | Forward |
GGAGCGAGATCCCTCCAAAAT |
| Reverse |
GGCTGTTGTCATACTTCTCATGG |
|
MAP3K8-3′UTR-WT | Forward |
CCGCTCGAGTACCAATCACAAGGATAATGC |
| Reverse |
ATTTGCGGCCGCATTATCCTTGTGATTGGTA |
|
MAP3K8-3′UTR-MUT | Forward |
CCGCTCGAGGCAACCGACCAAGGATAATGC |
| Reverse |
ATTTGCGGCCGCATTATCCTTGGTCGGTTGC |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction RT-qPCR
analysis
The total RNA was extracted from cells and tissues
using TRIzol® reagent (Invitrogen Life Technologies),
according to the manufacturer’s instructions. RT-qPCR analysis was
performed to detect the expression levels of miR-509-3p in the 10
human normal renal tissues and the 786-O and ACHN RCC cell lines. A
total of 1 μg total RNA was reverse transcribed into cDNA
using a miScript Reverse Transcription kit (Qiagen), according to
the manufacturer’s instructions. qPCR was performed using a SYBR
green assay (Takara Bio, Inc., Dalian, China) on a Roche
LightCycler 480 machine (Roche Applied Science, Mannheim, Germany),
according to the manufacturer’s instructions and using U6 as an
internal control. The 20 μl reaction mixture contained 10
μl 2X QuantiTect SYBR Green PCR Master mix, 2 μl 10X
miScript Universal Primer, 0.4 μl specific microRNA primer,
1 μl cDNA template and RNase-free water. The primers used
for miR-509-3p and U6 are listed in Table II, and were purchased from Promega
Corporation (Madison, WI, USA). The program for PCR was: 95°C for
15 min, followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec
and 72°C for 30 sec.
To determine the mRNA expression levels of MAP3K8,
RT was performed using Revert Aid First Strand cDNA synthesis kit
(Thermo Fisher Scientific, Vilnius, Lithuania), according to the
manufacturer’s instructions. The 20 μl PCR mixture contained
10 μl 2X QuantiTect SYBR Green PCR Master mix (Takara Bio,
Inc.), 1 μl cDNA template, 1 μl each primer and
RNase-free water. The program for PCR was: 95°C for 15 min,
followed by 45 cycles of 94°C for 15 sec, 57°C for 30 sec and 72°C
for 30 sec. The expression levels of MAP3K8 were evaluated using
the comparative threshold cycle (Ct) method (9). GAPDH was used as an internal control.
The primers used for MAP3K8 and GAPDH are listed in Table II. All reactions were performed in
triplicate.
Cell migration assay
Cell migration was examined using a wound scratch
assay, as described previously (9). The 786-O and ACHN cells (~500,000
each) were seeded into 6-well plates and, following 24 h incubation
at 37°C, were transfected with the miRNA-509-3p mimic, si-MAP3K8 or
negative control using Lipofectamine 2000 (Invitrogen Life
Technologies). Following 6 h incubation at 37°C, a sterile 200
μl pipette tip and marker was used to create a scratch in
the cell monolayer. The cells were then washed with
phosphate-buffered saline (PBS) three times and incubated at 37°C.
Images of the scratches were captured with a digital camera (DMIRB;
Leica Microsystems GmbH, Wetzlar, Germany) 0 and 24 h after the
scratches were made. The MIAS-2000 software program (Leica
Microsystems GmbH) was used to determine the distance of migration.
The experiments were performed in three independent repeats in
triplicate and were analyzed in a blinded-manner by at least two
observers.
Cell proliferation assay
The cell proliferation was determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, according to the manufacturer’s instructions. The 786-O and
ACHN cells were seeded into 96-well culture plates at a cell
density of 6,000 cells/well in growth medium (DMEM containing 10%
FBS) and transfected with 10 pmol miRNA-509-3p mimic, si-MAP3K8 or
negative control. The cell growth was assessed by adding 20
μl MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) to each
well, and incubating at 37°C for 4 h. The proliferation assay was
performed for 3 days and cell growth was examined every 24 h. The
reaction was inhibited by the addition of 150 μl dimethyl
sulfloxide (DMSO; Sigma-Alrdich). Following agitation for 15 min at
room temperature, the optical density (OD) of each sample, at a
wavelength of 490 nm, was measured using an Enzyme Immunoassay
instrument (Model 680; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). All assays were performed in triplicate.
Target gene prediction
Prediction of the miRNA target was performed using
computational algorithms, according to the base-pairing rules
between the miRNA and mRNA target sites, location of binding
sequences within the target’ 3′UTR, and conservation of target
binding sequences within associated genomes. In the present study,
genes were predicted using the miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk),
TargetScan v5.1 (http://www.targetscan.org), microRNA (http://www.microrna.org/microrna/home.do) and miRDB
(http://mirdb.org/miRDB/index.html)
computational algorithms.
Luciferase reporter assay
The present study generated luciferase reporter
plasmids containing the MAP3K8 3′UTR fragment and the miRNAs target
sequences inserted between the XhoI-NotI restriction
sites in the 3′UTR of the hRluc gene in the psiCHECK-2 luciferase
vector (Promega Corporation). To generate the psiCHECK-2-3′-UTR-WT
plasmid, primer sequences for the 3′UTR of MAP3K8 mRNA were
designed. In order to confirm the binding sites of the miR-509-3p,
two short fragments of the MAP3K8 3′UTR were synthesized, which
contained the potential binding sites and the matching sequences
(underlined in Table II). The
potential binding sites were mutated manually by exchanging the G
and T, A and C, to produce the psiCHECK-2-3′-UTR-MUT plasmid. The
forward and reverse sequences are shown in Table II (MAP3K8-3′UTR-WT and
MAP3K8-3′UTR-MUT′). These short fragments were cloned into the
psiCHECK-2 luciferase vector and the constructs were verified by
bidirectional sequencing between the XhoI-NotI
restriction sites in the 3′UTR of the hRluc gene in the psiCHECK-2
luciferase vector. Sequencing was performed by Invitrogen Life
Technologies. The luciferase reporter construct, together with the
miR-509-3p mimics or negative control, were subsequently
transfected into the 786-O and ACHN cells using Lipofectamine 2000
(Invitrogen Life Technologies). At 24 h post-transfection, the
firefly and Renilla luciferase activities of the cells were
detected using a Dual-Luciferase® (DLR™) Reporter Assay
system (Promega Corporation) on the Modulus™ Single Tube Multimode
Reader (Bio-Systems International, Beloit, WI, USA). Normalized
data were calculated as the quotient of Renilla/firefly
luciferase activities. These experiments were performed in
duplicate and were repeated at least three times.
Protein extraction and western blot
analysis
The proteins were extracted for western blot
analysis 48 h after transfecting the 786-O and ACHN cells with the
miRNA-509-3p mimics, si-MAP3K8 and negative control. The cells were
washed three times with PBS and lysed in radioimmunoprecipitation
buffer (Sigma-Aldrich) to extract the total cellular protein. The
protein content was determined using an Enhanced BCA protein assay
kit (Beyotime Institute of Biotechnology, Shanghai, China). The
total protein (30 μg) from each sample was separated by
electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels
prior to being transferred onto polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA) for 2 h. The membranes were blocked
for 1 h at room temperature using 5% non-fat milk and subsequently
incubated in Tris-buffered saline with 0.05% Tween-20®
(TBST; Ameresco, Solon, OH, USA), containing rabbit polyclonal
immunoglobulin (Ig)G anti-MAP3K8 (cat. no. 152104; 1:500; Abcam,
Hong Kong, China) or rabbit polyclonal β-actin (cat. no. 600-532;
1:5,000; Novus, Littleton, CO, USA) antibodies overnight at 4°C.
After 24 h, the membranes were washed with PBS three times and
incubated with peroxidase-conjugated goat anti-rabbit Ig G
secondary antibody (cat. no. 7074; 1:5,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 37°C for 2 h. The protein
expression was evaluated using chemiluminescence [Immun-Star™
Horseradish Peroxidase (HRP) Chemiluminescence kit; Bio-Rad
Laboratories, Inc.] and exposure to Kodak film (Kodak, Rochester,
NY, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation from at least three separate experiments. Statistical
significance was determined using Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses was performed using SPSS 16.0 statistical
software (SPSS, Inc., Chicago, IL, USA).
Results
miR-509-3p is downregulated and MAP3K8 is
upregulated in RCC cell lines
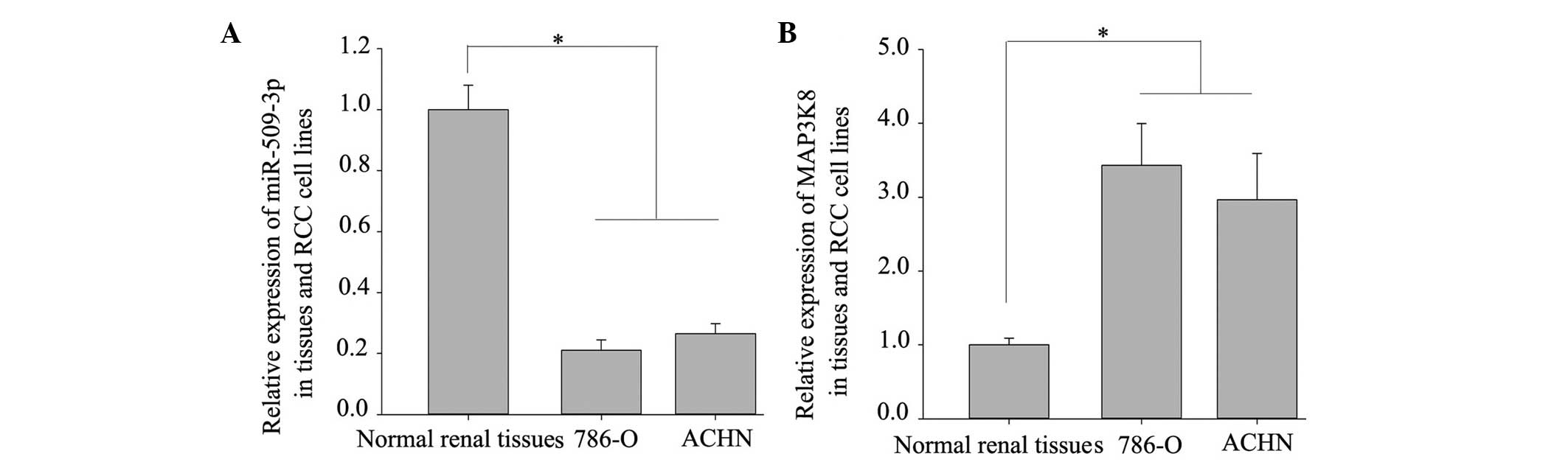
To evaluate the mRNA expression levels of miR-509-3p
and MAP3K8 in the 786-O and ACHN RCC cell lines, equal quantities
of total RNA from the normal adjacent tissues of 10 patients with
RCC were pooled together as a control. The mRNA expression levels
of miR-509-3p and MAP3K8 in each RCC cell line were compared with
the pool of 10 normal specimens by RT-qPCR. The results
demonstrated that all the cell lines downregulated the expression
of miR-509-3p (Fig. 1A) and
upregulated MAP3K8 (Fig. 1B),
suggesting that miR-509-3p acted as a tumor suppressor and MAP3K8
acted as an oncogene in the RCC cells.
Proliferative and migratory abilities of
RCC cells are regulated by miR-509-3p
To further confirm the role of miR-509-3p in RCC
cells, scratch and MTT assays were performed to evaluate the
proliferative and migratory capacities of the 786-O and ACHN cells
treated with the miR-509-3p mimics or negative control for 24 h. As
shown in Fig. 2A, cell migration
was significantly inhibited in the groups transfected with
miR-509-3p compared with those in the negative control group. The
inhibitory rates of migration were 31.68% in the 786-O cells
(P<0.05) and 28.66% in the ACHN cells (P<0.05), indicating
that miR-509-3p had a negative effect on cellular migration. The
MTT assay demonstrated that the relative cell proliferation in the
miR-509-3p transfectants was significantly decreased by 4.32 (24 h;
P<0.05), 12.14 (48 h; P<0.05) and 16.79% (72 h; P<0.05) in
the 786-O cells. Similarly, the proliferation of the ACHN cells was
inhibited by 5.83 (24 h, P<0.05), 12.1 (48 h; P<0.05) and
14.39 (72 h; P<0.05). The MTT assay results are shown in
Fig. 2B. These results
demonstrated that miR-509-3p markedly affected the migration and
proliferation of the RCC cell lines.
MAP3K8 is the target gene of
miR-509-3p
To predict the targets of miR-509-3p, four
computational algorithms (miRWalk, TargetScan, microrna and miRDB)
were used. From the potential targets, the present study focused on
the oncogene, MAP3K8. As shown in the Fig. 3A, MAP3K8 revealed putative target
sites at position 2397–2419 of the 3′UTR, with an exact match in
the seed region at position 2410–2419. This site is located in
conserved regions of the MAP3K8 3′UTR in Homo sapiens
(microRNA; http://www.microrna.org/microrna/home.do). To confirm
that MAP3K8 was a target of miR-509-3p, a luciferase reporter assay
was performed. The relative luciferase activity of the reporter,
which contained the wild-type 3′UTR, was significantly suppressed
when the miR-509-3p was cotransfected in the 786-O and ACHN cells
(Fig. 3B). By contrast, the
luciferase activity of the mutant reporter was unaffected following
cotransfection with miR-509-3p (Fig.
3B). Collectively, these results demonstrated that MAP3K8 was a
target gene of miR-509-3p and identified the site of interaction in
the 3′UTR of MAP3K8.
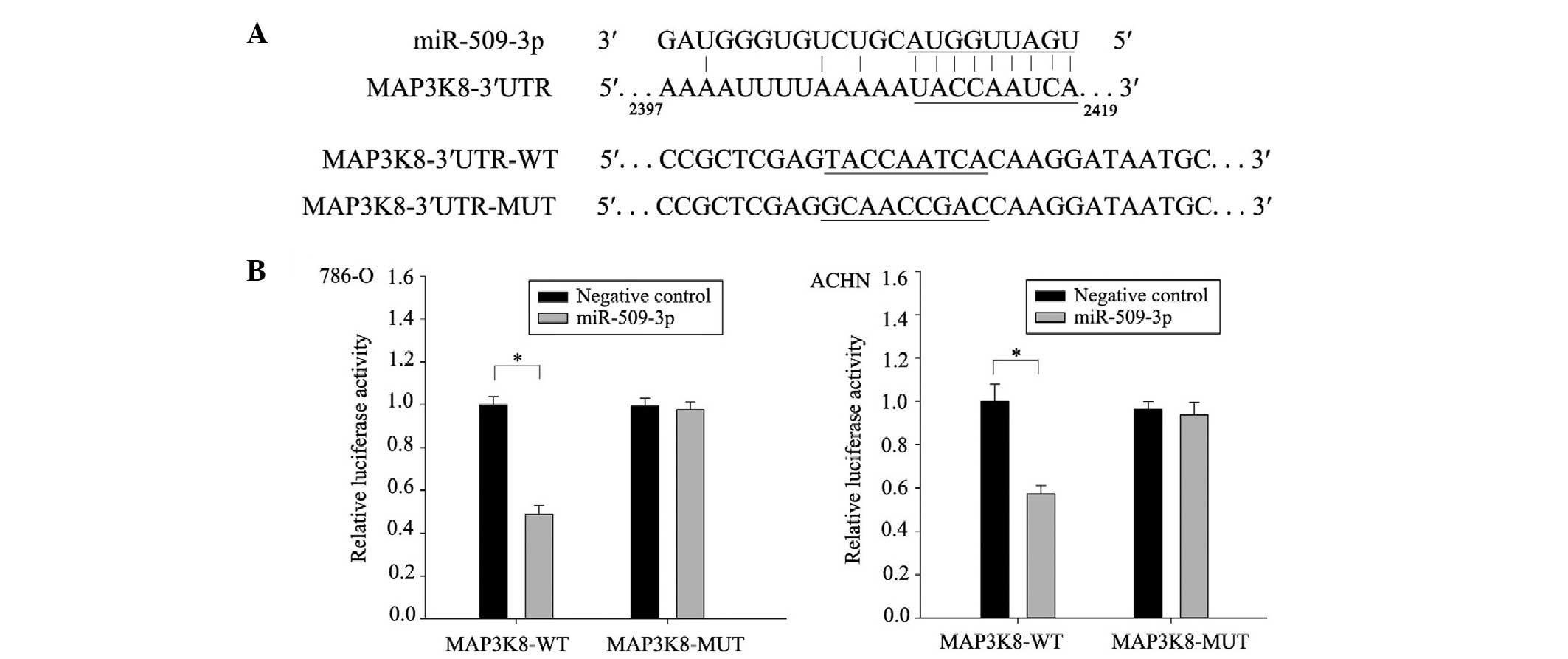 | Figure 3MAP3K8 is the target gene of
miR-509-3p. (A) Putative duplex formation between miR-509-3p and
the MAP3K8 3′UTR. The seed-recognizing sites are underlined. A
21-nucleotide-long fragment of the MAP3K8 3′UTR was synthesized,
containing WT potential binding sites. The MUT fragment was
generated by exchanging the G and T, A and C on the putative
binding sites. (B) Luciferase reporter constructs containing the
3′UTR of MAP3K8, together with the miR-509-3p mimics or negative
control, were transfected into the 786-O and ACHN cells. After 48
h, luciferase activity was detected. The relative luciferase
activity of the reporter, containing the WT 3′UTR was significantly
suppressed when miR-509-3p was cotransfected into the 786-O and
ACHN cells. By contrast, the luciferase activity of the MUT
reporter was unaffected by cotransfection with miR-509-3p. The
experiments were performed in duplicate and repeated three times.
Data are expressed as the mean ± standard deviation (*P<0.05,
compared with the negative control). RCC, renal cell carcinoma;
miR, microRNA; MAP3K8, mitogen-activated protein kinase kinase
kinase 8; UTR, untranslated region; WT, wild-type; MUT, mutant. |
MAP3K8 is downregulated by miR-509-3p in
the RCC cell lines
The RT-qPCR and western blot analyses were performed
to confirm whether the expression of miR-509-3p affected the
expression of endogenous MAP3K8 at the transcriptional and
translational levels. Consistent with the results from the
luciferase report assay, the cells transfected with the miR-509-3p
mimics exhibited decreased mRNA expression of MAP3K8 (Fig. 4A) compared with those transfected
with the negative control. The overexpression of miR-509-3p caused
a decrease in the protein expression levels of MAP3K8 in the two
RCC cell lines (Fig. 4B).
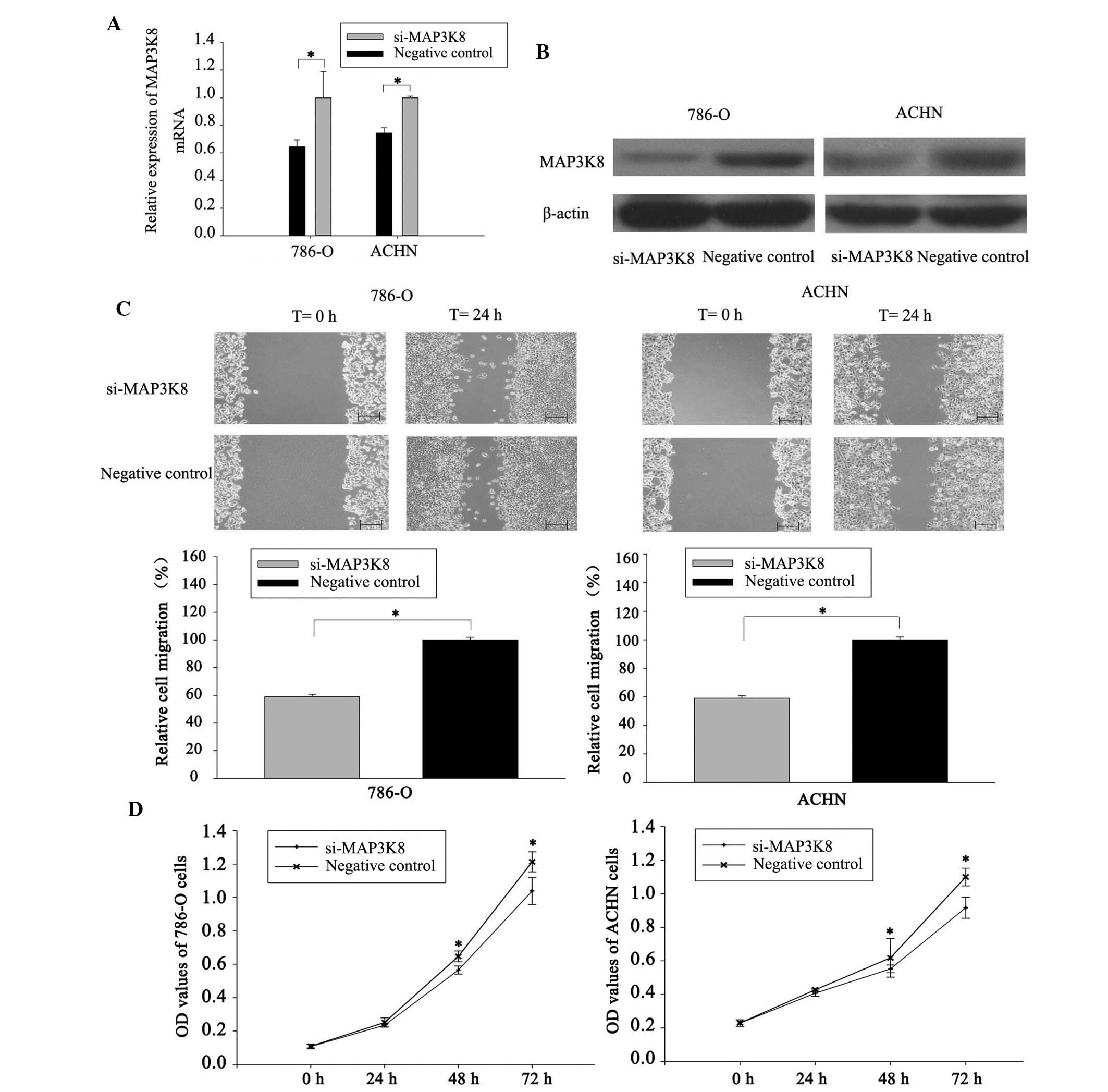
Effects of si-MAP3K8 transfection on the
RCC cells
To examine the significance of MAP3K activity in the
cell variability of RCC cells, loss-of-function analyses were
performed in the 786-O and ACHN cells transfected with the
si-MAP3K8 sequences. The mRNA and protein expression levels of
MAP3K8 were markedly repressed following si-MAP3K8 transfection
(Fig. 5A and B).
The scratch assays were performed to observe the
importance of MAP3K8 in cell migration. As shown in Fig. 5C, cell migration was significantly
inhibited in the groups transfected with si-MAP3K8 compared with
those in the negative control group. The inhibitory rates of
migration were 26.6% in the 786-O cells (P<0.05) and 40.92% in
the ACHN cells (P<0.05), indicating that knockdown of MAP3K8
inhibited the migration of the RCC cells.
To determine the potential role of MAP3K8 on the
proliferation of RCC cells, MTT assays were performed. The
si-MAP3K8 group and negative control group were measured at 0, 24,
48 and 72 h following transfection. The OD values demonstrated that
the proliferation of 786-O cells decreased by 6.88, 14.51 and 16.8%
(P<0.05), while the proliferation rates of the ACHN cells
decreased by 4.91, 12.01 and 19.95% (P<0.05) at 24, 48 and 72 h
after transfection, respectively. This suggested that silencing of
MAP3K8 suppressed the growth of the 786-O and ACHN cells compared
with the negative control (Fig.
5D).
Discussion
RCC is a highly vascularized tumor, which accounts
for 3% of all malignancies in adults and has the highest rate of
mortality of all urological malignancies (19,20).
However, the current treatment options of surgery, radiation and
chemotherapy are relatively ineffective in treating advanced RCC.
Therefore, it is vital to understand the molecular mechanisms
underlying the metastasis of RCC and to identify alternative
treatment strategies.
Previous studies have demonstrated that miRNAs
exhibit unique expression profiles in different types of cancer at
different stages, and are important in the initiation and
progression of several diseases. This suggests that miRNAs have a
crucial function in the occurrence and development of cancer
(21,22). Several miRNAs have been revealed as
either tumor suppressors or oncogenes in the development of RCC.
miR-210, miR-155 and miR-21 are upregulated and highly correlated
with patient survival rates in RCC (23). By contrast, miR-708, miR-141 and
miR-145 are downregulated in human RCC specimens (9,24,25).
The present study revealed that the expression levels of miR-509-3p
were downregulated in the 786-O and ACHN cells compared with the
normal adjacent RCC tissues. This result was consistent with those
of previous sequencing and RT-qPCR analyses in RCC tissues
(20). It was hypothesized that
miR-509-3p may be important as a tumor suppressor in RCC,
therefore, the present study investigated the functional
significance of miR-509-3p in RCC and found that miR-509-3p
inhibited cell proliferation and migration in 786-O and ACHN RCC
cells. Taken together, these results demonstrated the anticancer
effect of miR-509-3p, providing sufficient evidence to support the
tumor suppressive effect of miR-509-3p in RCC.
The results of the present study highlighted the
importance of miR-509-3p as a tumor suppressor in RCC, however, the
underlying molecular mechanisms remain to be elucidated. To improve
understanding of the tumor suppressive effect of miR-509-3p in
renal tumorigenesis, four computational algorithms were used to
predict the targets of miR-509-3p, which identified the MAP3K8 gene
as a novel target. The target gene and the binding sites of
miR-509-3p on the MAP3K8 3′UTR were confirmed using a luciferase
reporter assay.
Previous studies have demonstrated that MAP3K8 is a
member of the serine/threonine protein kinase family (17,26,27).
When overexpressed in cell lines, MAP3K8 activates the
extracellular signal-regulated kinase (ERK), JNK and p38 MAPK
pathways by inducing the phosphorylation and activation of the
respective MAP2K (27). MAP3K8 has
been the focus of investigations due to the close association
between the expression of MAP3K8 and tumor progression, growth and
metastasis. MAP3K8 is important in the promotion of androgen
depletion-independent (ADI) prostate cancer progression (15). The overexpression of MAP3K8
correlates with poor prognosis in patients with early-onset CRC
cancer (16). The present study
revealed that the expression of MAP3K8 was upregulated in RCC
cells, suggesting that it acted as an oncogene in RCC.
In the present study, miR-509-3p directly regulated
the expression of MAP3K8 in a luciferase reporter assay. The MAP3K8
oncogene was identified to be negatively regulated by miR-509-3p at
the post-transcriptional level through a specific target site
within the 3′UTR of the gene. This suggested that miR-509-3p may
exhibit tumor suppressive functions by regulating the oncogene in
RCC. Loss of miR-509-3p in RCC may lead to the upregulation of
MAP3K8, providing a selective growth and expansion advantage during
renal carcinogenesis.
The present study also identified that the
overexpression of miR-509-3p suppressed the mRNA and protein
expression levels of MAP3K8, and the knockdown of MAP3K8 inhibited
the migration and proliferation of the RCC cells. Taken together,
these results indicated that miR-509-3p may affect the MAPK
signaling pathway is a potential therapeutic target against the
MAPK signaling axis in preventing the development and progression
of RCC.
In conclusion, the results of the present study
demonstrated that miR-509-3p was significantly downregulated in the
RCC cell lines and appeared to function as a tumor suppressor in
RCC through the regulation of MAP3K8. Therefore, miR-509-3p may be
a potential target in the prognosis and treatment of RCC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81101922), the Medical Scientific
Research Foundation of Guangdong Province of China (nos. A2012584
and A2013606), the Science and Technology Development Fund Project
of Shenzhen (no. CYJ20130402114702124) and the fund of the
Guangdong Key Medical Subject.
References
|
1
|
Yamada Y, Hidaka H, Seki N, et al:
Tumor-suppressive microRNA-135a inhibits cancer cell proliferation
by targeting the c-MYC oncogene in renal cell carcinoma. Cancer
Sci. 104:304–312. 2013. View Article : Google Scholar
|
|
2
|
Mulders PF, Brouwers AH, Hulsbergen-van
der Kaa CA, van Lin EN, Osanto S and de Mulder PH: Guideline ‘Renal
cell carcinoma’. Ned Tijdschr Geneeskd. 152:376–380. 2008.in Dutch.
PubMed/NCBI
|
|
3
|
Hadoux J, Vignot S and De La Motte Rouge
T: Renal cell carcinoma: Focus on safety and efficacy of
temsirolimus. Clin Med Insights Oncol. 4:143–154. 2010. View Article : Google Scholar
|
|
4
|
Patel C, Ahmed A and Ellsworth P: Renal
cell carcinoma: A reappraisal. Urol Nurs. 32:182–190; quiz.
1912012.PubMed/NCBI
|
|
5
|
Yu ZH, Zhang Q, Wang YD, et al:
Overexpression of cyclooxygenase-1 correlates with poor prognosis
in renal cell carcinoma. Asian Pac J Cancer Prev. 14:3729–3734.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bouyssou JM, Manier S, Huynh D, Issa S,
Roccaro AM and Ghobrial IM: Regulation of microRNAs in cancer
metastasis. Biochim Biophys Acta. 1845:255–265. 2014.PubMed/NCBI
|
|
8
|
Xi Y: MicroRNA: A new player for cancer
chemoprevention. J Integr Oncol. 2:1052013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z,
Zhang S, Nie L and Yu Z: miR-145 functions as tumor suppressor and
targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J
Cancer Res Clin Oncol. 140:387–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Z, Ni L, Chen D, et al: Identification
of miR-7 as an oncogene in renal cell carcinoma. J Mol Histol.
44:669–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng H, Luo J, Hao H, Hu J, Xie SK, Ren D
and Rao B: MicroRNA-100 regulates SW620 colorectal cancer cell
proliferation and invasion by targeting RAP1B. Oncol Rep.
31:2055–2062. 2014.PubMed/NCBI
|
|
12
|
Xie J, Chen M, Zhou J, Mo MS, Zhu LH, Liu
YP, Gui QJ, Zhang L and Li GQ: miR-7 inhibits the invasion and
metastasis of gastric cancer cells by suppressing epidermal growth
factor receptor expression. Oncol Rep. 31:1715–1722.
2014.PubMed/NCBI
|
|
13
|
Salmeron A, Ahmad TB, Carlile GW, Pappin
D, Narsimhan RP and Ley SC: Activation of MEK-1 and SEK-1 by Tpl-2
proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J.
15:817–826. 1996.PubMed/NCBI
|
|
14
|
Krcova Z, Ehrmann J, Krejci V, Eliopoulos
A and Kolar Z: Tpl-2/Cot and COX-2 in breast cancer. Biomed Pap Med
Fac Univ Palacky Olomouc Czech Repub. 152:21–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong JH, Bhatia A, Toth Z, Oh S, Inn KS,
Liao CP, Roy-Burman P, Melamed J, Coetzee GA and Jung JU:
TPL2/COT/MAP3K8 (TPL2) activation promotes androgen
depletion-independent (ADI) prostate cancer growth. PLoS One.
6:e162052011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aparecida Alves C, Silva ID, Villanova FE,
Nicolau SM, Custódio MA, Bortoletto C and Gonçalves WJ:
Differential gene expression profile reveals overexpression of
MAP3K8 in invasive endometrioid carcinoma. Eur J Gynaecol Oncol.
27:589–593. 2006.
|
|
17
|
Tunca B, Tezcan G, Cecener G, et al:
Overexpression of CK20, MAP3K8 and EIF5A correlates with poor
prognosis in early-onset colorectal cancer patients. J Cancer Res
Clin Oncol. 139:691–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martínez-Salamanca JI, Huang WC, Millan I,
et al: Prognostic impact of the 2009 UICC/AJCC TNM staging system
for renal cell carcinoma with venous extension. Eur Urol.
59:120–127. 2011. View Article : Google Scholar
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai Q, Zhou L, Zhao C, Wan J, Yu Z, Guo
X, Qin J, Chen J and Lu R: Identification of miR-508-3p and
miR-509-3p that are associated with cell invasion and migration and
involved in the apoptosis of renal cell carcinoma. Biochem Biophys
Res Commun. 419:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Fu H, Wang Y, Tie Y, Xing R, Zhu J,
Sun Z, Wei L and Zheng X: MicroRNA-101 regulates expression of the
v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene
in human hepatocellular carcinoma. Hepatology. 49:1194–1202. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song T, Zhang X, Wang C, Wu Y, Cai W, Gao
J and Hong B: MiR-138 suppresses expression of hypoxia-inducible
factor 1α (HIF-1α) in clear cell renal cell carcinoma 786-O cells.
Asian Pac J Cancer Prev. 12:1307–1311. 2011.
|
|
23
|
Neal CS, Michael MZ, Rawlings LH, Van der
Hoek MB and Gleadle JM: The VHL-dependent regulation of microRNAs
in renal cancer. BMC Med. 8:642010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saini S, Yamamura S, Majid S, Shahryari V,
Hirata H, Tanaka Y and Dahiya R: MicroRNA-708 induces apoptosis and
suppresses tumorigenicity in renal cancer cells. Cancer Res.
71:6208–6219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu XY, Zhang Z, Liu J, Zhan B and Kong CZ:
MicroRNA-141 is downregulated in human renal cell carcinoma and
regulates cell survival by targeting CDC25B. Onco Targets Ther.
6:349–354. 2013.PubMed/NCBI
|
|
26
|
Johannessen CM, Boehm JS, Kim SY, et al:
COT drives resistance to RAF inhibition through MAP kinase pathway
reactivation. Nature. 468:968–972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim K, Kim G, Kim JY, Yun HJ, Lim SC and
Choi HS: Interleukin-22 promotes epithelial cell transformation and
breast tumorigenesis via MAP3K8 activation. Carcinogenesis.
35:1352–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|